Introduction
Anthropogenic introduction of exotic plants around the world has modified habitats and ecosystems (Essl et al. Reference Essl, Mang and Moser2011, Liebhold et al. Reference Liebhold, Brockerhoff, Kalisz, Nuñez, Wardle and Wingfield2017). The introduction of a non-native species can cause direct and indirect impacts, altering the natural characteristics of the ecosystem (Sousa et al. Reference Sousa, Andrade, Xavier, Silva and Albuquerque2017, Jeschke et al. Reference Jeschke, Bacher, Blackburn, Dick, Essl, Evans and Pergl2014, Richardson & Rejmanek Reference Richardson and Rejmanek2011). An invasive plant has characteristics that facilitate its colonization, such as high proliferation and growth capacity, absence of predators or pathogens, and can cause ecological changes, such as the alteration of soil nutrient cycles (Matos & Pivello Reference Matos and Pivello2009). Floristic surveys have indicated that vegetation in urban areas in Brazil is mostly composed of exotic species (Albuquerque & Duré Reference Albuquerque and Duré2013, Fabricante et al. Reference Fabricante, Santos, de Araújo and Cotarelli2017, Kramer & Krupek Reference Kramer and Krupek2012). The jackfruit (Artocarpus heterophyllus Lam.) is one of the most common exotic plant species in Brazil (Freitas et al. Reference Freitas, Magalhães, de Resende, da Costa Brasil, da Rocha Vivès, Pinheiro and Luz2017, Guimarães et al. Reference Guimarães, Silva and Corrêa2017, Sartori et al. Reference Sartori, Martins, Zaú and Brasil2018) and is native to Asian tropical forests; it grows well in humid climates and soils, reaching up to 20 m in height (Elevitch & Manner Reference Elevitch and Manner2006, Khan et al. Reference Khan, Zerega, Hossain and Zuberi2010, Lider Agronomia 2012, Saxena et al. Reference Saxena, Bawa, Raju and Yahia2011). In Brazil, this plant species produces fruits all year long, with higher maturation period between October and April, and a single tree produces on average 50 fruits per year, each fruit with a mass of 3–10 kg (Elevitch & Manner Reference Elevitch and Manner2006, Khan et al. Reference Khan, Zerega, Hossain and Zuberi2010, Lider Agronomia 2012, Saxena et al. Reference Saxena, Bawa, Raju and Yahia2011).
A contributing factor to a successful invasion of an exotic plant is the seed dispersal process (Howe & Miriti Reference Howe and Miriti2004). Zoochory is the predominant means of seed dispersal in tropical forests in Brazil (Jordano et al. Reference Jordano, Galetti, Pizo, Silva, Rocha and Bergallo2006, Mikich et al. Reference Mikich, Liebsch, Almeida, Miyazaki, Parron, Garcia, Oliveira, Brown and Prado2015, Silva et al. Reference Silva, Silveira, Aumond, da Silveira and Cademartori2017). Among these dispersing animals, frugivorous mammals play an important role in the dispersal of seeds, restructuring natural landscapes, consuming fruit pulp, avoiding the rotting of the seed, and transporting them either by handling or ingestion (Cáceres & Lessa Reference Cáceres, Lessa and Cáceres2012, Cantor et al. Reference Cantor, Ferreira, Silva and Setz2010, Jordano et al. Reference Jordano, Galetti, Pizo, Silva, Rocha and Bergallo2006). The diversity of mammals of the Atlantic Forest is composed of 321 species, of which 89 are endemic, presenting higher representation of the orders Didelphimorphia and Rodentia (Graipel et al. Reference Graipel, Cherem, Monteiro-Filho, Carmignotto, Monteiro-Filho and Conte2017). The marsupials and rodents, mostly frugivores and/or insectivores, are considered opportunistic animals for taking advantage of the food supply in the environment (Cáceres & Lessa Reference Cáceres, Lessa and Cáceres2012). Thus, a jackfruit invasion can maintain dense populations of small frugivorous mammals due to the high fruit production and its high caloric rate (Mello et al. Reference Mello, Moulton, Raíces and Bergallo2015). A large exotic plant, such as jackfruit, can alter the environment by suppressing the growth of herbaceous-shrub formations (Ziller Reference Ziller2001). With these characteristics, the jackfruit tends to generate homogeneity of the habitat through its dominance over native vegetation (Fabricante et al. Reference Fabricante, de Araujo, de Andrade and Ferreira2012, Ziller Reference Ziller2001). A habitat with reduced plant diversity can influence the richness and distribution of animal species (Kerr & Packer Reference Kerr and Packer1997, Lorenzón et al. Reference Lorenzón, Beltzer, Olguin and Ronchi-Virgolini2016, Ortega et al. Reference Ortega, Thomaz and Bini2018). Non-volant small mammals, for example, which use plant complexity as a camouflage strategy to escape potential predators and to obtain food resources, can be directly affected when the environment loses its plant diversity (Price et al. Reference Price, Green, Troscianko, Tregenza and Stevens2019, Stein et al. Reference Stein, Gerstner and Kreft2014).
Duas Bocas Biological Reserve (DBBR), am area protected by law located in the municipality of Cariacica in the state of Espírito Santo, has native areas where the jackfruit invasion occurs (Boni et al. Reference Boni, Novelli and Silva2009). Additionally, there are interaction records between mammalian species and jackfruit (Jose et al. Reference Jose, Mendes and Passamani2016). In this way, based on the potential interaction between animals (i.e. non-volant small mammals) and plants (i.e. jackfruit), it is possible to predict how the jackfruit affects mammals in DBBR. In this study, we evaluated how jackfruit affects non-volant small mammals’ assemblage in DBBR areas with different stages of conservation, including areas with high jackfruit (Artocarpus heterophyllus) density and areas without jackfruits. We aimed to answer the following questions: (1) What is the difference between the composition of non-volant small mammals among the studied areas? (2) Is there a difference in the species richness between areas with and without jackfruits? and (3) Is the abundance of non-volant small mammal species influenced by the jackfruit density? We expected to find a difference in the mammalian assemblage composition between the areas with and without jackfruit, and areas with higher jackfruit density will present a higher abundance of frugivorous mammals, while areas without jackfruit will present higher species richness.
Methods
Study area
We conducted the study at the Duas Bocas Biological Reserve (DBBR), located in the rural area of the municipality of Cariacica, Espírito Santo state, south-eastern Brazil, between the coordinates 20º18′31′′S and 40º20′26′′W (Figure 1). The reserve has an approximate area of 2,910 hectares (IEMA 2018), constituting an area protected by law formed by a fragment of the Atlantic Forest with predominant vegetation of Dense Ombrophylous Forest Sub-montana (Novelli Reference Novelli2010). The reserve has three major trails, with the composition and structure of the vegetation differing according to the jackfruit density and vegetation heterogeneity (Supplementary material 1).

Figure 1. (A) Map of Brazil, (B) the state of Espírito Santo, (C) the location of Duas Bocas Biological Reserve representing the sampling trails.
Methods of collection and analysis
We collected the data from March to October 2018, with monthly field expeditions. Each trail was subdivided into six sampling sites on a grid of 1 hectare with a minimum distance of 500 m between sites, with a total of 18 sampling sites. In each sampling site, we defined and simultaneously sampled six sampling stations that consisted in a plot of 10 × 10 m with a minimum distance of 50 m between stations. In each sampling station, we installed five live-traps, one Tomahawk (size: 45 × 21 × 21 cm) and four Shermans (size: 25 × 8 × 9 cm). The traps were arranged as follows: four Sherman traps at the corners of the sampling station plot (two placed 1.5 m high on lianas or tree trunks in the understorey and two placed on the ground) and a Tomahawk trap in the centre placed on the ground. All stations were sampled five times. Eight of the 18 sampling sites had jackfruits present.
We used a homogenized paste of banana, sardines, ground peanuts and cornmeal, as a bait for attracting mammals to the traps. The traps remained open for three consecutive nights in each field expedition and were checked every morning. For each captured animal we recorded the date of capture, the point at which it was captured, and identified the species. We marked the individuals with numbered ear tags to make it possible to recognize if the individual had been recaptured. After all the information was collected, each individual was released in the same place where it was captured. We used field guides, for example a mammals guide (Emmons & Feer Reference Emmons and Feer1997) and specific guides such as the Rodents Guide of Brazil (Bonvicino et al. Reference Bonvicino, Oliveira and D’Andrea2008) for species identification. The species were classified according to endemism, trophic guild and their nomenclature according to the annotated list of mammals of Paglia et al. (Reference Paglia, Fonseca, Rylands, Herrmann, Aguiar, Chiarello, Leite, Costa, Siciliano, Kierulff, Mendes, Tavares, Mittermeier and Patton2012).
Jackfruit density
In each 10 × 10 m sampling station grid, we counted the number of jackfruit trees. The obtained value of density in each sampling station was extrapolated to trees per hectare for the 18 sampling sites to be used in the analyses. The Represa Velha trail, located near a dammed lake, has an extension of 3500 m in length, and has a higher density of jackfruits, with an average of 15.5 jackfruit trees per hectare. The Porteira Preta trail, with an extension of 3800 m in length, has an average of 1.5 jackfruit trees per hectare. The Alto Alegre trail, located in the north-west of the reserve, is 4050 m long and presents the most preserved area of the reserve without the presence of jackfruits.
Data analysis
We performed Moran’s I (Dormann et al. Reference Dormann, McPherson, Araújo, Bivand, Bolliger, Carl, Davies, Hirzel, Jetz, Kissling, Kühn, Ohlemüller, Peres-Neto, Reineking, Schröder, Schurr and Wilson2007) to check for spatial autocorrelation. Values of Moran’s I range from −1 (indicating perfect dispersion) to +1 (perfect correlation). Moran’s I was computed using a permutation-based test (99 permutations at 5% significance level using the ‘moran.cp’ function in the ‘spdep’ package in R software; Bivand Reference Bivand2010). We did not find spatial autocorrelation in the residuals (Moran’s I = −0.64).
The observed and estimated richness was represented by the rarefaction curve to verify if the effort used was sufficient to represent the non-volant small mammals. We used the estimator Chao 1 due to the number of relatively rare species (Magurran Reference Magurran2013). The model with the estimators was made with 1000 randomizations and calculated in the software EstimateS® 9.2 (Colwell Reference Colwell2013).
We ordered the composition and abundance data (number of individuals first time captured) of the non-volant small mammal species in the DBBR using a Non-Metric Multidimensional Scaling analysis (NMDS, stress = 0.11) using the Bray–Curtis metric to calculate the similarity in the composition and frequency of records of the species between sites. The analyses were performed in R software version 3.4.4 using a ‘metaMDS’ function in Vegan package version 2.5-4 for community analysis (Oksanen et al. Reference Oksanen, Blanchet, Kindt, Legendre, Minchin, O’hara and Wagner2013). This analysis aimed to verify the existence of a pattern in the ordering of mammalian assemblage. ANOSIM was used separately to test for any difference between the sites with and without jackfruit. We fitted generalized linear model (GLM; Poisson error distribution) to understand how the jackfruit density influences the richness and abundance of species. We also tested the effect of each sampling site altitude in species richness and abundance. We used as predictor jackfruit density and altitude (in metres). We considered as plausible all models at ΔAIC < 2 from the best fitted model (Burnham & Anderson Reference Burnham and Anderson2002). We calculated model support using Akaike weights (wAIC, ranging from 0 to 1, with larger numbers indicating greater support; Burnham & Anderson Reference Burnham and Anderson2002). All analyses were performed in R software 3.4 (R Core Team 2017).
Results
We recorded 31 species of non-volant small mammals, 20 species of the Order Rodentia and 11 species of the Order Didelphimorphia (Table 1). The rarefaction curve reached asymptote, demonstrating that the effort used was sufficient to represent the non-volant small mammals in the DBBR (Figure 2).
Table 1. Non-volant small mammal species from Duas Bocas Biological Reserve, State of Espírito Santo, south-eastern Brazil. FO = Folivore; FR = Frugivore; GR = Granivore; HE = Herbivorous; IN = Insectivorous; OM = Omnivorous; PS = Piscivorous.
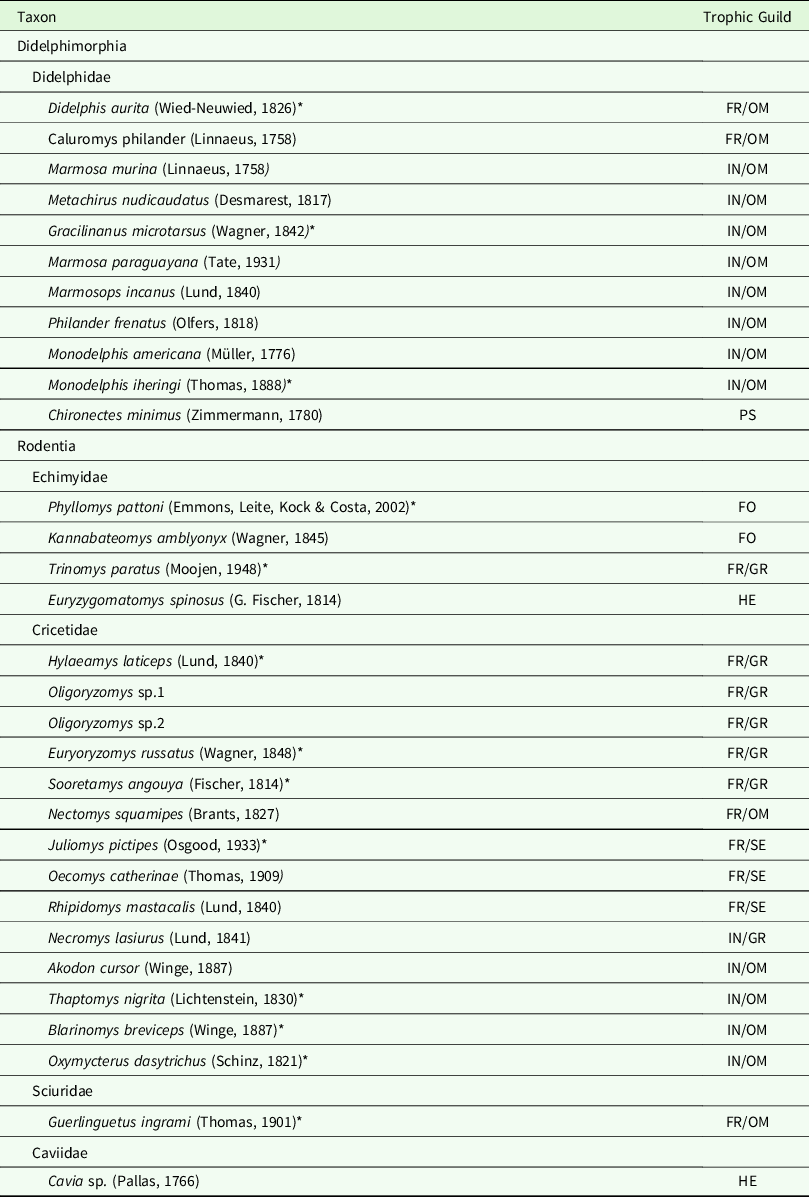
* Indicates endemic Atlantic Forest species

Figure 2. Rarefaction curve showing the estimated and observed richness in relation to the sampling effort employed in the Duas Bocas Biological Reserve, state of Espírito Santo, south-eastern Brazil.
The composition and structure of the non-volant mammal’s assemblage differed between the three localities sampled in the DBBR, grouping the sampling sites with higher similarity (Figure 3A). In addition to the differences in DBBR areas, the small mammal assemblage was also structured according to the presence and absence of jackfruits (Figure 3B). The difference between sites with and without jackfruits was confirmed by ANOSIM (R = 0.45, P < 0.001).

Figure 3. Non-metric multidimensional scaling (NMDS) showing (A) similarity between sampling areas in the richness and abundance of non-volant small mammals and (B) similarity between sampling areas with presence and absence of jackfruits in the State of Espírito Santo, south-eastern Brazil.
The jackfruit density found in the sampled sites influenced both mammalian richness and abundance (Table 2). Jackfruit density had a significantly negative effect on species richness (Figure 4A). On the other hand, the jackfruit density positively influenced the abundance of mammals (Figure 4B). We did not find an effect of altitude in the mammalian richness and abundance (Table 2).
Table 2. Generalized linear models (GLM; Poisson error distribution) that best explained richness and abundance of non-volant small mammals selected by AIC in the Duas Bocas Biological Reserve, Espírito Santo state, south-eastern Brazil.


Figure 4. (A) species richness and (B) total abundance per site of non-volant small mammals according to the jackfruit density in the Duas Bocas Biological Reserve, State of Espírito Santo, south-eastern Brazil. Symbols: Circle = sampling sites without jackfruit and triangle = sampling sites with jackfruit.
The non-volant small mammals assemblage, in terms of jackfruit density in the sampled areas (Figure 5), showed higher abundance of frugivorous species such as Didelphis aurita, Oligoryzomys sp.1 and Trinomys paratus in areas with higher jackfruit density. Insectivorous species such as Marmosa paraguayana, Monodelphis iheringi and Gracilinanus microtarsus were more abundant in areas without jackfruit.

Figure 5. Non-volant small mammals assemblage (bar values = total abundance per species in each sampling site) in relation to jackfruits density in the areas of study in Duas Bocas Biological Reserve, State of Espírito Santo, south-eastern Brazil.
Discussion
The structure of the non-volant small mammals assemblage in the three trails of DBBR differed in their composition with higher richness in the Alto Alegre trail. This could be related to the fact that this area is more preserved without jackfruit that tends to generate homogeneity of the habitat through its dominance over native vegetation (Fabricante et al. Reference Fabricante, de Araujo, de Andrade and Ferreira2012, Ziller Reference Ziller2001). Indeed, Banasiak & Shrader (Reference Banasiak and Shrader2016) suggest that communities of small mammals prefer more complex environments, because besides the higher availability of resources, vegetation cover helps reduce predation. Therefore, the plant structure in microhabitats could influence the composition of small mammals. We found similarities between the sampling sites of Represa Velha trail and the Porteira Preta trail with higher jackfruit density. While the Alto Alegre Trail, a site of preserved forest, showed similarity with the sampling sites of the Porteira Preta trail without jackfruit, which could also be related to a higher habitat heterogeneity for small mammals.
The non-volant small mammals were positively and negatively influenced by the jackfruits. In the study area, the climate and water availability are favourable for high fruit productivity all year round, making it a food resource for non-volant mammals even outside the jackfruit fruiting period. Some species of frugivorous-omnivorous marsupials have a diet based on food availability according to the season of the year (Cáceres and Lessa Reference Cáceres, Lessa and Cáceres2012, Casella Reference Casella2011, De Moura & Dos Santos Pires Reference De Moura and Dos Santos Pires2014), being favoured in an area where food is offered during both the dry and rainy seasons. Additionally, our data showed a higher abundance of small non-volant mammals, mainly frugivores, in areas with higher jackfruit densities. In fact, Mello et al. (Reference Mello, Moulton, Raíces and Bergallo2015) estimated that to maintain a population with two individuals of Trinomys dimidiatus, it is necessary to have 3.97 jackfruits per 100 m2 producing fruits monthly. Considering that we found an average density of 18.6 jackfruits per 100 m2 on the Represa Velha trail, this value could be enough to sustain the frugivorous populations in the DBBR. Therefore, the frugivorous species abundance in areas with a higher jackfruit density is justifiable. However, in these sites, jackfruit also plays a negative role through the action of allelopathic substances that cause significant changes in nutrient availability in the soil, and may decrease the diversity of small invertebrate species in areas with higher jackfruit density (Bergallo et al. Reference Bergallo, Bergallo, Rocha and Rocha2016, Fabricante et al. Reference Fabricante, de Araujo, de Andrade and Ferreira2012), negatively impacting the diet of mostly insectivorous species, which justifies the presence of a few insectivorous mammals in the areas with jackfruit.
Our results showed a higher richness of non-volant small mammals in the preserved forest area. There is a positive relationship between species richness and habitat complexity – a heterogeneous environment is one of the factors that can influence species richness (Grelle Reference Grelle2003). We observed that the higher the jackfruit density in the sampling sites, the lower the species richness of small mammals, which could be explained by habitat homogenization caused by jackfruit (Fabricante et al. Reference Fabricante, de Araujo, de Andrade and Ferreira2012, Ziller Reference Ziller2001). Non-volant small mammals prefer to forage for food in environments with higher vegetation cover, because they provide greater protection against predators (Banasiak & Shrader Reference Banasiak and Shrader2016). This may also explain why the richness of non-volant small mammals is higher in areas without jackfruits, where we observe higher vegetation cover in the soil (Supplementary material 2).
Jackfruit can affect both positively and negatively the DBBR assemblage of non-volant small mammals. For species with a habit of frugivory, the presence of jackfruit has a positive effect favouring these species. On the other hand, for insectivorous species, jackfruit represents an impact inhibiting the presence of these species in the area with high jackfruit density. The results presented are the first step in understanding the effect of this invasive species on the small mammals’ assemblage and initiating a monitoring of these species in areas affected by the presence of jackfruits. It is also important to consider evaluating the jackfruit effect on other species of larger mammals with known occurrence in DBBR such as agouti, paca, monkeys and peccary. Furthermore, management of jackfruits in the DBBR is required. This management process should be done gradually, assessing the impact that will occur in the population size of some species, especially for frugivores, found in areas where jackfruit density has been shown to be a strong influence on their abundance.
Supplementary materials
For supplementary material for this article, please visit https://doi.org/10.1017/S026646742000019X
Acknowledgements
This study was conducted with the research licence Process 76444341 – Authorization 003A-2017 provided by the ‘Instituto Estadual de Meio Ambiente e Recursos Hídricos – IEMA’. We thank the Duas Bocas Biological Reserve for logistical support.
Financial support
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. This study is a portion of the results of the Project ‘Vivendo na Floresta: Conservação da biodiversidade capixaba’. The authors benefitted from grants provided to HGB (process 307781/2014-3) and to CFDR (302974/2015-6 and 472287/2012-5) from CNPq and through ‘Cientistas do Nosso Estado’ Program from FAPERJ to CFDR (process No. E-26/102.765/2012 and E-26/202.920/2015) to HGB (process E-26/202.757/2017). ACF thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 for a scholarship and FAPERJ for a scholarship (process nº 240022 of E-26/202.198/2018 and process nº 255804 of Programa Pós-Doutorado NOTA 10 – 2020 “PDR102020”).









