Introduction
Date palm (Phoenix dactylifera L.), belonging to the Arecaceae family, is the main crop of the arid region of western Asia and North Africa (Munier, Reference Munier1973; Barrow, Reference Barrow1998; Zohary et al., Reference Zohary, Hopf and Weiss2012). It is the key species in the palm family, which consist of about 200 genera and more than 2500 species (El Hadrami and El Hadrami, Reference El Hadrami, El Hadrami, Ja and Priyadarshan2009; Jain et al., Reference Jain, Al-Khayri and Johnson2011). Throughout the previous three centuries, dates were introduced to Mexico, India, Pakistan, southern Africa, Australia and United States (Zohary et al., Reference Zohary, Hopf and Weiss2012). It represents about 1,235,601 ha and a living revenue of 10 million oases worldwide. In the oasis, date palm, just like other plants, is routinely exposed to an unpredictable combination of diverse stresses (Slama et al., Reference Slama, Abdelly, Bouchereau, Flowers and Savouré2015), which is even worse in the circumstances of climate change, soil salinization and environmental pollution. Plants commonly encounter severe growing conditions like low or high temperature, deficient or excessive water, high salinity, heavy metals and ultraviolet radiation (He et al., Reference He, He and Ding2018). These abiotic stresses can affect plant growth, causes irreversible damage and drives to death. They represent the main causes of crop yield penalty worldwide (Bray et al., Reference Bray, Bailey-Serres, Weretilnyk, Gruissem, Buchannan and Jones2000; Agarwal and Jha, Reference Agarwal and Jha2010).
Plants have to get around the stresses and develop powerful adaptive strategies to tolerate their adverse effects allowing them to survive and thrive. Therefore, plants are able to adapt their metabolism to diverse fluctuations in the environment throughout evolution (Maksymiec, Reference Maksymiec2007). Plant stress responses are dynamic and implicate cooperation amongst diverse regulatory levels, comprising modification of metabolism and gene expression for physiological and morphological adaptations; for instance, stress protein production, antioxidant up-regulation and compatible solute accumulation (Xiong et al., Reference Xiong, Schumaker and Jian-Kang2002; Krasensky and Jonak, Reference Krasensky and Jonak2011). Plant species vary in their tolerance to abiotic stress. Salt stress causes more than 50% yield losses in major crops around the world depending on the crop (Al-taweel et al., Reference Al-taweel, Abdel-Aziz, Rabea and Khaled2019) and affects the many metabolic and physiological processes of the plant, which can damage the cells.
Molecular techniques have been proposed to be appropriate powerful tools for the identification of some clonal variation, stress tolerance and establish genetic stability (Bennici et al., Reference Bennici, Anzidei and Vendramin2004; Khaled et al., Reference Khaled, El-Arabi, Sabry and El-Sherbiny2018). In recent times, many new promising alternative marker techniques, refining developments in the field of molecular biology, have developed. In this context, numerous approaches have been developed for the analysis of differential gene expression at the mRNA level in various plants. Recently, a new marker has been developed for the assessment of genetic diversity, called start codon targeted (SCoT) marker (Collard and Mackill, Reference Collard and Mackill2009). The start codon (ATG) and flanking sequences are highly conserved in the plant genes (Sawant et al., Reference Sawant, Singh and Gupta1999). In SCoT marker profiling, a single primer is used as in ISSR and RAPD markers. Conversely, this marker is more reproducible than the RAPD as it has a longer primer sequence (Mulpuri et al., Reference Mulpuri, Muddanuru and Francis2013). The SCoT marker has been used for the assessment of genetic diversity in P. dactylifera cultivars and gave reproducible results among the cultivars (Al-Qurainy et al., Reference Al-Qurainy, Khan, Nadeem and Tarroum2015; Rhouma et al., Reference Rhouma-Chatti, Choulak, Moussa, Chatti and Chatti2020). SCoT markers can be developed from the transcribed regions and might be related to the gene function as proven in mango (Mangifera indica L.) (Luo et al., Reference Luo, He, Hu, Yu and Ou2014) and sugarcane (Saccharum officinarum) (Wu et al., Reference Wu, Li, Yang, Fang and Song2013).
The cDNA-SCoT method is a new gene expression analysis method developed by Wu et al. (Reference Wu, Li, Yang, Fang and Song2013), compared with all other differential gene analysis methods available in the current research fields. It is relatively more efficient, rapid, inexpensive and simple to operate. Its data results are also easily reproducible (Luo et al., Reference Luo, He, Hu, Yu and Ou2014). For instance, even though the cDNA-AFLP and cDNA-SRAP methods are also reliable methods for gene expression analysis, the cDNA-SCoT method is much simpler since it needs fewer primer combinations to operate. This technique will certainly play an essential role in studying differentially expressed genes, finding new genes and exploring the molecular mechanism of resistance (Wu et al., Reference Wu, Li, Yang, Fang and Song2013).
The optimal environment is extremely variable for plant species. Harsh environments may be damaging for one plant species, however may possibly not be for another (Munns and Tester, Reference Munns and Tester2008). These facts designate that plant response to the environment are complex and that specific sensitivities exist throughout the multitude of plant species. Knowledge of molecular mechanisms in P. dactylifera L. under abiotic stress conditions is limited. Yaish and Kumar (Reference Yaish and Kumar2015) suggest that P. dactylifera L. can acclimate to extreme drought, heat and moderately elevate the stages of soil salinity. Therefore, in the present study, the experiments were carried out using seedlings from the elite cultivar ‘Deglet Noor’ to determine the performance for growth and yield-related traits and gene expression profiling under different stress levels.
Material and methods
Plant material, growth and experimental design
Seeds of ‘Deglet Noor’ cultivar were collected from the oasis of Tozeur in the South of Tunisia. The healthy seeds of P. dactylifera were cleaned, scraped, soaked for 48 h in lukewarm water and brushed to remove residues. Surfaces were sterilized with 75% ethanol for 3 min and then with sodium hypochlorite solution (12°) for 10 min and washed thoroughly four times with distilled water. Subsequently, the seeds were removed into sterilized wet Petri dishes and incubated at 37°C until germination, which was then transplanted into 2 litre pots. The pots were filled with a mixture of sand and peat moss (1:1). The sand was sieved, washed successively with tap water and rinsed with distilled water. The seeds were sown in plastic pots and watered at regular intervals to maintain moisture for better germination. The pots were maintained in the growth chamber at 26–27 °C, daylight and relative humidity of 72%.
The stress conditions were salt stress using 240 mM NaCl (four samples), salt stress using 50 mM CaSO4 (four samples), drought using polyethylene glycol (PEG, MW 6000) 82.5 g/l (four samples) and drought due to no irrigation (four samples). The experiment was carried out for 3 weeks along with four control samples (irrigated with distilled water) (Fig. 1). Leaves and roots were collected after each treatment and stored at −80°C for RNA isolation

Fig. 1. Different treatments on date palm seedlings in advance of transcriptomic analysis.
Biomass and morphological traits
Fresh leaf and root weight and shoot and root length were measured after 3 weeks of treatment. Each treatment was compared to control plants for the evaluation of their stress responses. The statistical analysis of the morphometric parameters was carried out using the software program IBM SPSS Statistics 20. The difference in variance between the treated groups was compared by an ANOVA test. The difference is significant at a p-value ≤0.05.
RNA extraction and differential gene expression analysis
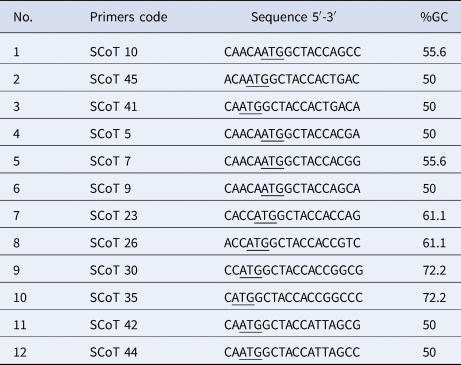
Total RNA was extracted from the control and stressed plants using the GF-1 Total RNA Extraction Kit (Vivantis, Malaysia) according to the directives specified in the manual. The quantity and quality were verified using the spectrophotometer (Gold S54T, Shanghai). High-quality cDNA was prepared using the PrimeScriptTM 1st strand cDNA Synthesis Kit (TaKaRa). The PCR reaction was performed in a total volume of 25 μl using the SCoT primers (Table 1) for the study of expression profiling. These primers were selected for their effectiveness in revealing polymorphism in date palms (Rhouma et al., Reference Rhouma-Chatti, Choulak, Moussa, Chatti and Chatti2020). The cDNA was diluted in RNase-free water to a working concentration of 50 ng for PCR amplification with SCoT primer (20 picomoles per reaction). PCR was performed in a 2720 thermal cycler (Applied Biosystems). The cycling profile was 94°C for 3 min, 45 cycles at 94°C for 1 min, 44.5°C for 30 s, 72°C for 1 min and a cycle of 72°C for 5 min. The amplified products were resolved on 2% TBE agarose gel. The oligo-dT anchored cDNA-SCoT patterns were compared for each stress treatment and control.
Table 1. SCoT primers used for PRC reactions
Isolation and sequencing of oligo-dT anchored cDNA-SCoT fragments
According to the results of the amplification, differential fragments were found based on their absence or differential intensity on an agarose gel, and then the polymorphic expressed sequence tags were cut from the agarose gel with a sharp scalpel. The target products were purified by DNA extraction using an agarose gel kit (EZ-10 Spin Column DNA Gel Extraction Kit, Bio Basic) and sequenced. The sequences were analysed for their homology with nucleotide sequences through BLASTX searches against the GenBank non-redundant public sequence database (http://www.ncbi.nlm.nih.gov/BLAST).
Results and discussion
Effect of stress on morphometric parameters:
In order to study the effect of salinity and drought on the morphometric parameters, of treated and control seedlings, we measured the length and weight of the root and aerial parts. The measures were carried out immediately after the date palm seedlings were removed from the culture bags and washed (Fig. 2). Statistical analysis showed no significant difference in morphological parameters (mass and length) (Fig. 3). Indeed, the absence of morphological differences between treated and untreated seedlings may be related to the short duration of stress and/or by the capacity of adaptation to drought and salinity of P. dactylifera which is superior to other plant species.

Fig. 2. Seedling after 20 days of stress. (A) Drought stress; (B) saline stress.

Fig. 3. Fresh leaves and root weight and length in Phoenix dactylifera L. grown in the green house at different saline (A) and drought (B) treatments. Data represent means of four replicates ± standard deviation.

Fig. 4. cDNA-SCoT marker profiling generated from individual plant leaves of Phoenix dactylifera L. at different stress treatments. Lane L: 100 bp ladder; Lane T-: negative control of PCR, lane C: control (seedling without treatment); lanes 1, 2, 3, and 4: seedling with drought or saline treatment. The arrows indicate some differentially expressed bands.
In fact, some cultivars of date palms have the capacity to grow close to the seashore where they are often exposed to seawater during tidal currents. Therefore, the date palm could be considered an exceptional halophyte plant and could possess a series of mechanisms of tolerance to salinity. Our results are in agreement with those described by Yiash et al. in 2017 where the cultivar Khalas subjected to severe salt stress for 10 days did not show morphological differences compared to the control seedlings. Moreover, Al Karusi et al. (Reference Al Kharusi, Sunkar, Al-Yahyai and Yaish2019) demonstrate that a saline treatment for a longer period (6 weeks) showed a 20 and 27% reduction in leaf area relative to the cultivars Umsila and Zabad and a decrease in root length and surface area. Other studies have shown that, under saline stress, other symptoms were visualized such as leaf tip burning (Al Kharusi et al., Reference Al Kharusi, Assaha, Al-Yahyai and Yaish2017).
On the other hand, it has been described that, under drought stress conditions, date palm develops several adaptation mechanisms including morphological changes in the aerial and/or root part. In 2016, Elshibli et al. (Reference Elshibli, Elshibli E and Korpelainen2016) showed that a date palm seedling displays multiple transformations in growth and morphology under drought-stressed conditions of 10 and 25% of plant capacity. In addition, a study of two cultivars developed in vitro under PEG-induced water stress allowed the selection of the most drought-tolerant cultivar (Djibril et al., Reference Djibril, Mohamed, Diaga, Diégane and Abaye B2005). The response to water stress is genotype-specific, as it is possible to identify and select the cultivars best adapted to improve yield in the most sensitive areas. Other studies carried out on date palm in open fields have shown the effect of water stress on different morphological parameters. Actually, Gribaa et al. (Reference Gribaa, Dardelle, Lehner, Rihouey and Burel2013) and Sabri et al. (Reference Sabri, Bouaziz, Hammani, Kuper and Douaik2017) reported a negative effect on date production in quantity and quality, in addition to the adverse effect on the aerial and root part.
Isolation and analyses of transcript-derived fragments (TDF) and function predictions
Analysis of gene expression is a central aim in most studies of molecular and cellular biology. It forms the basis for understanding plant growth, development and adaptation and permits the identification of precise regulator points of metabolism (Gupta and Chakrabarty, Reference Gupta and Chakrabarty2013). In this context, Oligo-dT cDNA-SCoT analysis was performed on RNA isolated from leaves and roots collected to identify genes response to stress treatments in P. dactylifera. A total of 12 SCoT primers were used for PCR amplification. SCoT polymorphism is a gene-targeted marker that can generate information correlated with biological traits compared with random DNA markers (Collard and Mackill, Reference Collard and Mackill2009; Xiong et al., Reference Xiong, Tang, Chen, Pan and Zhuang2009). Only those with stable amplification results and clear bands were selected. By using these 12 single primers, 108 cDNA fragments with the length of 100–3000 bp were amplified. Results show qualitative (presence or absence of bands) and quantitative differences (variability in band intensity). These results prove the effectiveness of the cDNA-SCoT technique. In fact, Al-Qurainy et al. (Reference Al-Qurainy, Khan, Nadeem, Tarroum and Gaafar2017) have shown its efficacy in the study of differential expression between date palm plants under salt stress induced by various concentrations of NaCl. In addition, this technique has been used to study genes expressed at different stages of development of sugar cane seedlings treated or not with gibberellic acid (Wu et al., Reference Wu, Li, Yang, Fang and Song2013) and results showed that a SCoT primer could produce three to 15 differentially expressed bands. Moreover, cDNA SCoT was equally used to study gene change and to understand the allopolyploidy evolutionism and the genetic mechanism of Arachis interspecific hybridization (He et al., Reference He, Tang, Jiang, Xiong and Huang2017). Due to high success rate, low false-positive rate, simple operation and low cost, cDNA-SCoT techniques were appropriate for analysing the variations of gene expression in those different plant species.
Thirty-three clear, bright and differentially expressed bands were selected and sequenced. After verification of the sequences obtained, it results that some of them do not present any significant homology. On the other hand, others present homologies with certain species and whose presence may be strongly associated with the stress applied. All of these sequences, their homologies and their possible functions are summarized in Table 2. TDFs sharing high homology with genes in the NCBI database could be divided into four types according to their functions, including resistance-related genes, signal transduction factor, transcription and translation factor-related genes and unknown functional protein-related genes. TDFs, expressed under drought or saline stress, are considered as not significant since they have unknown functional protein-related or are not associated to either biotic or abiotic stress tolerance.
Table 2. Function prediction of transcript-derived fragments (TDFs) by the cDNA start codon targeted polymorphism (cDNA-SCoT) technique

Features of genes differentially expressed during drought stress
Analysis of differentially expressed sequences, due to drought stress, showed homology with genes whose expression could be strongly associated with the applied treatment (Table 2). In fact, TDF1, expressed within the PEG-stressed stem, has homology with the sequence of P. dactylifera L. which codes for the protein DNA ligase 1-like controlling the repair of broken DNA following high levels of environmental stress (Waterworth et al., Reference Waterworth, Kozak, Provost, Bray and Angelis K2009). The expression of this gene indicates its role in stress response, as effective cellular response mechanisms have evolved to cope with DNA damage caused by water stress, including delay or arrest of the cell cycle and activation of repair pathways and DNA ligase ligation. TDF2, a fragment expressed at the PEG-stressed stem, has homology with the sequence of P. dactylifera. L, which codes for the eukaryotic translation initiation factor 3. This factor, described in Arabidopsis thaliana, is responsible for initiating the translation of a subset of mRNAs involved in cell proliferation. Stress has adverse effects on the growth of the seedling, consequently on cell proliferation. The expression of this factor aims to enhance cell proliferation in order to overcome water stress. Moreover, TDF3 which is expressed at the level of the stressed stem, due to the absence of irrigation, has a homology with the sequence of P. dactylifera L. which codes for a repetitive serine/arginine matrix protein 2. The latter interacts with other factors involved in messenger RNA maturation to activate the alternative splicing of numerous genes encoding factors involved in signal transduction, cell cycle and transcription (Yoshimura et al., Reference Yoshimura, Mori, Yokoyama, Koike and Tanabe2011). These results suggest the existence of an important transcriptome diversification under the effect of water stress. Finally, Chen et al. (Reference Chen, Li, Zhang, Yi and Yang2019) showed that late embryogenesis abundant protein (LEA) is a large and highly diverse family, and it plays a role in normal plant growth and development and protects cells from abiotic stress (drought, salt, heavy metals, heat and cold). The genes that code for LEA proteins are located in clusters on chromosomes 1, 2 and 10 of the Solanum tuberosum plant. In our study, TDF4 shows a similarity with chromosome 10 of Solanum tuberosum; we can deduce that our sequence corresponds to a gene coding for one of the LEA proteins involved in the response to water stress applied to the date palm seedling.
Features of genes differently expressed during saline stress
Plant salt tolerance depends on internal osmotic adjustment phenomena to maintain water with tissue turgidity. Depending on their response to saline conditions, plants can be divided into two types: (1) halophytic plants that tolerate high concentrations of salt (this tolerance is due to biochemical and molecular adaptation mechanisms mainly the compartmentalization of salt in the vacuole (Parida and Das, Reference Parida and Das2005)); and (2) glycophytic plants that are sensitive to high salt concentrations. These concentrations cause ionic toxicity and osmotic stress until the death of these plants.
Numerous regulatory genes are involved in different metabolic pathways of date palm exposed to saline stress (Table 2). In fact, analysis of differentially expressed genes under saline stress showed variability in the transcript-derived fragments. Indeed, TDF5 amplified by the SCoT 41 primer differentially expressed at the stem treated with NaCl shows homology with the gene encoding the Nucléolin 1-like protein (79.58%). This protein is multifunctional and is located mainly in the nucleoli, nucleoplasm, cytoplasm and cell membrane. It is involved in cell proliferation, ribosome biogenesis (rRNA synthesis), chromatin stability, DNA and RNA metabolism, stress response and signal transduction (Jia et al., Reference Jia, Yao, Zhao, Guan and Gao2017). Nucleolin overexpression has enabled the adaptation of transgenic rice to saline stress (Boonchai et al., Reference Boonchai, Udomchalothorn, Sripinyowanich, Comai, Buaboocha and Chadchawan2018). Differential expression of this structural gene, coding for a regulatory protein, in the stressed date palm may confer tolerance to salinity. TDF6, amplified by the SCoT 41 primer on the NaCl-treated stem, has homology to a predicted sequence of serine/arginine repetitive matrix protein expressed in P. dactylifera L. This protein is involved in the regulation of alternative splicing and transcriptome diversity in Arabidopsis in response to high light stress (Yoshimura et al., Reference Yoshimura, Mori, Yokoyama, Koike and Tanabe2011) which could be a key gene involved in the tolerance mechanism of date palm to saline stress. Moreover, TDF7 is homologous to the S-related kinase gene, actually observed in Arabidopsis lyrata. It is well known that kinase proteins are rapidly activated by osmotic stress and are involved in ABA signalling pathways (Lin et al., Reference Lin, Li, Zhang, Liu and Hsu C2020). The expressed sequence confirms the role of these proteins in the osmoregulation of the date palm to maintain its growth in the presence of environmental constraints. TDF8, expressed differentially in roots stressed by NaCl, corresponds to an element-binding protein 2-like sequence observed in P. dactylifera L. These elements are transcription factors allowing the activation of genes sensitive to heat stress and drought, which induces a high tolerance to these constraints in A. thaliana (Mizoi et al., Reference Mizoi, Kanazawa, Kidokoro, Takahashi and Qin2019). The expression of these elements in date palm increases the tolerance of the latter to saline stress. Finally, TDF9 gene is expressed in stems stressed by CaSO4. This gene is identical to the predicted Capsicum annum sequence of homeobox-leucine zipper protein HAT5 (transcription factors unique to the plant species) involved in plant growth, regulation of responses to biotic and abiotic stresses, and modulation of hormone action (Ribone et al., Reference Ribone, Capella, Arce and Chan2016). The expression of this gene in date palm allows its adaptation to CaSO4-induced stress.
Conclusion
Date palm is one of the most popular fruit crops in arid, semiarid, tropical and subtropical regions. It generally grows under hostile climatic circumstances and has therefore developed stress tolerance throughout its evolution. It is known to subsist under drastic drought, heat and high soil salinity rate (Yaish and Kumar, Reference Yaish and Kumar2015). Adaptation to these conditions includes evolutionary consequences detectable in a wide range of morphological and molecular multifunctional responses (He et al., Reference He, He and Ding2018). Genomic studies of the date palm over the last decade have led to the identification of several key genes associated with fruit colour and sugar accumulation (Hazzouri et al., Reference Hazzouri, Gros-Balthazard and Flowers2019). However, there is little understanding of the genetic aspects underlying different biotic and abiotic stresses. Analysis of stress-induced differential gene expression can provide valuable information on the adaptive mechanisms of date palms.
Our results showed that rapid and considerable gene expression variations began as early as abiotic stress threatened the date palm crop. Several types of gene expression changes were observed, including quantitative and qualitative (novel gene expression) variations. The cDNA-SCoT technique permitted us to successfully investigate the variations of gene expression in date palms. This study provides robust candidate genes for future functional research and a basis for improving abiotic stress tolerance in P. dactylifera crop. Indeed, genome-wide investigation of the structural diversity, phylogenetic relationships and functional attributes of those gene families is required. Furthermore, the expression of selected genes through quantitative real-time PCR will provide new insights into understanding the mechanism of salt and drought tolerance in date palm species.
Acknowledgements
This work was supported by grants from the Tunisian Ministry of Higher Education and Scientific Research.
Conflict of interest
None.








