Introduction
Durum wheat, Triticum durum Desf. (Dorofeev et al., Reference Dorofeev, Filatenko, Migushova, Udaczin and Jakubziner1979), is the basic ingredient of pasta, a staple for Italians, with an annual per capita consumption of about 23 kg per person. Since the start of the 1900s, breeding has played a key role in improving durum wheat quality and production (Giunta et al., Reference Giunta, Motzo and Pruneddu2007; Pronin et al., Reference Pronin, Börner, Weber and Scherf2020). More recently, the efforts of the scientific community were particularly focused on the sustainable improvement of yield and on meeting the growing market demands for high quality products, also by enhancing the content of bioactive compounds (Shewry et al., Reference Shewry, Charme, Branlard, Lafiandra, Gergelyd, Salgo, Saulnier, Bedő, Mills and Ward2012; Brouns et al., Reference Brouns, Geisslitz and Shewry2022). One of the factors limiting the progress of wheat breeding is the ‘genetic erosion’ of diversity due to the large-scale use of uniform and high-yielding cultivars (Van de Wouw et al., Reference Van de Wouw, Kik, van Hintum, van Treuren and Visser2010; Rascio et al., Reference Rascio, Picchi, Naldi, Colecchia, De Santis, Gallo and De Gara2015; Sansaloni et al., Reference Sansaloni, Franco, Santos, Percival-Alwyn, Singh, Petroli, Campos, Dreher, Payne, Marshall and Kilian2020). Selection of traits to further improve the food quality, security and safety of cereals, rely on sources such as wild progenitors (Triticum urartu; Aegilops speltoides), domesticated wheat (Triticum dicoccum; Triticum dicoccoides; Triticum spelta), and landraces (Zeven, Reference Zeven1998; Dwivedi et al., Reference Dwivedi, Ceccarelli, Blair, Upadhyaya, Are and Ortiz2016; Rascio et al., Reference Rascio, Beleggia, Platani, Nigro, Codianni, De Santis and Fragasso2016; Mefleh et al., Reference Mefleh, Conte, Fadda, Giunta, Piga, Hassoun and Motzo2019; Rufo et al., Reference Rufo, Alvaro, Royo and Soriano2019).
Landraces are plant populations adapted to local agroclimatic conditions which are named, selected and maintained by traditional farmers and are not improved through conventional breeding (Camacho Villa et al., Reference Camacho Villa, Maxted, Scholten and Ford-Lloyd2005). Generally, they have high genetic heterogeneity, while the ‘varieties’ or ‘cultivars’ selected for a particular attribute or combination of attributes, are clearly distinct, uniform and stable in their characteristics (Zeven, Reference Zeven1998). In the common language but also in literature, several definitions and synonyms have been used for ‘landrace’ such as ancient variety, local variety, ecotype, traditional cultivar, race or farmer variety (Zeven, Reference Zeven1998). The landraces or varieties which are naturally adapted to local and regional conditions are defined by the EU Directive 2009/145/EC as ‘Conservation Varieties‘. For these reasons their cultivation falls within the EC policies to protect, encourage, support both the diversification of cultivated and transformed product supply and to improve the sustainability of production ((Regulations (EEC) nos. 2092/91, 2078/92, 20/92 and 2082/92).
At the beginning of the 1900s, several botanists (Percival, Reference Percival1921; De Cillis, Reference De Cillis1927; Draghetti, Reference Draghetti1927) used the plural term ‘Saragolle’ to indicate a pool of morphologically similar Italian ‘durum wheats’ or ‘macaroni wheat’, mainly used for pasta production. ‘Saragollio’ (Fiore, Reference Fiore2013), ‘Saragolla’ (Percival, Reference Percival1921; Draghetti, Reference Draghetti1927) and ‘Saragollo’ (Hume, Reference Hume1923) wheats were cultivated in southern Italy at least since 1600 (Fiore, Reference Fiore2013) and used until the advent of the breeding era started at the beginning of 1900s by the Italian agronomist and plant breeder Strampelli (De Cillis, Reference De Cillis1942; Deidda et al., Reference Deidda, Motzo, Giunta and Fois2001; Scarascia Mugnozza Reference Scarascia Mugnozza2005). The same landrace used in different regions may undergo changes to its population structure over time, due to different cultivation environments and practices, farmer selection and/or the introduction of other cultivars. For this reason, if specific actions to protect local typicality are not implemented, there is a high risk of extinction, change of name and/or loss of old wheat populations (Van de Wouw et al., Reference Van de Wouw, Kik, van Hintum, van Treuren and Visser2010; Bindi et al., Reference Bindi, Conti and Belliggiano2022; Raggi et al., Reference Raggi, Pacicco, Caproni, Álvarez-Muñiz, Annamaa, Barata, Batir-Rusu, Díez, Heinonen, Holubec and Kell2022).
Currently several farmers are marketing production of ‘ancient Saragolla’ wheat for their own families or for the local market. The reason for this rediscovery of Saragolla wheat lies in the belief, disseminated on the internet, that Saragolla wheat is the Italian equivalent of the wheat of the pharaohs, or ‘Kamut’, and marketed as a healthier and tastier food (https://casasapori.shop/en/traditional-products/italiam-durum-wheat-saragolla/). Autochthonous Saragolla wheats from Puglia and Lucania Regions have been already included in the Regional or National Register of the Genetic Resources. Fuelling confusion, in 2004 the name Saragolla was used to register a modern cultivar resulting from crosses between the ‘Iride’ cultivar and the ‘0114’ elite line by the ‘Produttori Sementi Bologna’ company.
The complex situation caused by the simultaneous existence in the market of the exotic Kamut-like Saragolla wheat, the modern Saragolla cultivar and old Saragolla wheat landrace populations induced the local authorities of Abruzzo to fund the SARAB project: ‘Characterization of ancient Saragolla populations from the Abruzzo Region’. The project aim was to obtain a detailed botanical characterization of the Saragolla wheats currently cultivated in Abruzzo, to describe their distinctive traits and hence register them as ‘the Saragolla conservation variety/ies from Abruzzo’.
Characterization of old Italian durum wheats using agronomic and quality traits have been widely performed (Porceddu, Reference Porceddu1976; Zeuli and Qualset, Reference Zeuli and Qualset1987; Pecetti and Annicchiarico, Reference Pecetti and Annicchiarico1998; Fares et al., Reference Fares, Menga, Codianni, Russo, Perrone, Suriano and Rascio2019), as have molecular approaches (Figliuolo et al., Reference Figliuolo, Mazzeo and Greco2007; Mangini et al., Reference Mangini, Margiotta, Marcotuli, Signorile, Gadaleta and Blanco2017; Fiore et al., Reference Fiore, Mercati, Spina, Blangiforti, Venora, Dell'Acqua and Sunseri2019). Morphological cataloguing of autochthonous wheats from North African countries (Orlov, Reference Orlov1923; Ducellier, Reference Ducellier1930; Laumont and Erroux, Reference Laumont and Erroux1961) and from Sicily (De Cillis, Reference De Cillis1942; Porceddu et al., Reference Porceddu, Vannella and Perrino1981; Perrino and Hammer, Reference Perrino and Hammer1983) has been documented, too, but to our knowledge there have been no studies on the botanical varieties comprising the Saragolla landraces from the other Italian regions.
There are seven main classifications of the Triticum L. (Lyapunova, Reference Lyapunova2017) genus. In most of them durum wheat is considered a subspecies of Triticum due to its genetic structure (van Slageren, Reference van Slageren1994). Intraspecific diversity within durum wheat can be catalogued using morphology based taxonomic ranks or morphotypes, consisting of a variable combination of botanical varieties or forms (Percival, Reference Percival1921; Flyaksberger, Reference Flyaksberger1935; Laumont and Erroux, Reference Laumont and Erroux1961; Dorofeev et al., Reference Dorofeev, Filatenko, Migushova, Udaczin and Jakubziner1979). Due to variability (even within the same classification scheme) depending on the selection of traits examined (Lyapunova, Reference Lyapunova2017), morphotypes must be monitored over time using standardized classification criteria to allow efficient identification and maintenance of populations.
The aim of this work was to obtain the quantitative botanical characterization of ‘Saragolla’ wheat currently cultivated on 11 farms in Abruzzo, and to identify one or more populations that could eventually be registered as conservation varieties. To better define the distinctive traits of Saragolla from Abruzzo, a comparison with the registered ‘local Saragolla wheat from Puglia’ was performed. The morphological similarity of the current Saragolla, with the one described in Italy at the beginning of the 1900s (Percival, Reference Percival1921; Draghetti, Reference Draghetti1927), was also determined. To estimate how well diversity has been preserved, the number and types of durum wheat botanical varieties of currently cultivated Saragolla were compared to those resulting from studies performed in the Mediterranean basin, dating back to the first half of the 1900s (De Cillis, Reference De Cillis1942 in Porceddu et al., Reference Porceddu, Vannella and Perrino1981; Boudour et al., Reference Boudour, Gherroucha, Boukaboub, Bouchtab, Baka, Samra, Hocine, Amar, Karima, Mebarek and Kheireddine2011).
Materials and methods
Twelve seed samples of Saragolla durum wheat, here marked as S1, S2…….., S12, were used. Eleven seed samples whose weight ranged from 2 to 5 Kg were produced by 11 farms located in different provinces of the Abruzzo Region (Italy), at altitudes from 100 to 1000 m above sea level (see Rascio et al., Reference Rascio, Codianni, Paone, Fiorillo and Marone2021 for locations). The samples from Abruzzo were delivered to the CREA-CI in 2018 and information pertaining to the selection of the participating farms, visual inspection of the source material prior to collection and date of collection was not recorded and available to the Authors. The twelfth sample (S12) of Saragolla belongs to the ex situ working collections of CREA-CI, stored at 4 °C and 40% RH, and periodically reproduced to preserve seed viability over the last 50 years. It has been included in the list of the Puglia Regional Resources in 2020. (https://filiereagroalimentari.regione.puglia.it/documents/1662405/2055807/grano-saragolla_91.pdf/ed4df68d-fb00-7ce0-1529-0594c40035cf?t=1659445456997 with the name: ‘local Saragolla wheat from Puglia’.
In 2019, all 12 seed samples were sown in the CREA-CI's experimental fields in Foggia, Italy, located at 72 m above sea level, using a randomized block design with 3 repetitions and a sowing density of 250 viable seeds/m2. The plots measured about 10 m2 each (1.30 m × 7.0 m), with 8 rows that were 7.5 m long and 0.17 m apart. Cultivation was carried out according to the usual agronomic practices (Fares et al., Reference Fares, Menga, Codianni, Russo, Perrone, Suriano and Rascio2019). At harvest, all plants in the central row of each plot were collected and morphologically examined. Soft wheat (Triticum aestivum L. subsp. aestivum) and durum wheat were separated from wild barley (Hordeum spontaneum Koch), oat (Avena sativa L.) and ‘hulled’ or indehiscent plants of ‘Farro wheat’ taxa (van Slageren and Payne, Reference van Slageren and Payne2013). Soft and durum wheat were differentiated based on leaf pubescence, ear presence, straw cross section, glume pubescence, grain length, glume dorsal keel, crosswise folds at the glume base and traits of the spikelet. The durum seeds obtained from plants in the remaining rows were hand harvested and threshed to remove barley (Hordeum L.) and oat (Avena L.) contamination and 12 plots were sown again (in 2020) using a sub-sample of 250 cleaned seed, in 5 × 5 m long rows, 30 cm apart. At the second harvest, a random sample of 10–20 durum wheat plants was taken from every plot and added to those collected during 2019 to verify the presence/absence of new durum botanical varieties and/or new morphotypes in addition to those observed in the previous year.
During the two years of characterization, about 1000 plants were examined by two and in uncertain cases, also by three experts. The number of barley, farro, durum and soft wheat plants, observed within the central row of every experimental plot, was counted and varied depending on the seed viability and on differences for the in-field performances of the original samples. The species composition of every sample was quantified following the taxonomical classification of Pignatti (Reference Pignatti1982). After two years of sample regeneration, 434 durum wheat plants were totally examined, and categorized according to their botanical variety based on a combination of the five traits used in almost every classification system of wheat: presence or absence of awns; presence or absence of pubescent glumes; glume colour (white, red); awn colour (white, red or black) and kernel colour (white or red) (Percival, Reference Percival1921; Flyaksberger, Reference Flyaksberger1935; Dorofeev et al., Reference Dorofeev, Filatenko, Migushova, Udaczin and Jakubziner1979; Vavilov, Reference Vavilov1992; Zuev et al., Reference Zuev, Amri, Brykova, Pyukkenen and Mitrofanova2013). A subsample of 96 plants representing each botanical variety was examined in greater depth using the following 20 morphological traits:
1. Seed length
2. Length of seed hair
3. Colour of grain
4. Culm solidity
5. Awn colour
6. Glume colour
7. Pubescence of glume
8. Shape of lower glume beak
9. Lower glume beak length
10. Lower glume shoulder width
11. Nerve presence on outer glume
12. Prominent keel on glume
13. Awn tip colour
14. Awn base colour
15. Awn/spike length ratio
16. Form of ear
17. Prominent dorsal ridge of seed
18. Spike laxity (side view) seeds/10 cm
19. Spike length
20. Plant height
The qualitative traits were measured using the value scale ranging of the International Union for the Protection of New Varieties of Plants (UPOV, 2012) test guideline, dated 28/03/2012. The percentage of each botanical variety within the total of morphotypes characterized, was calculated, and compared to two previous studies performed (with the same botanical classification) in Sicily (De Cillis, Reference De Cillis1942 in Porceddu et al., Reference Porceddu, Vannella and Perrino1981) and Algeria (Boudour et al., Reference Boudour, Gherroucha, Boukaboub, Bouchtab, Baka, Samra, Hocine, Amar, Karima, Mebarek and Kheireddine2011).
Statistics
The 96 characterized morphotypes were subjected to stepwise discriminant analyses, as run with STATISTICA (StatSoft Inc.), firstly using as classification categories the 9 groups of botanical varieties and secondly the 12 farm samples as autochthonous genotypes. The forward stepwise selection method, the F-test of significance of the partial regression coefficient of the last variable entering the model, and the Wilks’ lambda statistical test were used. A tolerance of 0.01 was applied to eliminate the variables providing superfluous information, along with those previously included in the model. The contribution of each variable to the discrimination was examined by comparing the standardized b coefficients. The graphs of the individual scores of canonical variables for the two principal functions were constructed. Mahalanobis distances between groups and their significance level probability values were calculated to measure the spaces between each group's centroids.
Results
Species composition
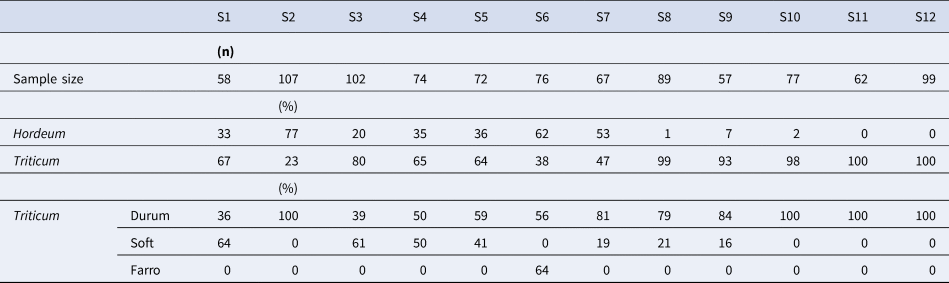
Almost all the 11 Saragolla seed samples sown in 2019 were highly heterogeneous communities of cereals, being a mixture of different species including durum wheat, soft wheat, farro and barley (Table 1), while oat was more rarely observed. Barley accounted for about 80% of plants in S2 and was absent or almost absent in S8, S9, S10, S11 and S12. By examining the percentage composition of the plants belonging to the Triticum genus, the maximum content of soft wheat was detected in the S1, S3, S4 and S5 samples; percentages equal to or less than 20% were present in all the others; a high content of farro (64%) was found in S6.
Table 1. Number of plants collected from each population sample (sample size), percentage composition of Hordeum and Triticum plants and percentage composition of the different species belonging to the Triticum genus observed in the central row of each experimental plot of Saragolla wheats from Abruzzo (S1 to S11) or Puglia (S12) and cultivated in the field of CREA-CI (Foggia) during 2019
Durum wheat botanical composition
Nine of the 22 different botanical varieties of durum wheat known to Percival in 1921 were also observed within the 12 Saragolla samples and a representative for each of them is shown in Fig. 1. This equals the number of botanical varieties observed at the beginning of 1900 within 32 durum wheat genotypes from all over the Sicily Region (De Cillis, Reference De Cillis1942 in Porceddu et al., Reference Porceddu, Vannella and Perrino1981). However, 17 botanical varieties were distinguished within 846 accessions from Algeria (Boudour et al., Reference Boudour, Gherroucha, Boukaboub, Bouchtab, Baka, Samra, Hocine, Amar, Karima, Mebarek and Kheireddine2011), where no breeding work was made during the second half of 1900.

Figure 1. Example of ear types observed within Saragolla wheats from Italy's Abruzzo and Puglia Regions, and the botanical variety to which they belong. (A) Botanical varieties with glabrous glumes. (B) Botanical varieties with pubescent glumes.
Most of the Saragolla samples from Abruzzo were of the italicum botanical variety (32%) with red glumes and red awns, followed by the valenciae (14%) and leucurum (10%) with white glumes and awns (Table 2). The italicum variety was found in every sample except for S12. The genotype quantitatively prevailing in most Saragolla populations from Abruzzo had an average height of 125 cm, with rather compact, lightly pigmented spikes, hairy glumes, red or brown-red awns and yellow-amber and elongated grains (Rascio et al., Reference Rascio, Codianni, Paone, Fiorillo and Marone2021). The affine and the very similar leucurum botanical varieties were the main component of S12 and were also observed in every Abruzzo landrace except for S5, S7 and S11. Leucurum and leucomelan were the most abundant in collections from Sicily and Algeria, respectively, while both contained reichenbachii which was absent from all Saragolla samples. Blue-black ear (provincial, obscurum, coerulescens or libycum) and beardless varieties (australe and sub-australe) were rare in all collections.
Table 2. Percentage botanical composition of autochthonous Saragolla wheat from 11 Abruzzo farms (S1–S11) and its comparison with the botanical composition of Saragolla from Puglia (S12) or the old durum wheats from Sicily (De Cillis, Reference De Cillis1942 in Porceddu et al., Reference Porceddu, Vannella and Perrino1981) and Algeria (Boudour et al., Reference Boudour, Gherroucha, Boukaboub, Bouchtab, Baka, Samra, Hocine, Amar, Karima, Mebarek and Kheireddine2011)

The degree of dissimilarity among the 9 botanical varieties was evaluated by a stepwise discriminant analysis, using the whole dataset of 20 morphological traits and the 9 groups of botanical varieties as classification categories. The Wilks’ Lambda and the F values (online Supplementary Table S1) proved that the model had a high discriminatory power, with 2 out of 8 discriminant functions accounting for 88% of the explained variance. All the Mahalanobis distances between botanical varieties were highly significant (online Supplementary Table S2), but the largest occurred between leucurum or affine and melanopus varieties and the smallest ones between valenciae and italicum. The biplot of canonical discriminant variables (Fig. 2) showed larger point dispersion around the centroids of the italicum, leucurum, valenciae and affine varieties, suggesting that a high degree of morphological variability exists among the genotypes included in these groups.

Figure 2. Scatter diagrams of the canonical scores reported by the nine groups of botanical varieties in the discriminant functions.
Based on the highest absolute values of the standardized coefficients of the canonical variables (online Supplementary Table S1), the glume pubescence and the awn colour were the main traits that, horizontally and vertically respectively, contributed to the separation of the nine groups. Other traits relevant to the botanical variety identification were the nerve presence on the outer glume (very evident in leucomelan genotypes), the mucrone length (longest for apulicum) and the ear density (highest for leucomelan).
Affinities among Saragolla populations
Using the 12 samples as classification categories, the affinity degree among them was analysed. The results of the stepwise discriminant analysis showed a significant discriminatory power of the model. The first two out of eleven discriminant functions accounted for 51% of the explained variance, with 15/20 parameters (online Supplementary Table S3) that most contributed to the differentiation among populations. Based on the probability values of Mahalanobis distances (online Supplementary Table S2) the S12 genotype group from the Puglia Region differed from those belonging to the Abruzzo populations, except S1 and S11. On the contrary, S11 did not significantly differ from the other Abruzzo samples except S4. The biplot of the canonical variable coefficients, separately constructed for each farm sample (Fig. 3), showed that S4 exclusively distributed in the I^ quadrant. S1, S6 and S7 mainly occupied the right side of the diagram and hence were essentially different from S3, S5 and S9, falling over the left quadrants. The standardized values of the canonical coefficients (online Supplementary Table S3) indicate those variables that most differentiated the sample populations falling in the left and right side of the diagram: glume colour, awn/ear length ratio, glume shoulder width and prominent dorsal ridge of seed. The traits that mostly vertically separated the populations were glume pubescence, stem solidity and plant height.

Figure 3. Scatter diagrams of the canonical scores reported in the discriminant functions by the 11 groups of autochthonous Saragolla wheats from Abruzzo and by the one from Puglia Region.
Discussion
Abruzzo and Puglia are two Regions historically dedicated to the cultivation of durum wheat, linked by the herd migration and probably by the exchange of seeds between farmers. The botanical characterization of the autochthonous wheats is mainly requested by the Local Authorities to protect territorial production.
The Saragolla wheats from Abruzzo were found to be a mixture of plants belonging to different genera, with wild barley accounting for 80% of seed in some samples. The undesired weed infestation is a frequent problem for cereals (Zhang et al., Reference Zhang2021). It can be attributed mainly to the existence of a wild barley seed bank in the soil which has the same growth period of wheat, an earlier seed dispersal and great seed ability to remain dormant in the soil (Hamidi and Mazaheri, Reference Hamidi and Mazaheri2012). The barley infestation affects the harvest quality and raises the cost of cultivation because of the work necessary for cleaning the grain. The lack of effective herbicides for barley control is a major obstacle for the conservation of Saragolla durum wheat, which is the main interest of farmers and local authorities in the Abruzzo Region.
At least 9 of the 22 different botanical varieties of durum wheat known to Percival in 1921 can be found within the 12 Saragolla population samples. Every botanical variety includes various forms differing for other morphological traits, in addition to those used for Percival's classification. We used the same classification system as previous botanical characterization studies of durum wheat (De Cillis, Reference De Cillis1942 in Porceddu et al., Reference Porceddu, Vannella and Perrino1981; Boudour et al., Reference Boudour, Gherroucha, Boukaboub, Bouchtab, Baka, Samra, Hocine, Amar, Karima, Mebarek and Kheireddine2011), carried out during the first half of the 1900s, which allowed us to make a diachronic comparison of the botanical variety diversity of the currently cultivated Saragolla with those observed at the beginning of 1900 in Sicily and Algeria, two important sites for durum wheat diversity conservation. Sicily has a rich heritage of durum wheat varieties, because it was the first arrival point of durum wheat during the westward migration from the first Near Eastern centre of origin, while North Africa was a secondary centre of diversity (Vavilov, Reference Vavilov1992). Within the old Sicilian collections, 9 botanical varieties were distinguished over a total of 32 genotypes coming from the entire region (De Cillis, Reference De Cillis1942 in Porceddu et al., Reference Porceddu, Vannella and Perrino1981). Using slightly different morphological classifications, the number of botanical varieties of several old Algerian collections of durum wheat ranged from 22 (Orlov, Reference Orlov1923) to 29 (Ducellier, Reference Ducellier1930). A more recent study (Boudour et al., Reference Boudour, Gherroucha, Boukaboub, Bouchtab, Baka, Samra, Hocine, Amar, Karima, Mebarek and Kheireddine2011) showed that the number of Percival's botanical varieties was 17, within 846 Algerian accessions. Considering that from 1962 (the beginning independence) to 2011, there was no amelioration strategy (Boudour et al., Reference Boudour, Gherroucha, Boukaboub, Bouchtab, Baka, Samra, Hocine, Amar, Karima, Mebarek and Kheireddine2011), it seems that Algeria underwent a heavy loss of durum diversity. Conversely, the detection of 9 different botanical varieties of durum wheat, within 96 samples belonging to the same landrace, suggests that a high degree of genetic diversity still exists in the 11 Saragolla populations from the Abruzzo Region, especially considering that the original seed collections were of poor quality (Rascio et al., Reference Rascio, Codianni, Paone, Fiorillo and Marone2021) and were contaminated with wild barley and soft wheat. Similar evidence was obtained by molecular marker analysis (Figliuolo et al., Reference Figliuolo, Mazzeo and Greco2007) of a Saragolla landrace from the Basilicata Region.
Most of the samples from Abruzzo consisted of durum and soft wheat and the finds of 9 taxonomically different botanical varieties, in variable mixtures within them, may have been the result of some interspecific hybridization (Matsuoka, Reference Matsuoka2011; Sahri et al., Reference Sahri, Chentoufi, Arbaoui, Ardisson, Belqadi, Birouk, Roumet and Muller2014). Most of the Saragolla plants belonged to the italicum botanical variety. An italicum morphotype, with an average height of about 125 cm, rather compact, lightly pigmented spike, hairy glumes, red or brown-red awns and yellow-amber and elongated grains, was observed within all the populations from Abruzzo. As this morphotype was distributed all over the territory, but was not observed within the Puglia samples, it could be considered the typical ‘Saragolla local variety of the Abruzzo Region’. The leucurum botanical variety, to which the Saragolla population cultivated in Italy at the beginning of the 1900s belongs (Percival, Reference Percival1921; Draghetti, Reference Draghetti1927), was also observed in 8 of the 11 Abruzzo samples. The leucurum variety together with the very similar affine variety, made up most of the S12 population from the Puglia Region and is probably the oldest Sicilian botanical variety (Porceddu et al., Reference Porceddu, Vannella and Perrino1981). It could be hypothesized that it first arrived from Sicily to Puglia, the closest geographic region, and then to Abruzzo, where, over time, may have been mixed with other varieties having better characteristics of adaptability, productivity and/or qualitative traits, as was likely the case in Sicily (Porceddu et al., Reference Porceddu, Vannella and Perrino1981).
Results of this work also showed that the key morphological traits, generally used for classification of durum wheat, have great systematic importance to undisputedly identify the botanical varieties within the Saragolla populations. In fact, the multivariate analysis showed a highly significant distance between the populations composing the 9 groups of botanical varieties, despite the large variability for the other morphological traits (not used for the botanical classification) that above all characterized the italicum, leucurum, valenciae and erythromelan varieties. Glume pubescence and awn colour were the main morphological traits that contributed to distinguishing the 9 botanic groups. Other relevant traits to botanical variety identification were the nerve presence on the outer glume (very evident in leucomelan genotypes), the mucrone length (longest for apulicum) and the ear density (highest for leucomelan). The mucrone length is a very stable trait (Porceddu et al., Reference Porceddu, Vannella and Perrino1981) and ear density is an important agronomic trait, as well as being useful for botanical classification (Vavilov, Reference Vavilov1951).
Although further molecular analyses are necessary to verify if morphological similarity corresponds to genetic similarity, the Mahalanobis distances (morphological differences) between the 11 Abruzzo groups were generally shorter, than those that separated each of them from the population from Puglia. Some exceptions were also observed: the S1 and S11 populations were very similar to the S12 population from the Puglia Region; S4 was the only population that differed from almost all the others, the main characteristic being short height. It may carry the Norin 10 dwarfing gene which can be found in many modern varieties and transferred to durum wheat from T. aestivum (2n = 6 × = 42) by breeding work starting in 1956 (Lebsock, Reference Lebsock1963). Hence, the low height is particularly indicative of the presence of recently released varieties and therefore of a lower integrity of conservation of the original population.
Appropriate information must be given to the farmers about the distinctive traits of ‘Saragolla’ from Abruzzo, but also on the effects that any agronomical practice and selection may have on the in situ conservation of their food crops. By comparing the results of this work with those of further future studies, it will be possible to evaluate with time, the effectiveness of the in situ conservation actions undertaken in the Abruzzo Region. (Hammer et al., Reference Hammer, Knüpffer, Xhuveli and Perrino1996; Barry et al., Reference Barry, Pham, Béavogui, Ghesquière and Ahmadi2008; Veteläinen and Maxted, Reference Veteläinen and Maxted2009).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262123000345.
Acknowledgements
The authors wish to thank the farm owners that provided the studied materials, Dr. Agostino Sacchetti, Dr Maurizio Odoardi, Dr Daniela Codoni, and Dr Nicola Bonifacio (Department of Rural Development and Fisheries Policies – Promotion of Knowledge and Innovation in Agriculture – DPD022) of the Abruzzo regional authorities, for their valuable contribution to the ‘SARAB project: Characterization of ancient Saragolla populations from the Abruzzo Region’.







