Introduction
In the last decades, a new interdisciplinary science called biomimetics has emerged that has significantly influenced the design of certain new materials. Nature, as a source of inspiration, can give us ideas and concepts to implement new functional properties. One example is the self-cleaning property (superhydrophobicity) of certain plant leaves, better known as the Lotus Effect [Reference Solga1, Reference Koch2]. This term comes from the lotus leaf (Nelumbo nucifera), the best-known self-cleaning surface in nature. On this kind of leaf, water droplets roll over the leaf surface and collect dirt and other particles from the surfaces. The superhydrophobicity of these leaves is caused, in general, by a hierarchical surface structure, built by a randomly oriented small hydrophobic wax structure on the top of convex cell papillae. The wetting condition of the solid surface is a particular property of materials and depends on both surface energy and surface topography. Many papers have been written to show how superhydrophic surfaces with periodic and random patterns can be made, but few describe and characterize biological self-cleaning surfaces [Reference Guo and Liu3]. These papers usually analyze them by using optical profilers and scanning electron microscopy with specific sample preparation (for example, fixation) and in some cases atomic force microscopy. The main conclusion of those works is that binary structures (hierarchical microstructures and nanostructures) and unitary structures (basically nanostructures) are found in superhydrophobic plant leaves. However, no information regarding the general micro-nano structural pattern of biological surfaces on the x-y plane has been reported. These surfaces seem to have a random pattern in most of the cases or at least a vague arrangement of its constitutive elements whose morphologies can be represented by geometrical figures (for example, hexagonal/pentagonal polygons, circles, and straight lines).
In the present work, the two-dimensional Fourier transform is introduced as a tool for describing the topographical pattern of the epidermis, which is composed of different features (for example, epidermal cells and papillae, which form the microstructure or micro-pattern, and epicuticular wax, which is the nanostructure or nano-pattern). This technique, called Rotated Image with Maximun Average Power Spectrum (RIMAPS), allows identification of the main directions of the micro-nano structural pattern and its distribution on the x-y surface. This technique has recently been applied in the description of technological and biological surfaces [Reference Fuentes and Favret4, Reference Favret5, Reference Favret6, Reference Favret7, Reference Favret and Löthman8, Reference Favret9, Reference Fuentes, Favret, Favret and Fuentes10]. Therefore, the aim of the present work is to describe, using RIMAPS, the micro-nano patterns of binary structures found on superhydrophobic (ultra non-wettable) leaves, leaving for future work its comparison with non-wettable leaves.
Materials and Methods
Leaves (fresh samples) from Xanthosoma violaceum Schott, Oryza sativa L., Brassica oleracea L., and Tropaeolum majus L. (Figure 1) were analyzed. We chose these species because they have sculptured leaf surfaces that are quite different. The static contact angles (CA) of these four leaves are between 155º and 165º, and the sliding angles are below 10º, which means they are superhydrophobic surfaces. Figure 2 shows a water drop lying on this kind of surface. A scanning electron microscope (FEI Quanta 200), low-vacuum, was used to obtain images of the morphology. In all cases, observations were made on the central part of the leaves, avoiding the main vein (see Figure 1).

Figure 1: A Tropaeolum majus plant. The data were taken from the central part of the leaf (black box).

Figure 2: A water drop lying on a Tropaeolum majus leaf.
The RIMAPS technique consists of rotating the image using algorithms of commercial software and calculating the x-step of the two-dimensional Fourier transform for each y-line of the new image obtained after rotation. As a consequence, averaged power spectra are obtained for each angular position. The corresponding maximum values are plotted as a function of rotation angle to obtain the RIMAPS spectrum. For a deeper explanation of this technique see reference 4. Basically, the peaks of the RIMAPS spectrum indicate the main angular directions of the topographic pattern and the arrangement of the micro and nano elements that constitute the leaf surface. Because for a full 360º rotation the RIMAPS spectra are symmetric, only the first 180° will be presented in the graphs.
Results
The SEM images of Oryza sativa leaf (adaxial side) indicate a binary structure (microstructures and nanostructures) on its surface (Figures 3a and 3b). The microstructure is formed by the epidermal cells (think of them as rectangles), bumps, stomas, and prickles (see Figure 3b). The epidermal cells are located in a linear arrangement parallel to the apex (the longer side of the cell is parallel to the apex). All graminae have this type of structure. The water droplets can roll off easily along the apex direction, but movement is much harder along the perpendicular direction. The differences between the two sliding angles (4º and 12º) are due to the anisotropic arrangement of the bumps [Reference Guo and Liu3]. The nanostructure is given by the epicuticular wax found between the bumps.

Figure 3a: SEM image of an Oryza sativa leaf. Arrows indicate the main direction (1) of the pattern, which coincides with the apex direction and its perpendicular (2). (Original magnification: 500×).

Figure 3b: Same sample as Figure 3a, but with a higher magnification. Prickles (I), bumps (II), and stomas (III) are seen (original magnification: 2,000×).
The RIMAPS spectra for both Figures 3a and 3b show an interesting difference (Figure 4). Whereas the RIMAPS spectrum of Figure 3a shows a main direction approximately in 5º–10º, which coincides with the apex direction, a secondary direction is found approximately in 100°–110° in the spectrum of Figure 3a, caused by the shorter side of the epidermal cells. In the case of Figure 3b, we have a higher magnification (2,000×), which means we are looking at a smaller area compared to the previous case. The one-dimensional pattern that predominates in Figure 3a seems to disappear. The RIMAPS spectrum detects other angular directions, which were not clearly detected in Figure 3a (35º, 65º, 75º, 120º, and 160º).

Figure 4: RIMAPS spectra of Figures 3a (black line: 500×) and 3b (red line: 2,000×). Arrows indicate the main direction of the pattern and its perpendicular.
Figure 5 shows the microstructure of the Tropaeolum majus leaf (adaxial side), which also has a binary structure. The morphology and distribution of the epidermal cells are different from Oryza sativa. It has a convex epidermis, and there is more than one main direction of the pattern. The RIMAPS spectrum (Figure 6) coincides with this assumption for a 400× magnification. There are two global maxima, one around 65º and the other around 130º. If we magnify the visual field (1,600×), the maximum around 130º disappears, leaving only one global direction. It is important to point out that the width of these maxima at their base is between 60º and 70º, different from the case of Oryza with a base width of 40º. This is due to the more chaotic or isotropic arrangement of the epidermical cells of Tropaeolum compared to the case of Oryza. A less angular width of the maximum means a more linear pattern.

Figure 5: SEM image of a Tropaeolum majus leaf. Epidermal cells are seen. Arrows 1 and 2 indicate the two main directions of the pattern, obtained by RIMAPS (original magnification 400×).

Figure 6: RIMAPS spectra of a Tropaeolum majus leaf with two different magnifications (black line: 400×; red line: 1,600×). Arrows 1 and 2 indicate the two main directions of the pattern, obtained by RIMAPS.
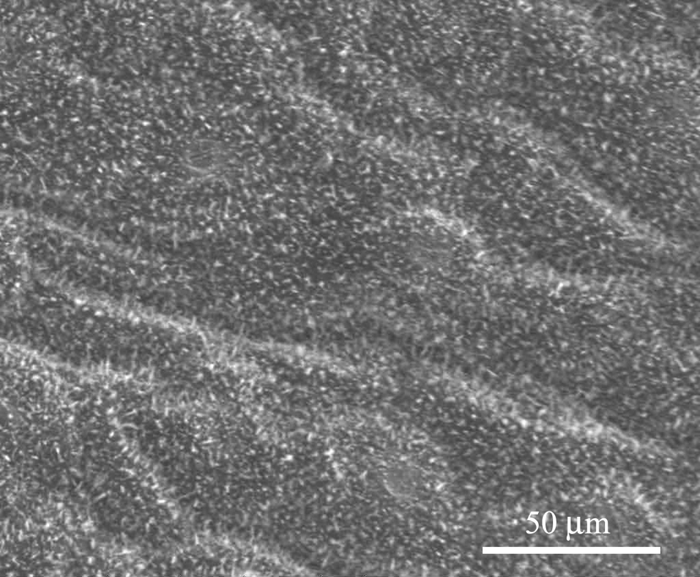
Figures 7 and 8 show the microstructure of Xanthosoma violaceum (abaxial side) and Brassica oleracea (adaxial side) leaves, respectively. The main characteristics of the topography of Xanthosoma violaceum are papillae, stomas, and wax, similar to the case of Colocasia esculenta and Nelumbo nucifera, which exhibit the Fakir effect (the drops sit on the top of the bed of micro-nano elements) [Reference Favret and Löthman8, Reference Quéré and Reyssat11]. The peaks observed in the RIMAPS spectrum (Figure 9) are caused by the orientation of the arrangement of the micro elements of the surface. Brassica oleracea has a totally different microstructure. Its leaf surface has almost a planar cell sculpture, but it is densely covered with wax, which caused the Fakir effect. The amount and density of wax is dependent on the humidity conditions in the air during cultivation [Reference Koch12]. The x-y dimensions of the wax are below 1 μm, which leads us to believe that in the case of Brassica we are dealing with a unitary structure instead of a binary structure. We used, in this case, a higher magnification, necessary to reveal the nanostructure. The RIMAPS spectra of Xanthosoma and Brassica are quite similar (Figure 9), indicating that the arrangement of the microstructure of Xanthosoma is almost equivalent to the nanostructure of Brassica. The RIMAPS spectrum of Tropaeolum majus is also compared to the spectra of Xanthosoma and Brassica, showing similarities in the angular range 40º–160º.

Figure 7: SEM image of a Xanthosoma violaceum leaf. Papillae (I), stomas (II), and epicuticular folders (III) are observed (original magnification: 500×).
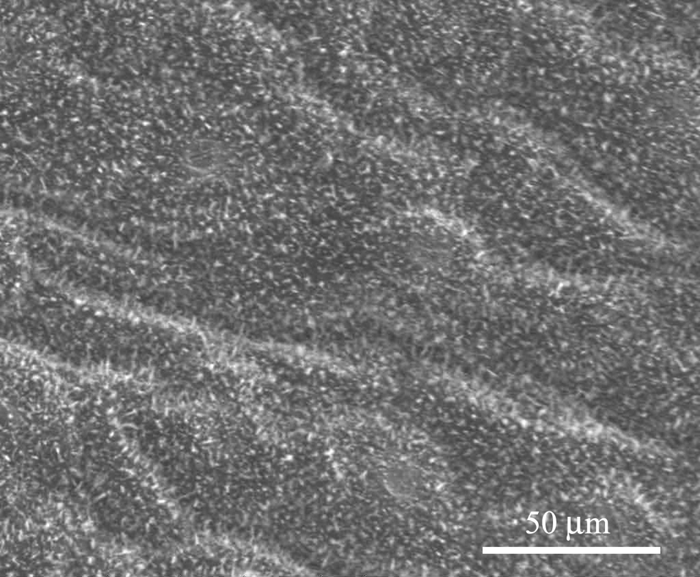
Figure 8: SEM image of Brassica oleracea leaf. Epicuticular wax is observed (original magnification: 1,600×).

Figure 9: Comparison between the RIMAPS spectra of Tropaeolum majus (black line), Brassica oleracea (red line), and Xanthosoma violaceum (blue line) leaves.
Discussion
The species analyzed are superhydrophobic, and it is clear from the micrographs that the topography patterns of their leaves are not unique. However the existence of micro-nano elements on the surface seems to be a common characteristic between them, verifying the conclusion of previous works [Reference Koch2, Reference Guo and Liu3] that sculptured surfaces are needed to have a superhydrophobic condition. So far, the RIMAPS results of the biological species analyzed show that the distribution of the elements that constitute the microstructure and nanostructure of the leaf surface are quite similar, in spite of what we see on the micrograph. Perhaps this result goes beyond superhydrophobic leaves and is common to any type of leaf. In future research work, comparison with wettable leaf surfaces will be carried out.
More research needs to be done to answer questions such as “Is the topography pattern of superhydrophobic leaves a main element for having the self-cleaning condition?” We do not yet know the answer. However, we can imagine that the arrangement and size of the micro-nano elements of the surface are essential for having the self-cleaning condition.
The results show the robustness of RIMAPS analysis to distinguish and characterize the arrangement of the micro-nano patterns of leaf surfaces. This method is an auxiliary tool for the biologist to study surface variations of organisms. The use of RIMAPS opens a wide range of possibilities by providing tools for the quantitative description of leaf surfaces. In addition, this technique allows researchers to distinguish similarities and differences that occur in different environments and the quantitative parameters that represent an organismal lineage.
Conclusions
The RIMAPS results obtained so far highlight the following conclusions: a) Different arrangements of micro-patterns are found on superhydrophobic leaves; b) For a given species, the topographical pattern shows differences depending on the size of the area studied; c) Oryza sativa has a linear micro-pattern for low magnifications; d) Brassica oleracea has a nano-pattern almost similar to the micro-pattern of Xanthosoma violaceum; e) The nano-pattern (epicuticular wax) seems to be isotropic, which means that the arrangement of the topographic features has multiple main directions.
Acknowledgments
The authors wish to thank Dr. Patricia Bozzano and Mrs. Adriana Domínguez for their work on the scanning electron microscope.