Introduction
Seasonal allergic rhinitis (or hay fever), an antigen-mediated inflammation of the nasal mucosa that may extend into the paranasal sinuses because of an allergic reaction to allergens such as pollens, is the most common atopic disorder. Its prevalence ranges from 10 to 40 per cent of all adults, with higher rates in Western countries.Reference Katelaris, Lee, Potter, Maspero, Cingi and Lopatin1,Reference Brożek, Bousquet, Agache, Agarwal, Bachert and Bosnic-Anticevich2 Its prevalence has had a nearly 2-fold increase in the last 20 years, with more significant rises in countries with formerly low prevalence.Reference Brożek, Bousquet, Agache, Agarwal, Bachert and Bosnic-Anticevich2,Reference Schwindt and Settipane3 This increase is paralleled by an increase in co-morbidities such as asthma.Reference Lunn and Craig4 Seasonal allergic rhinitis is characterised by a runny nose, congestion, sneezing and sinus pressure. It is usually a non-life threatening issue, but it is considered a major chronic respiratory disorder because of its high incidence and impact on the quality of life, healthcare costs, mood, social functioning, work or school performance, and sleep.Reference Brożek, Bousquet, Agache, Agarwal, Bachert and Bosnic-Anticevich2,Reference Zuberbier, Lötvall, Simoens, Subramanian and Church5–Reference Sardana and Craig7
Seasonal allergic rhinitis occurs because of a type I hypersensitivity following the release of the granule-stored mediators such as proteases, histamine, lipid mediators and cytokines from mast cells.Reference Theoharides and Kalogeromitros8 These mast-cell mediators can sustain or amplify the inflammation by supporting the inflow of inflammatory cells, thereby increasing tissue inflammation. The only long-lasting treatment (i.e. immunotherapy) has variable efficacy and duration; therefore, other treatments are frequently utilised in order to control symptoms.
First-line management is usually based on the identification and avoidance of the causing allergens, coupled with decongestants and second-generation anti-histamine drug use. Second-line interventions consist of anti-leukotrienes, steroids and anti-cholinergic drugs. Immunotherapy still represents a third-line treatment, mostly because of its cost. It is well-known that first-generation anti-histamines may cause side effects like drowsiness, dizziness, headache, loss of appetite, stomach upset, vision changes, irritability, dry mouth and dry nose.Reference Baena-Cagnani9,Reference Hardjojo, Shek, van Bever and Lee10 Second-generation anti-histamines have poor efficacy in the management of more severe casesReference Baena-Cagnani9 and in treating perennial rhinitis, because symptoms, predominantly nasal obstruction, are not histamine-mediated.Reference Greiner, Hellings, Rotiroti and Scadding11,Reference Turner and Kemp12 Topical nasal corticosteroids are commonly prescribed; however, the safety of these compounds remains controversial. The main concerns derive from dose-related systemic adverse effects associated with long-term treatments (e.g. adrenocortical function suppression, growth in children and bone metabolism alterations).Reference Baena-Cagnani9,Reference Al Sayyad, Fedorowicz, Alhashimi and Jamal13 Since commonly employed drugs may not only induce known adverse reactions but do not have an impact on symptom recurrences, natural anti-allergic products could represent a further useful tool in anti-allergic treatment.
Prior research suggests that quail egg has a high protein content with anti-allergic, anti-inflammatory and anti-cancer activities.Reference Knoops, Louahed, Van Snick and Renauld14–Reference Bruttman18 Several studies suggested that daily oral quail egg administration may weaken allergic asthma and rhinitis symptoms.Reference Truffier15,Reference Benichou, Armanet, Bussière, Chevreau, Cardot and Tétard16 Furthermore, it has been shown that quail egg has therapeutic potential in modulating the inflammatory response and reducing the manifestations of food allergy induced eosinophilic oesophagitis disease.Reference Knoops, Louahed, Van Snick and Renauld14 Quail egg has an anti-allergic action via inhibiting the activation of mast cells. As histamine, tryptase, T helper 2 cells and pro-inflammatory-related cytokines are related to several allergic and inflammatory disorders, downregulating their mast cell secretion could prove useful. In this randomised, controlled trial, we aimed to compare the efficacy and immediate tolerance of oral quail eggs as a supplement of nasal mometasone spray in a randomised, controlled trial setting.
Materials and methods
A Consolidated Standards of Reporting Trials compliant, open-label, randomised, controlled trial with a parallel-group design was conducted to evaluate the efficacy and immediate tolerance of a one-month treatment regime with a commercially available zinc and quail egg dietary supplement combined with mometasone nasal spray in improving seasonal allergic rhinitis symptoms. The study was implemented from February to September 2019 at the University of Catania. The Consolidated Standards of Reporting Trials checklist for randomised, clinical studies is shown in Table 1 in the supplementary material, available on The Journal of Laryngology & Otology website.
Table 1. Baseline demographic and clinical profile of patients
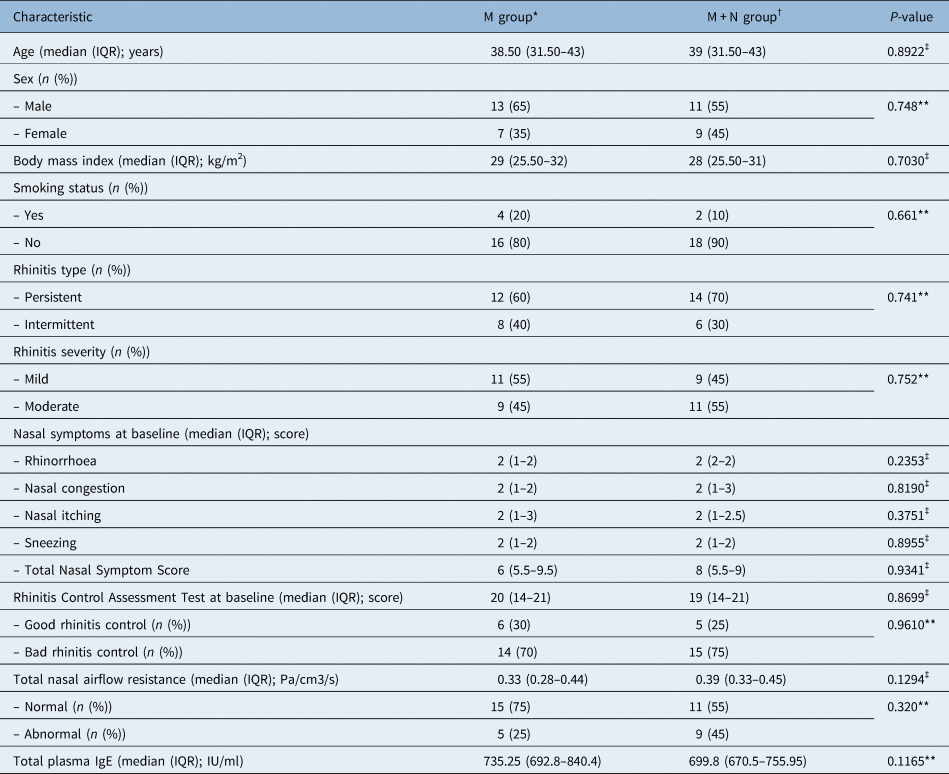
*n = 20; †n = 20; ‡Mann–Whitney U test was used; **Fisher's exact test was used. M = mometasone; M + N = mometasone + quail egg and zinc tablets; IQR = interquartile range
Patients were eligible to participate if the following inclusion criteria were met: (1) age equal to or more than 18 years and (2) with a recent diagnosis of mild to severe seasonal allergic rhinitis to the most common inhalant allergens (Lolium perenne, Phleum pratense, Secale cereale, Holcus lanatus, Parietaria Judaica, Artemisia vulgaris, Olea europaea and Alternaria tenuis) prevalent in the geographic area in which the study was carried out and (3) no ongoing treatment for seasonal allergic rhinitis.
Diagnosis and severity of seasonal allergic rhinitis are based on the Allergic Rhinitis and its Impact on Asthma guidelines in combination with positive skin test reactions to suspected allergens and a positive determination of allergen-specific serum immunoglobulin (Ig) E levels (ImmunoCAP, Pharmacia Diagnostics AB, Uppsala, Sweden). Specific IgE values of equal to or more than 0.35 kU/L were considered indicative of aeroallergen sensitisation. All patients underwent the same common inhalant allergen panel evaluation composed of 12 items. An allergen schedule specific for Southern Italy can be seen in Table 2 in the supplementary material, available on The Journal of Laryngology & Otology website.
Table 2. Mean change in nasal symptom score by treatment group

*n = 20; †n = 20; ‡independent t-test was used; **statistically significant value. M = mometasone; M + N = mometasone + quail egg and zinc tablets; SD = standard deviation
Exclusion criteria were: any other chronic medical condition (including uncontrolled asthma), pregnancy, concomitant respiratory tract infection, administration of systemic or topical antihistamine, leukotriene receptor antagonist, or decongestant drugs within the week before the study participation, systemic or topical corticosteroid use within the month before the study participation, and known sensitivities to any of the ingredients of the study product.
The researchers employed a convenience sampling design to select study participants. Patients who satisfied the inclusion and exclusion criteria were invited to participate in the study. Because of the lack of previously published studies comparing the two treatments of interest that could be used for a priori sample size computation as well as the limited resources available to the researchers, the attained statistical power was examined instead, given a finite sample of 20 patients for each treatment arm. Post-hoc analysis showed that the study achieved 61 per cent power in detecting a significant difference in the mean change in Total Nasal Symptom Score between the two groups based on the following parameters: (1) effect size of 0.73 (mometasone group: 2.95 ± 1.73; mometasone + quail egg and zinc tablets group: 4.50 ± 2.44), (2) alpha equal to 0.05 and (3) a sample size of 40 (20 for each group). Eligible patients were invited and were referred to the primary investigator for study procedure orientation and informed consent administration. A statistician not related to this study generated the allocation schedule before the start of the study by using a statistical computing web programming tool (available at www.graphpad.com/quickcalcs). A simple randomisation technique was used to allocate the patients to two groups in a 1:1 ratio. The allocation schedule was concealed from study researchers until study termination.
Patients were assigned a number sequentially according to their study entry by the primary investigator. Patients received the treatment assigned to their number based on the allocation schedule. G*Power 3.1.9.2 software was used for power analysis.
A Consolidated Standards of Reporting Trials compliant diagram with patient allocation and analysis is shown in Figure 3 in the supplementary material, available on The Journal of Laryngology & Otology website. Patients were randomly allocated into two groups: the mometasone nasal spray group and mometasone nasal spray + quail egg and zinc tablets group. The mometasone nasal spray group received 100 mcg mometasone nasal spray (Nasonex, Merck, Kenilworth, USA) and were instructed to administer two sprays in each nostril once daily for four weeks. Patients assigned to the mometasone nasal spray + quail egg and zinc tablets group were instructed to take Narivent (DMG, Rome, Italy) oral soluble tablets, which contain quail egg homogenate and zinc (average values for 2 tablets consist of 84 mg quail egg powder and 1.5 mg zinc), twice a day, morning and evening, preferably between meals, for four weeks in addition to the mometasone nasal spray. Patients were also informed that the tablets should be chewed or sucked slowly until completely dissolved in the mouth. Patients were allowed to use cetirizine 5 mg (once daily) as rescue medication during the treatment period.
At their first visit, the primary investigator obtained the following baseline demographic and clinical data: age, sex, body mass index, smoking status, rhinitis type and rhinitis severity based on the Allergic Rhinitis and its Impact on Asthma guidelines.Reference Brożek, Bousquet, Agache, Agarwal, Bachert and Bosnic-Anticevich2
The following measures were also obtained at baseline and at the end-of-study visit to assess efficacy. (1) Total Nasal Symptom Score, which included four parameters related to symptoms (rhinorrhoea, nasal congestion, nasal itching, sneezing). The intensity was calculated by total and individual nasal symptoms score using a numerical score scale (0, no symptoms; 3, severe).Reference Ellis, Soliman, Steacy, Boulay, Boulet and Keith19 (2) A Rhinitis Control Assessment Test, which is a self-administered instrument, was used for evaluating symptom control in patients with rhinitis. This had a 5-point Likert scale (‘never’ to ‘extremely often’), with scores ranging from 6 to 30 and scores less than 21 indicating bad rhinitis symptom control.Reference Nathan, Dalal, Stanford, Meltzer, Schatz and Derebery20 (3) Total nasal airflow resistance (right + left; Pa/cm3/s) was measured with active anterior rhinomanometry (Rhino Pocket ED 200; Euro Clinic, Castel Bolognese, Italy) by using a standard protocol. Total nasal airflow resistance reflects the resistance of both sides of the nasal cavity. Normal value range is 0.10–0.40 Pa/cm3/s.Reference Ottaviano and Fokkens21 (4) Total plasma IgE (IU/ml) was also measured.
Furthermore, at each visit patients underwent a general otolaryngological examination and a flexible nasal endoscopy. Patients were asked to return one month after treatment initiation to assess the outcomes. The use of cetirizine rescue doses, as well as the presence of adverse events, were recorded at follow up.
This study was conducted in accordance with the Declaration of Helsinki and the note for guidance on good clinical practice (International Conference on Harmonisation-Good Clinical Practice). The study was approved by the Ethics Committee of the School of Medicine (Azienda Sanitaria Provinciale 3 Catania; approval number: 077/19). All patients signed written informed consent before their enrolment. Moreover, the trial was registered by the German Clinical Trials Register (code: DRKS00023981).
Data were encoded in Excel® spreadsheet software by the researcher. Stata MP® (version 14) statistical software was used for data processing and analysis. Continuous data were presented as mean and standard deviation or median and interquartile range depending on data distribution. The Shapiro–Wilk test was used to assess data normality. Categorical data were presented as frequency and percentage. Independent t-test or Mann–Whitney U test was used to analyse continuous variables. Fisher's exact test was used to analyse categorical variables. P-values equal to or less than 0.05 were considered statistically significant. The intention-to-treat principle was implemented.
Results
Forty patients were included in the study as planned, and 20 patients were randomised to each treatment arm (see supplementary Figure 3). Table 1 compares the demographic and clinical profile of patients by treatment group. The median age of all patients was 38.5 years (range, 19–51 years). Most patients were male (60 per cent). There was no significant difference observed between the two groups in terms of median age, sex, median body mass index, smoking status, rhinitis type, rhinitis severity, nasal symptom score, Rhinitis Control Assessment Test, total nasal airflow resistance and total plasma IgE (p > 0.05).
Table 2 compares the mean change in the nasal symptom scores between the two groups. The mean reduction in rhinorrhoea, nasal itching and sneezing scores was found to be higher in the mometasone + quail egg and zinc tablets group; however, statistical significance was only observed in sneezing and nasal itching scores. The mean change in total nasal symptom score was also found to be significantly higher in the mometasone + quail egg and zinc tablets group.
Among the secondary outcomes (Table 3), only the change in Rhinitis Control Assessment Test score was significantly different between the two groups. A higher proportion of participants in the mometasone + quail egg and zinc tablets group had good rhinitis control and normal total nasal airflow resistance as compared with those in the mometasone only group, but the results were statistically significant only in the first case. None of the participants in the mometasone + quail egg and zinc tablets group required rescue medication (cetirizine). Only 2 patients (10 per cent) for each group developed adverse events (2 patients in the mometasone + quail egg and zinc tablets group had nasal dryness; one patient in the mometasone only group developed nasal dryness and one had epistaxis). No significant difference in the proportion of patients who developed adverse events was observed. All patients completed the intended one-month treatment regime and no patients were lost to follow up.
Table 3. Secondary outcomes by treatment group
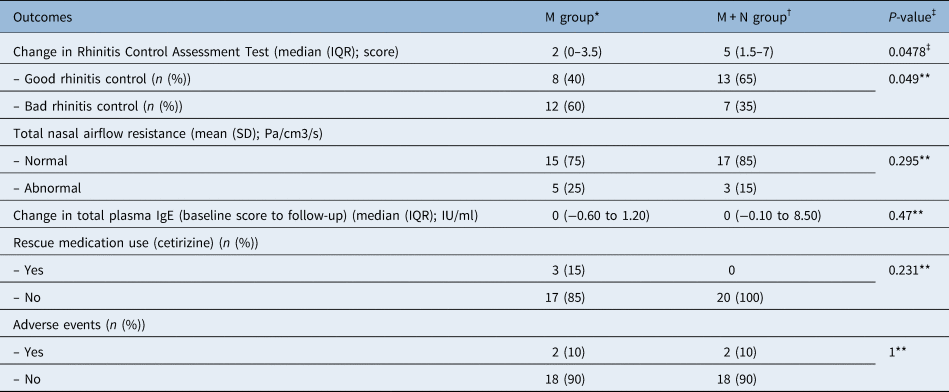
*n = 20; †n = 20; ‡Mann–Whitney U test was used; **Fisher's exact test was used. M = mometasone; M + N = mometasone + quail egg and zinc tablets; IQR = interquartile range; SD = standard deviation; Ig = immunoglobulin
Discussion
In our randomised, clinical trial, we found a significantly higher mean reduction in nasal itching and sneezing scores and mean change in total nasal symptom score in the mometasone + quail egg and zinc tablets group. Among the secondary outcomes, the change in the Rhinitis Control Assessment Test score was significantly different between the two groups. A significantly higher proportion of participants in the mometasone + quail egg and zinc tablets group had good rhinitis control when compared with those in the mometasone only group.
In line with our findings, two previous clinical trialsReference Benichou, Armanet, Bussière, Chevreau, Cardot and Tétard16,Reference Syrigou, Psarros, Makris, Grapsa and Syrigos22 supported the efficacy and safety of a quail egg dietary supplement for the symptomatic treatment of seasonal allergic rhinitis symptoms. In contrast with those results, our trial showed a significant improvement in sneezing scores following administration of the drug but not in nasal congestion. This is most likely because of the fact that we used nasal steroids in both groups. There is increasing evidence that intranasal corticosteroids provide a better effect than anti-histamines for nasal blockage,Reference Mygind, Dahl, Nielsen, Hilberg and Bjerkex23 and this would explain the lack of difference in nasal blockage between the two groups. This is also supported as well by the absence of significant difference in nasal airflow resistance. The use of quail egg as an adjunct to an already commonly employed and effective therapy, such as topical mometasone, allowed our study to give more relevance to the role of quail egg as an adjunct to baseline therapy, in contrast with prior studies on this subject.
Despite quail egg not appearing to be very effective for obstructive symptoms, it was indeed effective in terms of sneezing and itching scores. This could be because of its potential in inhibiting the cascade of immune-related allergic responses and blocking allergens before they can activate the immune cells. In contrast, common over-the-counter medications such as anti-histamines block the activity of histamine after it has been released during an allergic attack.Reference Syrigou, Psarros, Makris, Grapsa and Syrigos22,Reference Aaronson24
The use of natural dietary supplements, with a lower adverse events risk profile and no intrinsic contraindications other than known component allergy, might prove a useful integration for symptomatic management of seasonal allergic rhinitis.Reference Aaronson24 Quail egg consists mainly of water, proteins (especially ovomucoids and ovoinhibitors), fats, minerals and carbohydrates.Reference Prelipcean, Prelipcean and Teuşan25 In vitro data showed that these proteins may act as inhibitors of serine proteases,Reference Feeney, Means and Bigler17,Reference Vergnaud, Bruttmann, Louwagie and Morel26 which present in some outdoor and indoor antigens causing tissue injury and IgE-mediated allergic response. Thus, quail egg seems to attenuate the periodic manifestations of an allergic reaction.Reference Benichou, Armanet, Bussière, Chevreau, Cardot and Tétard16,Reference Widmer, Hayes, Whittaker and Kumar27 Besides, toxicological research including an acute and repeated oral administration on rats as well as in vitro studies demonstrated good tolerability of quail egg without genotoxic or mutagenic problems.Reference Bruttman18
Another study demonstrated that oral quail egg succeeded in reducing immune reactions and manifestations of peanut-sensitised mice with eosinophilic oesophagitis-like disorder, confirming the apparent anti-allergic role of quail egg.Reference Lianto, Han, Li, Ogutu, Zhang and Fan28 It was observed that farmers with quails had fewer allergic manifestations than the general population in the same area.Reference Truffier15 Of note, cases suffering from outdoor and indoor allergens were given quail egg powder or placebo. It was shown that consumption of quail egg resulted in symptom relief with good tolerability.Reference Bruttman18 Not only preclinical studies but also some clinical trials have investigated the efficacy and safety of quail egg. Benichou et al. found good symptomatic relief with no side effects in cases with induced manifestations of seasonal allergic rhinitis.Reference Benichou, Armanet, Bussière, Chevreau, Cardot and Tétard16 Syrigou et al.Reference Syrigou, Psarros, Makris, Grapsa and Syrigos22 found improved nasal flow and patency in individuals with active symptoms as documented by the statistically suggestive increase of peak nasal inspiratory flow in comparison with pre-interventional values. A significantly reduced visual analogue scale score was shown for all allergic rhinitis associated symptoms, which appeared more for nasal than ocular symptoms. It is worth mentioning that both peak nasal inspiratory flow and visual analogue scale score improvements had statistical significance within 15 to 30 minutes from quail egg administration, except for watery eyes, which took longer to respond. Quail egg led to better nasal patency and breathing than placebo.Reference Benichou, Armanet, Bussière, Chevreau, Cardot and Tétard16
Zinc, a pivotal mineral in quail egg, has a well-established role in several physiological processes because of its immunoregulatory, anti-inflammatory and anti-oxidant functions.Reference Zalewski, Truong-Tran, Grosser, Jayaram, Murgia and Ruffin29 A suggested anti-inflammatory mechanism of zinc includes suppression of the interaction between a cell surface antigen of neutrophils, leucocyte-related antigen I and intracellular adhesion molecule I, another cell surface antigen that is expressed not only by inflammatory cells but also by the nasal epithelium and which regulates the inflammatory response in seasonal allergic rhinitis.Reference Zalewski, Truong-Tran, Grosser, Jayaram, Murgia and Ruffin29–Reference Demoly, Sahla, Campbell, Bousquet and Crampette31 Oral zinc supplement was found to inhibit inflammation of the epithelial airway in animals,Reference Lu, Xin, Tang and Shao32 to enhance lung functions in individuals with cystic fibrosis,Reference Demoly, Sahla, Campbell, Bousquet and Crampette31 to benefit cases with atopic asthma and common cold, a lower respiratory tract infection, pneumonia, and tuberculosis, and to reduce respiratory tract infection rates in children.Reference Mohamed, Rushdy and Abdel-Rehim33
It was observed that quail egg albumen played the most effective role compared with quail egg yolk in modulating mast cell degranulation by suppressing the release of β-hexosaminidase, histamine, tryptase, and pro-inflammatory cytokines and upregulating the release of interleukin-10. It was shown that the lowest levels of quail egg albumen already had a significant inhibitory effect on modulating these mediators. Quail egg yolk also showed a significant therapeutic effect on modulating these mediators despite being not as strong as quail egg albumen. However, quail egg yolk showed a greater significant inhibition effect as compared with quail egg albumen on modulating the cytokines. The augmentation of T helper 2 cytokines in a higher concentration of quail egg was not surprising as it is largely known that quail egg itself contains many described egg allergens, which also may act on immune pathway regulation to provide benefit in the occurrence of allergy reactions. Besides, the effective effect of quail egg yolk on modulating T helper cytokines is likely because of its high nutrient contents, which may also play an important role as anti-allergic agents.Reference Tolik, Poławska, Charuta, Nowaczewski and Cooper34 The anti-allergic and anti-inflammatory activity and mast cell stabilising role of quail egg appears to be because of the inhibition of allergic mediator secretion, depletion of endoplasmic reticulum calcium ion store and intracellular calcium ion influx generation through the inhibition of the proteinase-activated receptor-2 downstream signalling transduction pathway.Reference Knoops, Louahed, Van Snick and Renauld14
Our study has several intrinsic design limitations. First and foremost, its pilot nature led to a brief effectiveness evaluation period. Although one month of therapy was enough to suggest a superiority of quail egg and zinc supplement versus mometasone alone, longer study periods are required to assess long-term positive and negative side effects. Secondly, the open-label design did not allow us to rule out the possible placebo effect related to the oral tablet supplementation. As the greater symptom improvement in the mometasone + quail egg and zinc tablets group is indeed marginal, gaining insight on the possible placebo effect induced by our dietary supplement is pivotal in order to gain definitive information on its potential. This potential bias needs to be addressed in future studies. Again, the small sample size, coupled with the short study period, did not allow us to draw solid conclusions on the safety profile of quail egg but only gain some insight on its immediate tolerance profile. This is especially true if we aim to put potential side effects in context with allergies to eggs of other species (such as hen), which have not been explored in the present study population. Furthermore, we were unable to define patient subgroups based on specific IgE levels as they were not evaluated in our patient pool.
• Most available treatments manage seasonal allergic rhinitis with some side effects and without reducing recurrence
• Natural anti-allergic products could represent an interesting addition for seasonal allergic rhinitis management
• Quail egg has a high protein content with anti-allergic and anti-inflammatory activities, which may weaken allergic asthma and rhinitis symptoms
• Quail egg benefits appear to be a result of the inhibitory effects on mast cell degranulation by suppressing proteinase-activated receptor-2 downstream in the signalling transduction pathway
• Quail egg, as an add-on therapy to intranasal steroids, has potential to be an effective and safe adjunct to steroids alone in seasonal allergic rhinitis management
It is interesting, and potentially a source of bias, that the median age in our study is rather high, probably as a consequence of the specific profile of patients usually accessing our out-patient department for second or third-level opinion in uncontrolled rhinitis. Undoubtedly, such peculiarity must be taken into account when weighting the results of our study.
Lastly, the compound nature of the oral supplement given to patients did not allow us to separate the effects of quail egg and zinc, which may have acted synergically. A further minor point is the lack of specific laboratory tests, which might have helped to shed more light on the pharmacological effects of the oral supplement, a limitation we plan to overcome in future larger studies. Nevertheless, given the lack of better-designed and more powerful studies comparing quail egg supplementation of topical steroids, the briefly described limitations do not hamper the overall validity of the study. Still, we are aware that in order to definitely prove the role of quail egg in allergic rhinitis, these shortcomings need to be addressed in larger and longer placebo-controlled trials.
Conclusion
Our clinical trial preliminarily shows that the use of quail eggs as an add-on therapy to intranasal steroids might have the potential to become an effective and safe adjunct to steroids in seasonal allergic rhinitis management. The use of such natural dietary supplements, with their encouraging risk profile, might represent a further advancement in seasonal allergic rhinitis management. Our preliminary results on quail egg are encouraging and call for further, larger and possibly placebo-controlled studies. Furthermore, future studies delving into the mechanisms of action of active ingredients of quail egg are required to identify its therapeutic potential and anti-allergic effects and might represent the first step in developing studies comparing quail egg, or other natural dietary supplements, against topical steroid therapy and not only as an adjunct.
Competing interests
None declared
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022215122001219.





