A panel of the world's leading economists recently ranked providing ‘micronutrient supplements for children’ as the best investment for advancing global welfare, particularly in developing countries( 1 ). Fe deficiency is the most common nutritional deficiency in the world and is considered a major cause of anaemia, particularly during infancy and early childhood( 2 , 3 ). According to the WHO, more than 750 million children suffer from Fe deficiency and anaemia, with the majority coming from developing countries( 2 , 3 ). While little has been reported on the prevalence of Ca deficiency, studies in developing countries describe a growing public health concern with low Ca intakes resulting in bone disorders among infants and children( Reference Combs, Hassan and Dellagana 4 – Reference Thacher, Fischer and Pettifor 6 ).
The inhibitory effect of dietary Ca on Fe absorption is well described( Reference Galan, Hercberg and Soustre 7 – Reference Hallberg 13 ). However, few studies have been conducted in paediatric populations and none to our knowledge have examined this nutrient–nutrient interaction among anaemic infants. Moreover, the studies performed were of short duration (often less than 1 month), carried out in developed countries, included the use of Fe-fortified infant formula, and used healthy infants and children with adequate Fe and Ca intakes( Reference Galan, Hercberg and Soustre 7 – Reference Dalton, Sargent and O'Connor 15 ). Interestingly, some studies, particularly those of longer duration, do not demonstrate this nutrient–nutrient interaction( Reference Lonnerdal 16 ).
In developing countries, providing a diet that is nutritionally adequate for children 6 to 24 months of age is difficult to achieve( Reference Mensah and Tomkins 17 ). This is because the majority of complementary foods consumed are based on plants, cereals or roots that have low micronutrient content, poor micronutrient bioavailability and contain high amounts of phytates, oxalates, dietary fibre and polyphenols that are known to inhibit the absorption of Fe and Ca( Reference Mensah and Tomkins 17 – Reference Lind, Lonnerdal and Persson 19 ). Studies assessing the habitual Ca intake in Bangladesh report that all age groups obtain only 30 % of the Adequate Intake, with milk and dairy products not often consumed in the typical diet( Reference Islam, Lamberg-Allardt and Karkkainen 20 , Reference Arnaud, Pettifor and Cimma 21 ). The prevalence of Fe-deficiency anaemia (IDA) is extremely high throughout South Asia and is reported to be 55 % among children younger than 5 years of age in Bangladesh( 22 ).
Community-based trials in several regions including Northern Canada, Asia, Africa and Central America have demonstrated that the use of micronutrient powder (MNP; Sprinkles™) over an 8-week period, daily or weekly or flexibly, can effectively increase Fe intake and Hb concentration in anaemic children between the ages of 6 and 24 months( Reference Lundeen, Schueth and Toktobaev 23 – Reference Christofides, Schauer and Sharieff 28 ). However, the original Sprinkles MNP formulation did not contain Ca because of the postulated negative effect on Fe absorption( Reference Hyder, Haseen and Rahman 29 ). In the current study, we used a new Sprinkles MNP formulation which included Ca in addition to Fe, Zn, folic acid, ascorbic acid and vitamin A. Our primary objective was to investigate whether dietary Ca intake could be increased to its Adequate Intake in anaemic infants aged 6 to 11 months through home fortification with Ca- and Fe-containing Sprinkles MNP without compromising the expected Hb response.
Experimental methods
Study area and participants
The study was conducted in twenty-six rural villages of Kaliganj, a sub-district in the Gazipur region of Bangladesh, from April to June 2010. The villages were selected on the basis of discussions and approval from sub-district executives, a thana nirbahi officer and village elders/leaders. Kaliganj is a fair representation of rural Bangladesh with its high population density, fertile agricultural land, susceptibility to seasonal flooding, and limited access to health-care and education services. Similar to other rural settings of Bangladesh, the study area is known to have widespread malnutrition and poverty, with women and children bearing most of the burden( Reference Hyder, Haseen and Rahman 29 , Reference Shakur, Choudhury and Hyder 30 ). Kaliganj is not an area with endemic malaria or hookworm (hookworms affect fewer than 2 % of children under the age of 2 years), but it does have a high prevalence of IDA (approximately 87 %) among children 6 to 24 months of age( Reference Hyder, Haseen and Rahman 29 – Reference Sarker, Mahmud and Davidsson 31 ). The habitual intake of Ca in children of this age group is reported to be only 30 % of the Adequate Intake( Reference Arnaud, Pettifor and Cimma 21 ).
Eligible infants between the ages of 6 and 11 months were screened on the basis of the following inclusion criteria: (i) Hb concentration between 70 and 100 g/l; (ii) consuming complementary food in addition to breast milk at least once daily; (iii) free from acute or chronic illness; (iv) afebrile; (v) weight-for-age Z-score >−3 based on the WHO growth standard; (vi) no history of Fe or Ca supplementation prior to two weeks of recruitment; and (vii) being permanent residents of their village. All infants diagnosed with severe anaemia (Hb < 70 g/l) were referred to a local health-care facility for treatment.
Study design
The study was a randomized, double-blind, controlled trial with an intervention period of 8 weeks. This duration was based on previous Sprinkles MNP studies, which demonstrated that daily, weekly or flexible administration could effectively increase Fe intake and Hb concentrations in anaemic children between the ages of 6 and 24 months( Reference Lundeen, Schueth and Toktobaev 23 – Reference Christofides, Schauer and Sharieff 28 ). Upon completion of the baseline assessment, infants were randomly assigned to one of two intervention groups: (i) Sprinkles MNP with Ca (Ca-MNP) or (ii) Sprinkles without Ca (MNP). Individual randomization was performed using sealed opaque envelopes with intervention group designations. Sachets were labelled with one of two numeric codes to indicate the formulation inside. All individuals involved in the study (including parents, field workers and research staff) were blinded to the group assignments and sachet numerical codes for the duration of the study and data analysis.
The primary outcome measure of the study was change in Hb concentration (baseline to end). Data on dietary intake using an FFQ and 24 h recall, adherence to the intervention and side-effects were also collected. Determination of the sample size was based on the primary outcome of the study. The standard deviation of Hb concentration was estimated to be 15·1 g/l, based on a previous Sprinkles MNP study conducted on individuals of the same background( Reference Ip, Hyder and Haseen 25 ). With a type I error of 5 %, power of 90 % and the assumption of a 10 % loss to follow-up, the sample size was determined to be fifty infants per intervention group to detect a 10·5 g/l difference in Hb concentration both within and between groups at 8 weeks of follow-up.
Micronutrient powder formulations
Two MNP formulations were used in the study; the composition of each is shown in Table 1. The micronutrient content in both interventions was based on previous bioavailability and dose–response studies using Sprinkles MNP, the Recommended Nutrient Intakes published by the WHO/FAO and the Dietary Reference Intakes of the Institute of Medicine( Reference Hyder, Haseen and Rahman 29 , 32 , 33 ). Although there is a discrepancy between the Institute of Medicine and WHO/FAO regarding the recommended intake of Ca for infants aged 6 to 11 months, the higher WHO/FAO value was used as the current study was conducted in a country that does not share similar dietary patterns to those of the developed world( Reference Lutter and Dewey 34 , 35 ). The micronutrient dosages did not exceed the Tolerable Upper Intake Level set by the Institute of Medicine( 32 ). Fortitech Inc. (New York, NY, USA) provided the MNP premix formulations, while Renata Pharmaceuticals (Dhaka, Bangladesh) packaged the powder into sachets and conducted quality control of the micronutrient content. The packages of the MNP interventions were identical, as was the appearance of their contents.
Table 1 Composition of micronutrients in the micronutrient powder (MNP) sachets

RE, retinol equivalents.
Study procedures
Mothers of selected infants were visited in their respective homes to verify eligibility. The study protocol was explained in detail and written informed consent was obtained. Sachets were delivered to the study participants weekly and mothers were instructed to administer the daily dose in a single semi-solid meal. In an attempt to ensure adherence, households were visited on alternate days to inquire about the level of MNP consumption, coach the mothers on using the MNP and address any issues related to the study. On the days that the mothers were not visited, a self-coaching picture guide and a compliance calendar were used to ensure proper use and adherence of the MNP.
At recruitment, baseline information on household demographics and the infant's breast-feeding practices were obtained from mothers using validated questionnaires. Anthropometric and Hb measurements were performed at the start and end of the study. An adjustable wooden length-board was used to measure length to the nearest 0·1 cm and all weight measurements were performed using a digital scale (model HD-305; TANITA Corporation, Tokyo, Japan), which recorded to the nearest 0·1 kg. Mothers were weighed both separately and with their infants to determine infant weight. To exclude individual variation, all anthropometric measurements were performed twice with the mean value of the two measurements taken. The anthropometric data and age of the infant were used to calculate the weight-for-age Z-score using the WHO Anthro version 3·0·1 software package. Hb was determined on capillary blood obtained from a finger prick using a portable haemoglobin spectrophotometer, HemoCue Hb 201+ analyser (HemoCue AB, Ängelholm, Sweden), and standardized techniques( Reference Cohen and Seidl-Friedman 36 ). The WHO cut-off for anaemia in children 6–59 months of age is Hb < 110 g/l( 37 ). However, the functional consequences of IDA become apparent at Hb concentration of 100 g/l. Thus, we used Hb concentration between 70 and 100 g/l to define anaemia in the present study( Reference Grantham-McGregor and Ani 38 ).
Infant food consumption was assessed at the start and end of the study. The FFQ was designed to collect information on foods containing Fe and Ca likely to be consumed by infants aged 6–11 months. For the 24 h dietary recall questionnaire, standardized measuring utensils including cups and spoons were used to determine the estimated portion sizes of food consumed. Feeding episodes included all meals reported to have been given by mothers to their infant during the morning, mid-morning, afternoon, evening and night. The recorded food items were coded and the equivalent weight of raw food was calculated using a conversion table for Bangladeshi foods developed by Hellen Keller International( 39 ). The nutritional content for a single feeding of breast milk was also provided in this table and mixed dishes were disaggregated into individual foods prior to analysis. To calculate the amount of Ca and Fe obtained from complementary foods, we multiplied the grams of each food consumed by the micronutrient content (mg/100 g) using the ‘Tables of Nutrient Composition of Bangladesh Foods’ database( 39 ). These values were then compared with the Recommended Nutrient Intakes provided by the FAO/WHO for Ca and Fe( 33 ).
Throughout the 8-week intervention, a weekly morbidity, mortality and adherence questionnaire was administered by field workers. During home visits, any episodes of fever, coughing, difficulty breathing, nausea, vomiting, loose motion, constipation, blackened stool and lack of appetite over the previous week were documented. Side-effects from a list including nausea, vomiting, retching, stool consistency and changes in stool colour within the past 12 h were also recorded. Adherence was assessed by counting the number of used and unused MNP sachets from the preceding week and through specific questions regarding the sharing and daily use of sachets.
Statistical analysis
Data were entered, stored and analysed using the statistical software package SAS version 9·1. The primary clinical outcome, change in Hb, was analysed using an intention-to-treat model for all randomized infants. A one-sample Kolmogorov–Smirnov test was used to determine normality of the main outcome variable and other continuous variables. To compare the change in mean Hb concentration, dietary intakes of Ca and Fe, reported health complications, adherence, anthropometric measurements, and socio-economic and demographic characteristics between groups, the independent-samples Student's t test (on continuous variables) and the χ 2 tests (for categorical variables) were performed. Pearson and Spearman correlation matrices were generated to identify continuous and non-continuous variables significantly associated with a change in Hb concentration between groups. Significant variables were then included as candidate predictors in multivariate analyses. An interaction between intervention group and baseline Hb was included in the final model to examine whether the difference in Hb was independently associated with intervention group and Hb baseline status, or whether baseline Hb status modified the association between the difference in Hb and groups. The effect of potential confounding variables was assessed in all multivariate models. Hb concentration was treated as a dichotomous variable (anaemic v. non-anaemic) and the prevalence of anaemia post-intervention was compared between groups using the χ 2 test. Linear regression analyses of factors associated with dietary Fe and Ca intakes were also conducted. If the distribution of a dependent variable in a linear regression model was skewed, log transformation was performed. In all analyses, statistical significance was defined as P < 0·05.
Ethics approval
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by Research Ethics Committees at The Hospital for Sick Children (Toronto, Canada) and BRAC University (Dhaka, Bangladesh). Written informed consent was obtained from all mothers.
Results
Study attrition
Of the 100 infants enrolled in the study, five were lost to follow-up due to family migration. Consequently, a total of ninety-five infants (forty-eight in the MNP and forty-seven in the Ca-MNP group) completed the final assessment, including Hb, dietary and anthropometric measurements (Fig. 1).
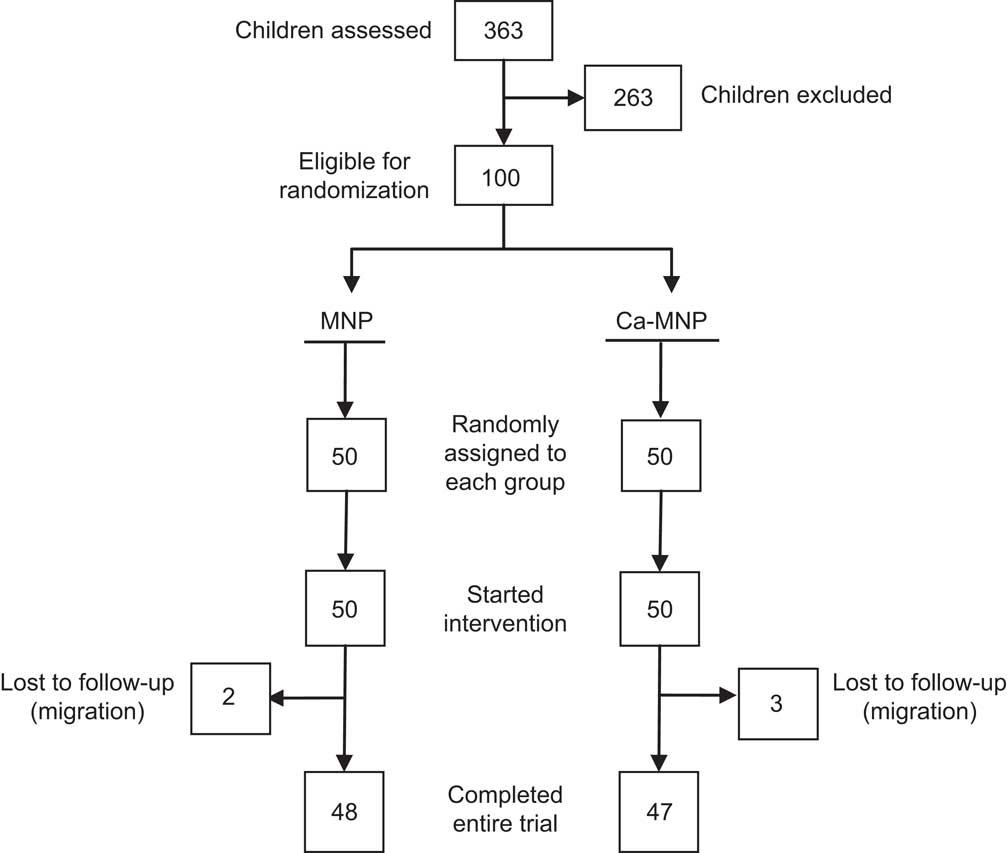
Fig. 1 Flow diagram illustrating the number of infants screened, excluded and randomly assigned, and the temporal pattern of dropouts, for each group
Baseline characteristics
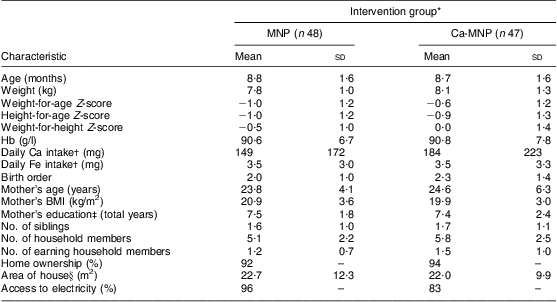
At baseline, the groups did not differ in terms of demographic characteristics (Table 2). The ratios of males to females were 26:22 (MNP group) and 20:27 (Ca-MNP group), and all mothers reported breast-feeding their infants. The mean Hb concentration was also similar between groups (MNP, 90·6 (sd 6·7) g/l v. Ca-MNP, 90·8 (sd 7·8) g/l; P = 0·90). The infants’ weight-for-age Z-scores at baseline are presented in Table 2 and were not different between groups at the start and end of the study (P = 0·34).
Table 2 Infant and household sociodemographic characteristics by intervention group at baseline; infants aged 6–11 months (n 95) from twenty-six rural villages of Kaliganj sub-district, Gazipur region, Bangladesh, April–June 2010
MNP group, received Sprinkles micronutrient powder containing Fe, Zn, folic acid, ascorbic acid and vitamin A; Ca-MNP group, received the same powder with Ca.
*Values are presented as mean and standard deviation, or percentage where indicated. Intervention groups were compared using the independent-samples Student's t test for all variables except sex, home ownership and access to electricity for which a χ 2 test was performed. Groups did not differ for any of the variables (P < 0·05).
†Data on baseline Ca and Fe intakes were missing from one infant in each group.
‡Data on mother's education were missing from nine infants in the MNP group and six infants in the Ca-MNP group.
§Data on area of house were missing from four infants in the MNP group and three infants in the Ca-MNP group.
Effect of Sprinkles micronutrient powder on Hb concentration
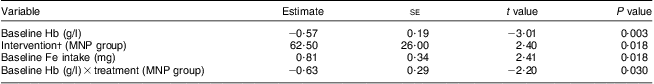
After the 8-week intervention, there was a significant increase in the mean Hb concentration in both groups (MNP, 13·3 (sd 12·6) g/l v. Ca-MNP, 7·6 (sd 11·6) g/l; P < 0·0001), with a significantly greater increase in infants who received the MNP intervention (P = 0·024; Table 3). Fifty-three per cent (50/95) of infants improved from an anaemic to a non-anaemic state (Hb > 100 g/l) post-intervention, but the two groups differed significantly in the likelihood of recovery (Fig. 2). Infants in the Ca-MNP group were more likely to remain anaemic (OR 3·2; 95 % CI 1·4, 7·5). Baseline Hb (Pearson's r = −0·53; P < 0·0001), baseline dietary Fe intake (Pearson r = 0·29; P = 0·004), treatment group (Spearman's ρ = −0·25; P = 0·017) and the change in Fe intake from baseline to end point (Pearson's r = −0·24; P = 0·025) were variables significantly correlated with a change in Hb between groups. In the adjusted multivariate model, baseline Hb, baseline Fe intake, intervention group and the baseline × intervention group interaction were all significant predictors of the change in Hb (Table 4).
Table 3 Hb concentrations and the percentage of non-anaemic infants by intervention group at baseline and study end point; infants aged 6–11 months (n 95) from twenty-six rural villages of Kaliganj sub-district, Gazipur region, Bangladesh, April–June 2010

MNP group, received Sprinkles micronutrient powder containing Fe, Zn, folic acid, ascorbic acid and vitamin A; Ca-MNP group, received the same powder with Ca.
*Values are presented as mean and standard deviation, or percentage where indicated.
†Groups were compared using the independent-samples Student's t test.
‡Within-group analysis was performed using the paired t test.
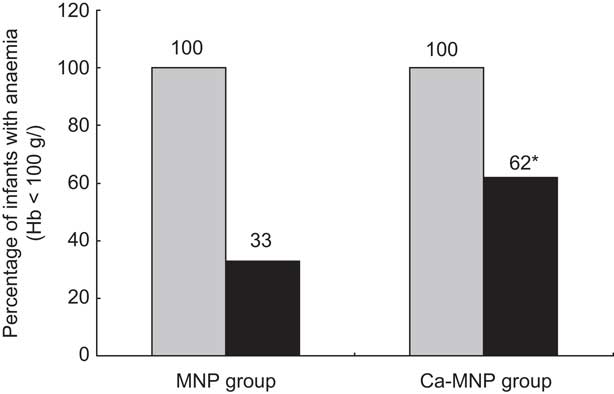
Fig. 2 Number of infants anaemic (Hb < 100 g/l) at baseline (![]() ) and remaining anaemic (
) and remaining anaemic (![]() ) at the end of the 2-month intervention period by intervention group (MNP group, received Sprinkles micronutrient powder containing iron, zinc, folic acid, ascorbic acid and vitamin A; Ca-MNP group, received the same powder with calcium); infants aged 6–11 months (n 95) from twenty-six rural villages of Kaliganj sub-district, Gazipur region, Bangladesh, April–June 2010. *Significantly more infants in the Ca-MNP group remained anaemic (χ
2 test): P = 0·0076
) at the end of the 2-month intervention period by intervention group (MNP group, received Sprinkles micronutrient powder containing iron, zinc, folic acid, ascorbic acid and vitamin A; Ca-MNP group, received the same powder with calcium); infants aged 6–11 months (n 95) from twenty-six rural villages of Kaliganj sub-district, Gazipur region, Bangladesh, April–June 2010. *Significantly more infants in the Ca-MNP group remained anaemic (χ
2 test): P = 0·0076
Table 4 Multivariate analysis of variables associated with change in infants’ Hb levelsFootnote *; infants aged 6–11 months (n 95) from twenty-six rural villages of Kaliganj sub-district, Gazipur region, Bangladesh, April–June 2010
MNP group, received Sprinkles micronutrient powder containing Fe, Zn, folic acid, ascorbic acid and vitamin A; Ca-MNP group, received the same powder with Ca.
* All listed variables included in final model; model R 2 = 40·8 %.
† MNP group change in infants’ Hb = 13·1 g/l; Ca-MNP group change in infants’ Hb = 7·4 g/l; difference between the two groups = 5·7 g/l.
Infants' dietary intake
The mean number of daily meals was 3·6 (sd 1·2) at baseline and 4·0 (sd 1·1) at end point. Foods consumed at baseline and end point respectively included: cereals, found in 39·7 % and 38·0 % of meals; meat accounted for 1·4 % and 3·5 %; dairy products represented 10·9 % and 13·4 %; fruits and vegetables were found in 24·4 % and 23·6 %; and miscellaneous items accounted for the remaining 24·1 % and 21·5 %. There was no significant difference between groups in dietary intake of Ca and Fe at either baseline (Ca, P = 0·39; Fe, P = 0·95) or end point (Ca, P = 0·22; Fe, P = 0·64). Additionally, the change in dietary Ca and Fe intake within groups did not differ. Both the mean Fe and Ca intakes obtained from complementary foods and breast milk fell significantly short (P < 0·05) of the WHO/FAO recommended intakes at baseline and end point (Table 5).
Table 5 Mean baseline and end-point iron and calcium intakes from complementary foods and breast milk by intervention group, compared with recommended nutrient intakes; infants aged 6–11 months (n 95) from twenty-six rural villages of Kaliganj sub-district, Gazipur region, Bangladesh, April–June 2010

MNP group, received Sprinkles micronutrient powder containing Fe, Zn, folic acid, ascorbic acid and vitamin A; Ca-MNP group, received the same powder with Ca.
*Fe and Ca intake values are presented as mean and standard deviation.
†All Fe and Ca intake values are represented in mg/d and calculations were based on 24 h recall questionnaires. The WHO/FAO( 33 ) RDA for Fe (9·3 mg/d) and Ca (400 mg/d) for infants 6 to 11 month of age were used to compare with calculated values. Using the independent-samples Student's t test, both the mean Fe and Ca intakes obtained from complementary foods and breast milk fell significantly short (P > 0·05) of the WHO/FAO recommended amounts at baseline and end point.
‡Recommended values are Adequate Intakes based on WHO/FAO values.
Adherence and side-effects
Adherence was determined by calculating the percentage of unused sachets out of the total assigned. On the basis of the combined data from the weekly monitoring visits, adherence in the MNP group (98·1 (sd 3·3) %) was not significantly different from adherence in the Ca-MNP group (98·4 (sd 2·8) %; P = 0·63). Sharing of sachets with a non-study child was not reported and there were no differences in the occurrence of reported side-effects between groups (56·3 % v. 59·6 %). Reported side-effects were predominantly mild to moderate in nature and consisted of constipation, darkened stools, nausea, vomiting and retching.
Discussion
To our knowledge, the present study is the first to examine the impact of combined Fe and Ca home fortification on Hb status in anaemic infants. One group of infants received a micronutrient powder containing Ca, Fe, Zn, folic acid, ascorbic acid and vitamin A, while the other received the same powder without Ca. Although both groups demonstrated a significant increase in Hb concentration from baseline to the study end point, the response was significantly lower in the group receiving Ca. Similarly, post-intervention, the group receiving Ca had a higher rate of anaemia compared with the Ca-free group. These results suggest that the addition of Ca to the MNP blunted Fe absorption.
The literature on the impact of dietary Ca on Fe and anaemia status is variable and thus non-conclusive. Results differ depending on whether adults or children were studied, whether the Ca was delivered in a single meal or longitudinally and whether the Ca was a component of the food or provided as a supplement( Reference Minihane and Fairweather-Tait 9 – Reference Dalton, Sargent and O'Connor 15 ). Even the mechanism by which Ca competes with Fe absorption is not fully understood( Reference Minihane and Fairweather-Tait 9 , Reference Hallberg, Rossander-Hulthen and Brune 12 , Reference Hallberg, Rossander-Hulten and Brune 40 – Reference Bendich 42 ). Moreover, there are no studies that have examined the impact of Ca on Fe status in anaemic subjects, where one would expect up-regulated Fe absorption. The few studies that have been completed in infants included healthy, Fe- and Ca-replete subjects( Reference Abrams, Griffin and Davila 14 , Reference Dalton, Sargent and O'Connor 15 ). In the current study, infants in both groups were anaemic with similar baseline Hb concentrations (mean 90 g/l). Using stable isotopic techniques, we have previously demonstrated that Fe absorption of microencapsulated ferrous fumarate present in Sprinkles MNP is dependent on the haematological status of the infant( Reference Tondeur, Schauer and Christofides 43 ). More specifically, infants with IDA absorbed approximately double the amount of Fe compared with those with Fe deficiency alone or Fe-replete infants. Although Fe absorption was not measured in the current study, we expected a robust change in Hb response since all infants were anaemic at baseline and all received Fe-containing MNP. Indeed, we observed this response in infants receiving the Ca-free MNP, but not to the same extent in those receiving MNP containing calcium. The prevalence of malaria is low in our study region and Helicobacter pylori infection has been proven to be neither a cause of IDA nor a reason for treatment failure of Fe supplementation in young Bangladeshi children; thus they could not account for this difference( Reference Hyder, Haseen and Rahman 29 , Reference Sarker, Mahmud and Davidsson 31 ). The dietary data demonstrated that the intake of Fe from food was low, but equal in both groups. Hence, differences in dietary intake of Fe would not explain the differential Hb response. After adjusting for all study variables, the only plausible explanation is the presence of Ca in the MNP formulation having an antagonistic interaction with Fe.
Nutrient dosage likely affects the interaction between Fe and Ca. Hallberg et al. have suggested a dose–response related reduction in Fe absorption with increases in Ca consumption, whereby no effect on Fe absorption is seen when less than 40 mg Ca is present in a meal and no further inhibition occurs when the Ca content exceeds 300 mg( Reference Hallberg, Brune and Erlandsson 11 , Reference Hallberg 13 ). In the current study, infants in the Ca-MNP group received 400 mg Ca daily added to a single meal, in addition to that provided by the food they ingested. If Fe absorption is maximally inhibited by 300 mg Ca, then the results we observed in this group are consistent with Hallberg's theory. However, we did not test for a Ca dose–response and the amounts of Ca and Fe used were based on the nutrient recommendations published by the WHO/FAO and International Nutritional Anemia Consultative Group( 33 , 44 ). It is important to note that previous studies showing the inhibitory effect of Ca on Fe absorption in a dose-related manner were conducted on healthy individuals in either single-meal or short-term studies( Reference Galan, Hercberg and Soustre 7 , Reference Gleerup, Rossander-Hulthen and Gramatkovski 10 , Reference Hallberg, Brune and Erlandsson 11 ). The effects of factors that change the bioavailability of Fe are often exaggerated in single-meal studies, while measurements based on the consumption of multiple meals are more likely to reflect the true nutritional impact of a nutrient–nutrient interaction( Reference Lynch 41 ). Unlike the single-meal studies mentioned above, the current study was conducted over two months and reflects a habitual intake of Ca. Under these circumstances, we believe the impact of Ca on Hb status to be quite robust. However, it must be noted that the daily Ca intake was not spread out across three meals, but provided in a single daily meal and the dose of Ca included in the sachet was equivalent to the total recommended intake for a whole day. Had the same dose of Ca been divided into three meals or lowered, the results may have been different.
When dietary Ca and Fe intakes were calculated, the estimated means from both breast milk and complementary foods were well below their Recommended Nutrient Intake for the given age group( 33 ). These findings are, however, comparable to those from previous studies examining Fe and Ca intake among Bangladeshi children( Reference Combs, Hassan and Dellagana 4 , Reference Combs and Hassan 45 , Reference Kimmons, Dewey and Haque 46 ). Food intake data also revealed a lack of diversity in infant diets, with plant foods based on cereals, vegetables and fruits being the main items consumed. A similar dietary pattern has also been reported among children aged 6–59 months in rural Bangladesh( Reference Rah, Akhter and Semba 47 ). The primary concern with these non-diversified diets is that they generally contain foods with a low concentration of Fe and Ca and high levels of phytates and oxalate which impede mineral absorption( Reference Mensah and Tomkins 17 – Reference Lind, Lonnerdal and Persson 19 ). Inadequate weaning practices resulting from a lack of education, unfounded cultural beliefs and, most importantly, insufficient access to nutrient-dense foods due to financial barriers might account for these findings( Reference Islam, Lamberg-Allardt and Karkkainen 20 , Reference Zlotkin, Christofides and Hyder 48 ).
Our study findings need to be viewed within some contextual limitations. Although infants were free from acute or chronic illness and afebrile, subclinical inflammation, which has been shown to independently decrease Hb concentration, was not assessed( Reference Grant, Suchdev and Flores-Ayala 49 ). Due to limited resources and study setting, serum Ca levels and indicators of Fe status were not measured. However, serum Ca levels only account for 1 % of the total body Ca and are often viewed as a poor biomarker for overall Ca assessment( Reference Moe 50 ). Regarding Fe status, of all the testing methods available, measuring Hb concentration is most often used to screen for anaemia because of its low cost, simplicity, speed of the procedure and better performance when compared with haematocrit assessment( Reference Mei, Parvanta and Cogswell 51 ).
The results of the current study suggest that Sprinkles MNP with Fe alone is more effective at improving Hb status and treating anaemia than the same formulation with Ca. Although the addition of Ca had a significant negative effect on the change in Hb concentration between groups, one must be careful not to over-interpret these results. When combined with Fe in the Sprinkles MNP, the group receiving Ca still displayed a significant improvement in Hb status. To our knowledge, no previous study has examined the nutrient–nutrient interaction between Fe and Ca in a paediatric population suffering from both anaemia and dietary Ca deficiency in a developing country. This makes our findings especially novel given the magnitude and growing prevalence of IDA and Ca deficiency in the developing world. Further research including a dose–response study with a similar Fe dose but lower amounts of Ca would advance our understanding of the long-term effects of Ca on Fe absorption in anaemic and Ca-deficient children.
Acknowledgements
Sources of funding: This research was funded by a grant from the HJ Heinz Company Foundation. W.U.K. received funding from a scholarship provided by the Ontario Graduate Scholarship program. Conflicts of interest: The authors have no conflicts of interest to report. Authors’ contributions: W.U.K. contributed to study conception and design, analysis and interpretation of data, drafting of the manuscript, administrative, technical and material support, and supervision. S.S. contributed to administrative, technical and material support, acquisition of data, and supervision. H.S. contributed to administrative, technical and material support, acquisition of data, and supervision. Y.A.S. contributed to statistical analysis and interpretation of data. D.W.S. contributed to administrative, technical and material support, and critical revision of the manuscript for important intellectual content. J.S.C. contributed to administrative, technical and material support. S.H.Z. contributed to study conception and design, analysis and interpretation of data, drafting of the manuscript, administrative, technical and material support, supervision, and obtaining funding. All authors read, edited and approved the final manuscript. Acknowledgements: The authors thank the staff at The Hospital for Sick Children in Toronto, Canada and the BRAC Research and Evaluation Division in Dhaka, Bangladesh for all their support.









