Introduction
The oldest remains of camelids have been found in North America in sediments dating back to about 45 million years ago (Ma) (Webb Reference Webb and Webb1974). Fossil and mitochondrial records (16 and 25 Ma, respectively) suggest that the radiation of camelids during the Miocene led to two divergent phylogenetic lines: the Camelini Tribe Gray, 1821 and the Lamini Tribe Webb, 1965 (Honey et al. Reference Honey, Harrison, Prothero, Stevens, Janis, Scott and Jacobs1998; Cui et al. Reference Cui, Ji, Ding, Qi, Gao, Meng, Yu, Hu and Zhang2007). Extinct members of the latter colonized South America after the formation of the Isthmus of Panama, 3.5 Ma. During the Pleistocene–Holocene transition (ca 10,000–12,000 years before present (BP)), extinct species of this tribe such as Hemiauchenia Gervais and Ameghino, 1880 and Lama gracilis Gervais and Ameghino, 1881, lost representativeness in the zooarchaeological record, whereas modern ones, such as the guanaco (Lama guanicoe Müller, 1776) and the vicuña (Vicugna vicugna Molina, 1782), became the only camelids recovered.
South American Camelids (SACs) have represented a key resource for human settlements, from the Central Andes to the Tierra del Fuego archipelago. Although there is no consensus on the phylogenetic history of domestic SAC species such as the llama (Lama glama Linnaeus, 1758) and the alpaca (Lama pacos Linnaeus, 1758) (Fan et al. Reference Fan, Gu, Guang, Marín, Varas, González, Wheeler, Hu, Li, Sun, Yang, Zhang, Gao, He, Munch, Corbett-Detig, Barbato, Pan, Zhan, Bruford and Dong2020; Diaz-Maroto et al. Reference Diaz-Maroto, Rey-Iglesia, Cartajena, Núñez, Westbury, Varas, Moraga, Campos, Orozco-terWengel, Marin and Hansen2021; among others), their emergence from the wild species mentioned above would have occurred from the Central Andes (Wheeler Reference Wheeler1995) up to its southern limit, the Argentine Puna, around 7,000 years BP (Yacobaccio Reference Yacobaccio2021). In the archaeological sites of Peru and the Puna, the evidence of a gradual process of intensification in the use of camelids culminates in the archaeological records of corrals dating back to ca 4,500 BP (Yacobaccio Reference Yacobaccio2021). Since the European colonization, wild SACs have been subjected to increasing stresses from human activity. In Patagonia, the invasion of exotic species since the beginning of the 20th century had a particular impact on wild SACs, along with the rapid establishment of extensive sheep farming (Martinic Reference Martinic1976). Currently, the greatest threats to the Patagonian guanaco continue to be their competition with sheep, their hunting, and the fencing of their territories (Baldi et al. Reference Baldi, Novaro, Funes, Walker, Ferrando, Failla, Carmanchahi, du Toit, Kockand and Deutsch2010), whereas in other regions, wild vicuñas and other guanacos populations suffer similar threats (Baldi et al. Reference Baldi, Acebes, Cuéllar, Funes, Hoces, Puig and Franklin2016; Arzamendia et al. Reference Arzamendia, Acebes, Baldo, Rojo and Segovia2019; Carmanchahi et al. Reference Carmanchahi, Panebianco, Leggieri, Barri, Marozzi, Flore, Moreno, Schroeder, Cepeda, Oliva, Kin, Gregorio, Ovejero, Acebes, Schneider, Pedrana and Taraborelli2019). Likewise, domestic SACs have also suffered the effects of the European colonization, and studies have estimated that their genetic variability has been reduced by 80% during the first 100 years since the colonization (Wheeler Reference Wheeler1995).
Biological invasions and their effects on native communities are widely documented (Vitousek et al. Reference Vitousek, D’antonio, Loope, Rejmánek and Westbrooks1996; Simberloff et al. Reference Simberloff, Martin, Genovesi, Maris, Wardle, Aronson, Courchamp, Galil, García-Berthou, Pascal, Pyšek, Sousa, Tabacchi and and Vilà2013). Importantly, the invasion of free-living animals can also lead to the simultaneous invasion of their parasites, as well as to an increase in the prevalence of indigenous parasites for which they are potential hosts (Torchin et al. Reference Torchin, Lafferty, Dobson, McKenzie and Kuris2003; Kelly et al. Reference Kelly, Paterson, Townsend, Poulin and Tompkins2009). For example, parasitological studies performed in guanacos during the last 40 years have shown a parasite richness made up of numerous helminths typical of domestic mammals (revised in Fugassa Reference Fugassa2020). Thus, it is difficult to discern whether some of the species that currently parasitize SACs have a native or a foreign origin. At present, the gastrointestinal parasite richness of SACs is dominated by nematode species (Leguía & Casas Reference Leguía and Casas1999). Strongylids (Subclass Secernentea von Linstow, 1905) are the most studied clade, probably both for their richness within SAC assemblages and for their health implications, whereas the taxonomic and ecological knowledge of enoplids (Subclass Adenophorea Linstow, 1905) that parasitize SACs remains scarce. The species of the Order Enoplida Filipjev, 1929 are grouped into two suborders, Dyoctophimina Chitwood, 1933 and Trichinellina, Hodda 2007, and only representatives of the latter have been reported in SACs (Superfamily Trichinelloidea Ward, 1907). Specifically, species of the Subfamilies Trichurinae Ransom, 1911 and Capillariinae Railliet, 1915, two of the three subfamilies recognized for the Family Trichuridae Ransom, 1911, have been reported in SACs. The former are soil-transmitted helminths that do not require intermediate hosts and whose eggs must mature in the environment before being ingested and hatching in the large intestine of a mammal (Anderson Reference Anderson2000), whereas the latter parasitize various organs of species of all classes of vertebrates and, specifically in SACs, have been found in the small intestine (Leguía & Casas Reference Leguía and Casas1999). Capillariid species are widely distributed throughout the world and are one of the most complex groups for their taxonomic and systematic analysis (Moravec et al. Reference Moravec, Prokopic and Shlikas1987).
The objective of this study was to analyze the richness and composition of helminths, specifically referring to trichurid species in SACs from a historical perspective. This approach can expose patterns that help to identify the mechanisms or events that determine them.
Materials and Methods
In order to establish the known diversity of trichurid nematodes in pre-Hispanic remains of SACs, as well as to identify their distribution patterns, a bibliographic search was carried out to find parasitological studies on paleontological and archaeological remains of SACs. A bibliographic search was also carried out in Google Scholar and Google to estimate the diversity of trichurids that parasitize modern SAC populations. For this, the descriptors ‘trichuris’ and the combinations with the common and scientific names of the four SAC species were used, both in Spanish and English; the same procedure was followed for capillariids, using ‘capillariid’ and ‘capillaria’ as descriptors.
In addition, a review of the morphometric information of the eggs of the Trichuris species found in archaeological and modern material from SACs was carried out to compare their dimensions. Likewise, eggs obtained from female Trichuris tenuis Chandler, Reference Chandler1930 isolated from llamas from the Argentine provinces of Jujuy and Tucumán (30 eggs in each case) were also used. To differentiate the eggs from the capillariids found in modern and archaeological material, the shape of the egg and the ornamentation of the eggshell were also considered because they are features of complementary taxonomic value (Moravec Reference Moravec2001). Specifically, for the eggs of the morphotype called Capillariinae gen. sp. 1, due to its similarity with the eggs of Calodium hepaticum Bancroft, 1893, the measures were compared with C. hepaticum eggs obtained from the liver of rats. Because homoscedasticity was rejected for each dependent variable using Levene’s test, one-way ANOSIM was applied. In all cases, the significance level p<0.05 and the free statistical package Past 4.09 (Hammer et al. 2001) were used.
In parasite ecology, some definitions (summarized in Bush et al. Reference Bush, Lafferty, Lotz and Shostak1997) must be adapted to the nature of the sample used (Watve & Sukumar Reference Watve and Sukumar1995). In the present study, parasite richness was defined as the number of taxa observed in each sampling unit, referring mostly to the intestinal infracommunity. Regarding prevalence, unlike the traditional definition (Bush et al. Reference Bush, Lafferty, Lotz and Shostak1997), we used the fecal prevalence to indicate the percentage of sampling units in which parasite taxa were detected. The information on the prevalence of trichurids in pre-Hispanic SACs was arranged in temporal periods: Late Holocene (up to 3,000 years BP), Middle Holocene (3,001–6,000 years BP), Early Holocene (6,001–9,000 years BP), and Pleistocene-Holocene transition (9,001–12,000 years BP). Dates were calibrated with Calib 8.2 (2 sigma) for the Southern Hemisphere (Hogg et al. Reference Hogg, Heaton, Hua, Palmer, Turney, Southon, Bayliss, Blackwell, Boswijk, Bronk Ramsey, Pearson, Petchey, Reimer, Reimer and Wacker2020) and using the probabilistic median. For publications in which only the dates calibrated with two sigma were reported, for each case, an arithmetical mean was estimated for the chart. The information on the prevalence was also arranged according to geographic origin of the samples: Patagonia and Central Andes.
Precautions regarding the zoological identification of coprolites were taken: samples associated with dates contemporary to the European colonization were included in the review but were excluded from the analysis of pre-Hispanic SAC parasitic assemblages because they could include invasive parasites from exotic species and even feces from introduced artiodactyls, such as goats and sheep. Considering that Eimeria macusaniensis Guerrero, Hernandez, Bazalar, and Alva, 1971 is a SAC-specific coccidian and that the nematode Lamanema chavezi Becklund, 1963 and the capillariid species Capillariinae gen. sp. 2 were frequently reported in publications on pre-Hispanic SAC parasites, the presence of E. macusaniensis or both nematodes mentioned was considered a complementary indicator for the identification of SAC coprolites.
Results and Discussion
Trichuris genus in SACs
The bibliographic search resulted in finding 17 publications related to parasites found in archaeological remains of SACs. These included one study on ectoparasites (Fugassa et al. Reference Fugassa, Petrigh and Martínez2017), one study on E. macusaniensis (Fugassa et al. Reference Fugassa, Sardella, Taglioretti, Reinhard and and Araújo2008a), and four unpublished studies (Fugassa Reference Fugassa2006; Bayer Reference Bayer2008; Taglioretti Reference Taglioretti2008; Taglioretti Reference Taglioretti2015). These 17 studies examined a total of 213 samples consisting mostly of individual coprolites and, on fewer occasions, series of coprolites or pools of them; only two of the samples consisted of enterolites extracted from camelid mummified remains (Leguia et al. Reference Leguía, Casas and Wheeler1995; Le Bailly et al. Reference Le Bailly, Goepfert, Prieto, Verano and Dufour2019). About 112 of these samples contained parasites that, due to their specificity, could be assigned to SACs and that came from archaeological levels associated with pre-Hispanic periods. However, since some publications did not specify the sample size (number of coprolites per sample), the sample set, defined as a sample belonging to a certain archaeological level and microsector, was used as the unit of analysis. A total of 52 sample sets were analyzed for pre-Hispanic SACs, 83% of which were positive for parasites. The genus Trichuris was present in SAC remains from all eight South American archaeological sites examined (Figure 1a) and in 28.8% of the 52 pre-Hispanic SAC sample sets (Table 1). In Patagonia, records of the genus Trichuris in SACs dated back to ca 11,000 cal. years BP, so its hosts could include extinct SAC species, such as L. gracilis and Hemiauchenia.
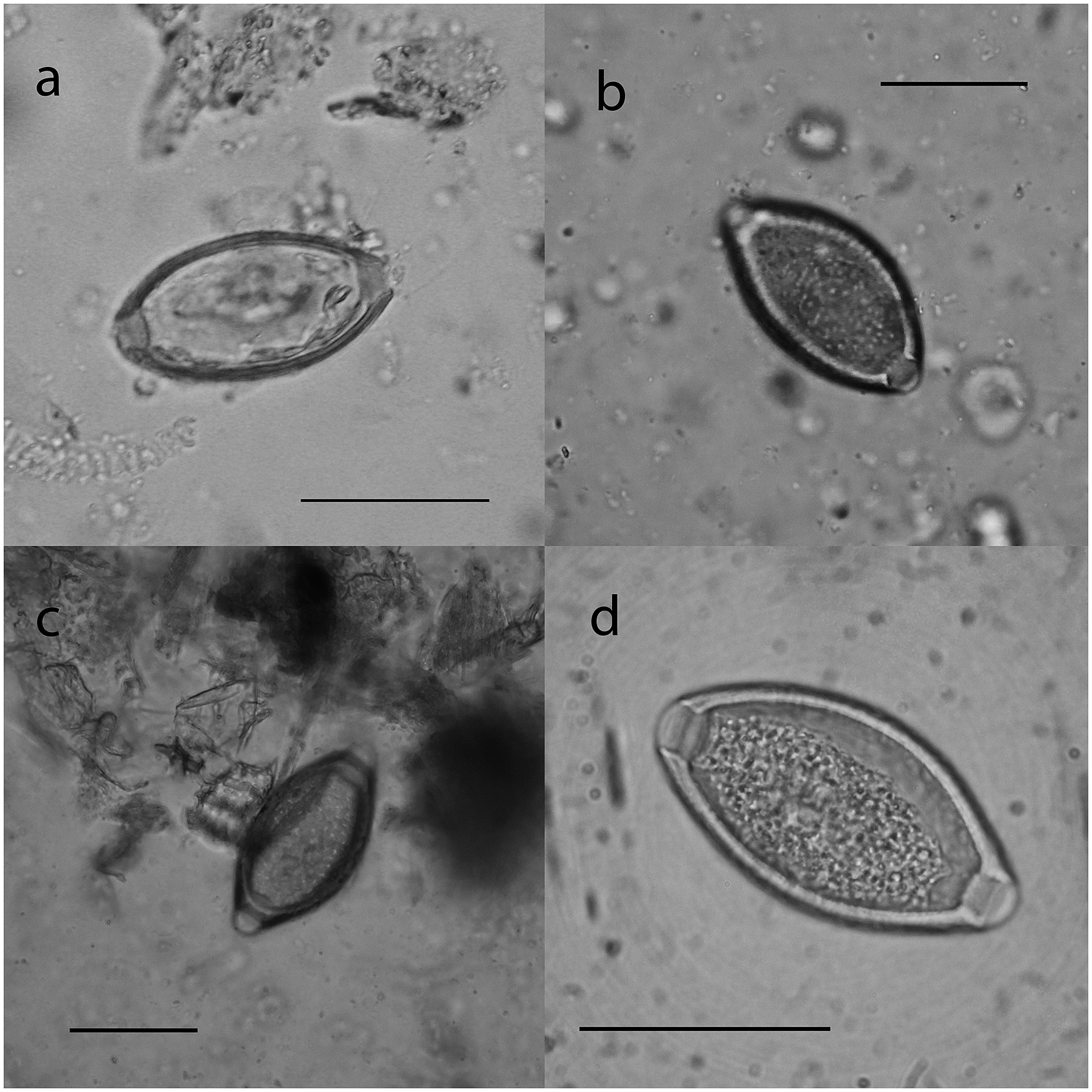
Figure 1. Trichuris eggs found in pre-Hispanic and modern SACs. Trichuris sp. egg with smooth eggshell in camelid coprolite (a), and Trichuris sp. 2 egg with hole eggshell in guanacos coprolite (b), in feces of modern vicuñas (Salta, Argentina) (c), and in T. tenuis female recovered of a llama (Jujuy, Argentina) (d). Scale bar = 40 μm.
Table 1. Parasitological reports of Trichuris sp. in archaeological remains from SACs; (?) Zoological origin not confirmed, (+) Arithmetic mean of two calibrated dates, (*) Revised identity in this work, (**) Mean, (WD) Without data

The genus Trichuris was reported in all SAC species and in all regions studied, although only 26 species-specific records were found and obtained from necropsies (Table 2). Six species were identified in SACs, although it has recently been suggested that Trichuris globulosa von Linstow, 1901 would represent a lineage of Trichuris ovis Abildgaard, 1795 (Callejon et al. Reference Callejón, Gutiérrez-Avilés, Halajian, Zurita, de Rojas and Cutillas2015). Only three of these species were identified in South American populations of SACs and only two (T. ovis and T. tenuis) were reported in all SAC species. All Trichuris species parasitize domestic ruminants, although T. tenuis is considered specific to SACs (Cafrune et al. Reference Cafrune, Aguirre and Rickard1999). In SACs, T. tenuis was first described in captive llamas in the USA (Rickard & Bishop Reference Rickard and Bishop1991a) and then subsequently reported in vicuñas and llamas from northwestern Argentina (Cafrune et al. Reference Cafrune, Aguirre and Rickard1999) and guanacos from Argentine Patagonia (Beldomenico et al. Reference Beldoménico, Uhart, Bono, Marull, Baldi and Peralta2003). However, this species was originally described in captive individuals of dromedaries in the USA (Chandler Reference Chandler1930) and later reported in sheep from Iran (Mirzayans Reference Mirzayans1974) and dromedaries from Australia (Beveridge & Green Reference Beveridge and Green1981). Regarding T. tenuis eggs, the analysis of the distribution of the length of the major axis of eggs obtained from females, as well as of those provided by various authors or recovered from archaeological sites, showed a wide range within some species and an overlapping of measurements among species (Figure 2). This may be due to various factors, such as the change in size that occurs as eggs mature (Yevstafieva et al. Reference Yevstafieva, Melnychuk, Nagorna, Stybel, Gutyj, Yatsenko, Petrenko, Nikiforova, Filonenko, Savenkova and Tahiltseva2021), and indicates that this feature is not reliable for the zoological identification of eggs and that a robust statistical comparison is not possible.
Table 2. Parasitological reports of Trichuris Roederer, 1761 species in modern SACs


Figure 2. Barplot of the length of Trichuris spp. eggs reported in modern and ancient SACs; (*) Pre-Hispanic Patagonian samples, (**) Pre-Hispanic Central Andes samples.
Eggs whose size, shape, color, and polar plugs corresponded to those of Trichuris spp. (Table 3), although they presented an eggshell covered by numerous ellipsoids to circular holes typical of capillariid species, were found in seven SAC archaeological samples (Figure 1b). Although this feature could be product of taphonomic effects, until now, this type of egg has only been found in SACs. Le Bailly et al. (Reference Le Bailly, Goepfert, Prieto, Verano and Dufour2019) also identified this feature and rejected the taphonomic hypothesis due to the regular pattern of this ornamentation in the eggs. These eggs (Trichuris sp. 2) had a wide geographic distribution, although with few reports (13.5% of the pre-Hispanic SAC sample sets) and at low densities. Therefore, because the features that characterize these eggs are unclear, they may be underestimated within both archaeological and modern samples. For example, Tietze et al. (Reference Tietze, Urquiza and Beltrame2021) and Ruiz Hurtado (Reference Ruiz Hurtado2016), in coprolites and modern feces respectively, reported Trichuris sp. whose images showed eggs with an irregular eggshell. Similar eggs have also been identified in wild vicuñas (Figure 1c), Argentine llamas, and Chilean guanacos (Fugassa pers. obs. Reference Fugassa, Petrigh and Martínez2017). The analysis of the eggshell surface of eggs obtained from T. tenuis females exhibited the ornamentation described for Trichuris sp. 2 (Figure 1d). No other Trichuris species with eggs with these traits have been reported. Members of the Subfamily Trichurinae are described as having a smooth eggshell, a criterion often used to differentiate them from eggs of the Subfamily Capillariinae (Traversa et al. Reference Traversa, Di Cesare, Lia, Castagna, Meloni, Heine, Strube, Milillo, Otranto, Meckes and Schaper2011), although this may not be a universal trait for the genus Trichuris.
Table 3. Ornamented eggs attributable to Trichuris sp. 2 in pre-Hispanic Southern American camelids (SACs); (*) Revised information here, (**) Mean value, (+) Arithmetic mean of two calibrated dates

As mentioned, Lamini camelids colonized South America about 3 Ma (Wheeler Reference Wheeler1995) and their prolonged isolation from Camelini species may also have led to divergence of their parasites. The atypical ornamentation of the eggs observed in T. tenuis females and in archaeological remains (Trichuris sp. 2) suggests that they correspond to the same species, although the initial reports of T. tenuis in various species of Old World modern artiodactyls refute the biogeographic history suggested. Helminth eggs such as Toxocara cf. canis and Eucoleus cf. aerophila have been found in pre-Hispanic Patagonian fox coprolites (Fugassa et al. Reference Fugassa, Fernández, Bellelli and Carballido Catalayud2022) in the same way as they are currently recovered from various Old World wild canids. A detailed analysis of the genome of the modern and pre-Hispanic populations would probably clarify whether both populations belong to the same species, after so much time of isolation. The eggs of Trichuris sp. 2 and those of modern T. tenuis probably represent vicariant populations, where modern T. tenuis would have entered South America along with the invasion of domestic hosts during the European colonization, whereas one or more native species could have become extinct or remain undetected, in wild relicts or as sibling species (sensu Nadler & de León Reference Nadler and De León2011).
Subfamily Capillariinae in SACs
In archaeological deposits, 63.5% of the pre-Hispanic SAC sample sets were positive for capillariid species. Eggs with marked morphological differences were identified, allowing them to be assigned to three different species (Table 4). Capillariinae gen. sp. 1 eggs (Figure 3a) were similar to those of C. hepaticum (Figure 3b). These eggs were also found in the human-occupied soil of the CCP7 cave with other parasites that indicated high SAC fecal contamination (Amalfitano et al. Reference Amalfitano, Petrigh and Fugassa2019). Capillariinae gen. sp. 1 has also been found in coprolites from predators with evidence of SAC consumption in Patagonia (Fugassa et al. Reference Fugassa, Denegri, Sardella, Araújo, Guichón, Martinez, Civalero and Aschero2006, Reference Fugassa, Beltrame, Bayer and Sardella2009) and also in rodents and pellets from raptor birds (Fugassa et al. Reference Fugassa, Sardella and Denegri2007; Beltrame et al. Reference Beltrame, Fugassa, Sardella, Civalero and Aschero2011; Fugassa Reference Fugassa2014). The statistical analysis of the dimensions of 843 eggs of this morphotype and of those recovered from coprolites of diverse zoological origin in Patagonia showed no statistically significant differences between them, so the authors assumed that it was a single species with a wide range of hosts (Taglioretti et al. Reference Taglioretti, Fugassa, Beltrame and Sardella2014). Although Capillariinae gen. sp. 1 eggs have been previously assigned to C. hepaticum, the scatterplot of the egg measurements observed for Capillariinae gen. sp. 1 and C. hepaticum eggs obtained from rats (Figure 4), as well as the multivariate analysis one-way ANOSIM, indicated a statistical difference between both groups of eggs (p = 0.0001). This, together with the life cycle of C. hepaticum (Moravec Reference Moravec, Prokopic and Shlikas1987), suggests that Capillariinae gen. sp. 1 and C. hepaticum are different species. As mentioned, in the archaeological sites examined, Capillariinae gen. sp. 1 was found in a wide range of hosts, in high prevalence in pre-Hispanic Patagonian SACs until about 12,000 years ago (Taglioretti Reference Taglioretti2015) and even in megafauna coprolites from the late Pleistocene (Ruíz Oyarzún et al. Reference Ruiz Oyarzún, Pérez-Espinoza, González-Saldías, Martin and Moreno2021). Camelids probably obtained this nematode from other mammals in the region because, until now, it has not been found in camelids from the pre-Hispanic Central Andes. The prevalence of Capillariinae gen. sp. 1 found in pre-Hispanic remains of SACs from Patagonia contrasts with the current prevalence, where only similar eggs were detected in captive SACs in Chile (Ruíz-Oyarzún et al. Reference Ruiz Oyarzún, Barrientos, Rodríguez, Almonacid, Barrientos, Painean, Ortiz and Ratto2017) and in llamas from the province of Salta, Argentina (Fugassa pers. obs. Reference Fugassa, Petrigh and Martínez2017). These two reports of eggs similar to those of Capillariinae gen. sp. 1 demand further studies that can inform whether the species is present in pre-Hispanic SACs or is another species recently acquired from European cattle, such as Aonchotheca bovis Schnyder, 1906.
Table 4. Parasitological reports of the Subfamily Capillariinae Railliet, 1915 in SACs archaeological remains; (*) Revised information here, (**) Mean value, (***) Historic sample (Petrigh et al. Reference Petrigh, Rindel, Goñi and Fugassa2019), (+) Arithmetic mean of two calibrated dates, (WD) Without data


Figure 3. Diversity of Capillariinae eggs reported in pre-Hispanic and modern SACs. (a) Capillariinae gen. sp. 1 in camelid coprolites (CCP7, Santa Cruz, Argentina), (b) Calodium hepaticum eggs recovered from rat liver, (c) Capillariinae gen. sp. 2 in camelid coprolites (CCP7, Santa Cruz, Argentina), (d) Capillariinae gen. sp. 3 in coprolite of guanacos (Sample 436, CCP7, Santa Cruz, Argentina). Scale Bar = 40 μm.

Figure 4. Scatterplot of the Capillariidae gen. sp. 1 eggs in SAC coprolites from CCP7 archaeological site, Patagonia (○), and Calodium hepaticum eggs recovered from liver rats (▲). Note: 95% ellipses.
Eggs assigned to Capillariinae gen. sp. 2 showed a more variable size than those of Capillariinae gen. sp. 1, as well as more curved, thin, yellowish-brown eggs with a coarse eggshell (Figure 3c). This species was found in pre-Hispanic archaeological remains in Patagonia as well as in the Central Andes and with a maximum age of about ca. 9,600 years. The eggs of Capillariinae gen. sp. 2 were assigned to different capillariids: Eucoleus sp. (Taglioretti et al. Reference Taglioretti, Fugassa, Rindel and Sardella2017; Amalfitano et al. Reference Amalfitano, Petrigh and Fugassa2019), Capillaria sp. (Leguia et al. Reference Leguía, Casas and Wheeler1995), and a species of the Subfamily Trichosomoidinae Hall, 1916 (Le Bailly et al. Reference Le Bailly, Goepfert, Prieto, Verano and Dufour2019). However, because capillariid species within the same genus do not have any uniformity in egg morphology, their assignment to the genus Eucoleus is arbitrary. The absence of an advanced embryonic stage does not allow assigning the species to a member of the subfamily Trichosomoidinae either (Anderson Reference Anderson2000). Although coprolites from various mammalian species in Patagonia were examined, Capillariinae gen. sp. 2 has been found to be exclusively present in SAC coprolites and puma coprolites with evidence of predation on camelids (Fugassa et al. Reference Fugassa, Beltrame, Bayer and Sardella2009). Although all reports of capillariids in modern SACs could not be identified to species, eggs similar to these have been identified in images from parasitological examinations of domestic SACs (Ballweber Reference Ballweber2009; Cebra Reference Cebra2014; Hyuga & Matsumoto Reference Hyuga and Matsumoto2016) – even diagnosed as Trichuris sp. (Kutzler Reference Kutzler2018), in wild guanacos from Mendoza, Argentina (Moreno et al. Reference Moreno, Schroeder, Taraborelli, Gregorio, Carmanchahi and Beldomenico2015), in vicuñas from Bolivia and Peru (Ruíz Hurtado Reference Ruiz Hurtado2016; Pacheco et al. Reference Pacheco, Pezo, Mathias, Tebaldi, Castelo-Oviedo and Lux-Hoppe2021), and in wild vicuñas from Catamarca, Argentina (Fugassa pers. obs. Reference Fugassa, Petrigh and Martínez2017). Therefore, it can be postulated that Capillariinae gen. sp. 2 is a helminth with a very specific parasitic relationship with Lamini camelids and that its oldest records date back to at least the late Pleistocene.
Capillariinae gen. sp. 3 eggs (Figure 3d) were only identified in pre-Hispanic camelid coprolites from the CCP7 site in Patagonia, Argentina (Table 4). This morphotype has no visual record in publications about ancient SAC and, although a similar image was identified in a coproparasitological examination of alpacas in Japan (Hyuga & Matsumoto Reference Hyuga and Matsumoto2016), it is insufficient to discuss its identity and its origin. These eggs are similar to others found in rodents in the region, so these cannot be ruled out as sources and, due to their low frequency, it cannot be ruled out that they represent post-depositional contamination from the soil.
Unlike reports of the genus Trichuris that parasitize SACs, reports of capillariids in modern camelid species could not be identified to species (Table 5), and there is no information on the identity of the species that parasitize them. Although the morphology of capillariid eggs may present features specific to each species (Moravec Reference Moravec2001), as mentioned, these features are not necessarily shared among species of the same genus. Likewise, a relationship between the structure of the eggshell and the location within the host has been suggested (Romashov Reference Romashov1985), although it has been recently questioned (Borba et al. Reference Borba, Enoki, Lopes-Torres, Machado-Silva and Iñiguez2021). Therefore, based on both quantitative and qualitative features, the usefulness of capillariid eggs for coproparasitological diagnosis is limited to reporting the operational taxonomic units (OTUs) – probably species – present and represents only an auxiliary trait for species identification.
Table 5. Capillarid reports in modern SACs; (WD) Without data

Trichurid prevalence in pre-Hispanic SACs
The prevalence of SAC trichurids in Patagonia was two-fold higher than that in the Central Andes (Figure 5). The analysis of the prevalence of each Subfamily showed that it was higher for the Subfamily Trichurinae in the Central Andes. The difference between regions was clearer for the prevalence of the Subfamily Capillariinae, which, in Patagonia, reached 100%. Capillariinae gen. sp. 1 is the species responsible for this contrast.

Figure 5. Prevalence of each species in Patagonia and Central Andes regions.
The prevalence of the genus Trichuris, considered as the sum of the prevalence of Trichuris sp. and the prevalence of Trichuris sp. 2, in the archaeological sites from Patagonia and Central Andes was almost 33.4% lower than that of capillariids (42.3% and 63.5%, respectively). A large part of the pre-Hispanic prevalence of capillariids, particularly in Patagonia, was due to Capillariinae gen. sp. 1. This contrasts with the uncertain presence of Capillariinae gen. sp. 1 in modern SACs. The absence of detailed descriptions in reports of trichurids in modern SACs prevents their specific identification. It is possible that Capillariinae gen. sp.1 has become extinct or remains in wild relicts, according with the trend in its prevalence from the late Pleistocene to the late Holocene (Figure 6a). This hypothesis and the possible responsible ecological processes will require more information.

Figure 6. Trichurid prevalence through time in pre-Hispanic Patagonian SACs. (A) Prevalence of each species found, (B) Prevalence of each Subfamily, (LH) Late Holocene, (MH) Middle Holocene, (EH) Early Holocene, (PHT) Pleistocene-Holocene Transition.
The analysis of these prevalences for the pre-Hispanic temporal blocks was only possible for Patagonia because the sample from the Central Andes corresponds to the late Holocene. The viability and embryonic development of eggs depend on various environmental factors. Trichuris trichiura eggs, for example, have low resistance to moderate humidity conditions and temperatures below 0 °C (Nolf Reference Nolf1932). Other species of the genus show similar features (Yevstafieva et al. Reference Yevstafieva, Melnychuk, Nagorna, Stybel, Gutyj, Yatsenko, Petrenko, Nikiforova, Filonenko, Savenkova and Tahiltseva2021). This could explain the low prevalence observed for Trichuris spp. in the early temporal blocks of Patagonia (Figure 6b). Regarding the highest prevalence of both capillariids -mainly Capillariinae gen. sp.1 (Figure 6a)- it is possible that this is due to the fact that their eggs have less requirements for their maturation. Another possible cause for its higher prevalence can their life stories; these may involve intermediate hosts, paratenic hosts, or reservoirs -this, possibly supported by the apparent low specificity observed by Taglioretti et al. Reference Taglioretti, Fugassa, Beltrame and Sardella2014- that reduce the pressures coming from climatic restrictions.
Conclusions
Parasitological studies of archaeological remains confirmed the pre-Hispanic presence of a Trichuris species in SACs. The pre-Hispanic presence of a capillariid specific to SACs was also corroborated. Its inclusion by Aguirre and Cafrune (Reference Aguirre, Cafrune, Suárez, Olaechea, Romero and Rossanigo2007) is based on the evidence obtained from camelid mummies (Leguía et al. Reference Leguía, Casas and Wheeler1995), and its illustration is compatible with the eggs of Capillariinae gen. sp. 2.
Now, the richness of the native trichurid in SACs has been extended by the inclusion of Trichuris sp. 2 and Capillariinae gen. sp. 1. Modern T. tenuis and Trichuris sp. 2 have been proposed as sibling species where the former would have invaded and colonized SACs during the European colonization, and the latter – the native species – would derive from Pleistocene camelids. Regarding Capillariinae gen. sp. 1, the assignment of its eggs to C. hepaticum through their morphometric analysis was refuted. Capillariinae gen. sp. 3 remains excluded from the trichurid richness composition in SACs because a single egg was found in only one sample.
Likewise, the integration of parasitological and paleoecological information also allowed us to propose a possible origin for some of them. Although the present analysis is exploratory, it exposes patterns and suggests hypotheses that should stimulate future research about the biogeographic history and current conservation status of SAC helminths. This information may be relevant, for example, to improve descriptions of the regional ecology during the Holocene and to identify threatened helminths that demand a place on conservation programs.
Acknowledgements
We thank Dr. Cumino (CONICET, IIPROSAM) for facilitating the infection of rats.
Financial support
This study was supported by CONICET (PIP 436), FONCyT (PICT 3664), and UNMdP (EXA 877).













