Introduction
Respiratory hazards, including different types of aerosols, gases, and vapors are an essential category of workplace hazards. These hazards could threaten employees’ health in various work environments, including medical centers, industrial centers, sewage treatment plants, service environments, etc. Reference Jahangiri, Neghab and Nasiri1,Reference Racz, Yamamoto and Eninger2 The last way to protect against respiratory hazards is to use respiratory protective equipment (RPE). One of the main characteristics of respirators to be efficient in respiratory protection is their fitting capability on the users’ faces. Reference Racz, Yamamoto and Eninger2
The fit testing procedure is mandated for tight-fitting respirators based on respiratory protection standards. This procedure aims to ensure the proper fit of a particular model and size of a respirator into the facial dimensions in the step of respirator selection. 3–6 Respirator fit is the ability of a device to interface with the user to protect the respiratory system against a hazardous atmosphere. Reference Rajhans and Pathak7 As the respirator demand increases during the pandemics of respiratory infectious diseases, paying attention to the importance of fit testing procedures considerably increases. Specifically, when huge numbers of health-care workers (HCWs) are intended to conduct fit testing procedures, the concern about the fit testing facilities would be increased considerably. 8
There are 2 types of respirator fit testing, including the Qualitative Fit Test (QLFT) and Quantitative Fit Test (QNFT), both used as recognition methods of fitting characteristics of respirators Reference Lam, Lee and Yau9 and provision of higher-level protection for the users. Reference Coffey, Lawrence and Campbell10 The QNFT decreases the test subjectivity and provides a numerical indicator of fit called the “fit factor,” using an instrument to measure the leakage into the respirator. 5 The advantages of the QNFT are as follows: no fit factor limit, documentation of numerical results, no chance of user deception or inability to taste/smell the solutions, and applicability to various classes of RPE. Reference Mullins, Danisch and Johnston11 Nevertheless, this method might be expensive and unavailable 12 and required some supplies, e.g., trained conductors, probed respirators or sampling adaptors, and annual factory recalibration. 3
The QLFT relies on a test subject’s olfactory or taste/smell response to a fit test challenge agent during a series of simulated work exercises while wearing a respirator. It uses 3 common challenge agents with a distinctive taste such as BitrexTM (bitter taste) and Saccharin (sweet taste), which are challenge agents for fit testing of particulate respirators (FFRs) or a specific smell such as Isoamyl acetate, a sweet-smelling vapor that is widely used as a fit test agent for elastomeric half facepiece respirators (EHRs) equipped with organic vapor (OV) cartridges. 5
The most important limitation of the QLFT is that it depends on the user’s reaction or subjective assessment. Noticeably, BitrexTM or Saccharin qualitative fit test kits consist of the sensitivity test solution, fit test solution, test hood, and nebulizers. 5 However, QLFT is more widely used because it is simpler to use, Reference Mullins, Danisch and Johnston11,Reference Mitchell, Wells and McGregor13 more accessible to transport Reference Mullins, Danisch and Johnston11 , faster to perform, Reference Mullins, Danisch and Johnston11,Reference Mitchell, Wells and McGregor13 and cheaper to set up and maintain Reference Mullins, Danisch and Johnston11 than the QNFT procedures; in particular, when epidemics or pandemics happen. Overall, commercial qualitative fit test kits containing fit test solutions might not be readily available during the pandemics, Reference Mitchell, Wells and McGregor13 when many subjects should be fit tested. Therefore, it is worthwhile to predict, feasible, and evaluate the possible alternatives for the components of fit test kits to be ready before the pandemics.
Numerous nebulizers, including medical and non-medical types, are produced and distributed in the marketplaces. All the nebulizers have a specific particle size or the Mean Mass Aerodynamic Diameter (MMAD) depending on their applications and purposes. For example, fit test nebulizers are a critical component of qualitative fit test kits. Based on the Occupational Safety and Health Administration (OSHA) standard 29 CFR 1910.134, DeVilbiss model 40 or equivalents could be used while undertaking the QLFT protocol. 14 However, the medical nebulizer DeVilbiss Model 40 has not yet been available in the marketplace. Currently, the DeVilbiss model 45 medical nebulizer with the MMAD of 5 μm is sold by the manufacturers. Reference Healthcare15 Moreover, according to the International Organization for Standardization (ISO) 16975-3: 2017, a handheld (manual) inhalation nebulizer with a MMAD of 2.50 μm with a 5 mL capacity shall be used for fit testing procedure. 6 The particle size of commercially manual fit test nebulizers (e.g., Allegro nebulizer) ranged from 0.30-5 μm (average MMAD: 2.65 μm). A variety of commercial nebulizers including the 3M FT-10 and TSI QfitTM are sold in the marketplaces. Although, these products are cheaper than the equipment used in the QNFT procedures; however, they are still high-cost and might not be easily available. Besides, there are vital problems to perform qualitative fit testing using the current manual nebulizers such as the investigator’s hand contact stress, leakage from the components (nebulizer bulb and reservoir), disconnection of nebulizer bulb from the remaining components (e.g., reservoir) by repetitively squeezing the bulb, high cost for conducting fit tests for huge numbers of subjects, and also the impossible provision of the imported manual nebulizers in Iran.
In this context, some previous studies were assessed regarding the commercial fit test solutions, Reference Honarbakhsh, Jahangiri and Ghaem16–Reference Hannum, Cycan and Jones21 synthetic fit test solutions, Reference Mitchell, Wells and McGregor13,Reference Fakherpour, Jahangiri and Yousefinejad22–Reference Fakherpour, Jahangiri and Yousefinejad24 and alternatives for fit test hoods. Reference Alison Bowry25,Reference O’Kelly, Arora and Pearson26 Generally, several studies regarding the QLFT procedure were performed using commercial nebulizers. Reference Coffey, Lawrence and Campbell10,Reference Honarbakhsh, Jahangiri and Ghaem16–Reference Coffey, Zhuang and Campbell18,Reference Hardis, Cadena and Carlson20,Reference Janssen, Luinenburg and Mullins27–Reference Hon, Danyluk and Bryce29 Furthermore, Huang et al. explored some medical nebulizers and found those nebulizers could be substituted for the OSHA-approved ones. Reference Huang, Hsu, Kuo, Chen and Chen30 Notably, some studies were carried out concerning the QLFT protocol using commercial nebulizers in Iran by Jahangiri et al. Reference Honarbakhsh, Jahangiri and Ghaem16,Reference Myong, Byun and Cho31,Reference Jahangiri and Oriad32 It seems that all of those studies were aimed to assess the feasibility of some substitutions for the QLFT protocols and introduce the cost-benefit and reliable solutions (options) to all respirator users in order to focus on the importance of conducting fit testing protocols and improving culture and attitude towards the fit testing procedures as one of the most important components of the respiratory protection program (RPP). Therefore, the feasibility assessment of alternatives for the components of commercial fit test kits could be benefited for the users all over the world when they undergo fit testing protocols; specifically, when the organizations (hospitals or industries, etc.) have no access to the quantitative fit testing equipment due to financial limitations, or when it is not feasible to conduct fit testing using the quantitative fit tester due to time or source limitations (e.g., during the respiratory infection outbreaks), or high challenge to perform QLFT procedure because of the possible problems arising from long time usage of the OSHA-approved manual nebulizers (e.g., hand contact stress, solution leakage or splashing, and disconnection of the nebulizer’s components). Considerably, the feasibility of substitution of available and cost-benefit nebulizers was not assessed until now in Iran. To do so, the objective of this study is to assess the equivalent and proportionate feasibility of one of the most common medical nebulizers in Iranian marketplaces compared to the OSHA-approved manual nebulizer used for respirator fit testing procedure.
Methods and Materials
Study Design
A randomized experimental study was conducted at Shiraz University of Medical Sciences, Iran. Ethical approval for the study was obtained by the Research Ethics Committee of Shiraz University of Medical Sciences (approval code IR.SUMS.REC.1399.417). The study participants were initially trained about the study procedures. After that, they signed a written informed consent form.
Exclusion Criteria
The participants with facial hair, including beard, stubble, facial deformity, surgery, getting a cold, cardiovascular or respiratory diseases, and those who were insensitive (had no allergy) to solutions used for fit testing were excluded from the study.
Participants
Due to the limited resources, a conceivable sample of 32 volunteers consisting of 15 males and 17 females with a mean age of 23.31±3.86 y were tested in the Industrial Safety laboratory of the School of Health, Shiraz University of Medical Sciences, Iran.
Respirators
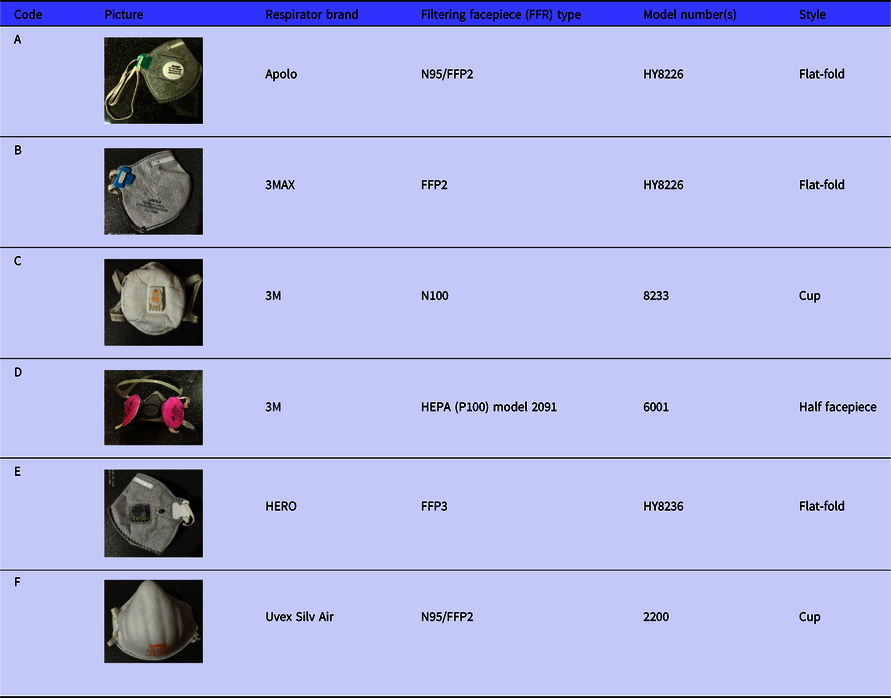
Due to the study’s resource and time constraints, a total of 6 brands of filtering facepiece respirators (FFRs) that were commonly used by Iranian users were tested in this study. All of the respirators were donned by the studied participants. They were randomly labeled from A to F (Table 1). The aim of the 6 FFRs was to accurately and conclusively assess and compare the Allegro and Accumed NF60 nebulizers during the QLFT procedure. All the studied respirators were one size fits all (OSFA), and the 3M half facepiece respirator model 6001 was only in medium size, randomly allocated to each participant.
Table 1. Features of the Studied FFRs
Instruments
The investigator searched for the varieties of nebulizers, including cosmetic and personal care products, medical nebulizers (drug delivery), sprays in the forms of pharmaceutical products, or some kinds of sprays used in the forms of food products. Finally, the Accumed NF60 nebulizer was selected due to its almost similar aerosol diameter to the Allegro one. The Accumed NF60 nebulizer is used widely to deliver medications to diseased respiratory individuals by health-care providers. 33
Two nebulizers were provided, and their features were described as follows: the OSHA-approved manual Allegro fit test nebulizer (Allegro Industries, Paramount, Calif.) with Buna-N (nitrile rubber) air bulb, which converts 5 mL of liquid into aerosol 0.30-5 μm (average MMAD: 2.65 μm) particle size and dimensions of 50 mm x 50 mm x 177 mm. Reference Bitter34 The Allegro nebulizer is made of a 50 mL-bulb which pumps the air into the 2 narrow passageways. The question-mark shaped insert is placed on the top of the 2 narrow passageways. The reservoir is the storage place of the test solution (sensitivity test or fit test solution). The black O-ring is used to prevent the solution leakage. When the bulb is squeezed manually by the investigator, then the solution placed in the 2 narrow passageways impacts into the question-mark shaped insert, and the aerosol-based solution is produced and passed through the nozzles. At last, the aerosol injects into the hole of the fit test hood while the subjects are being fit tested (Figure 1, left 2 panels).
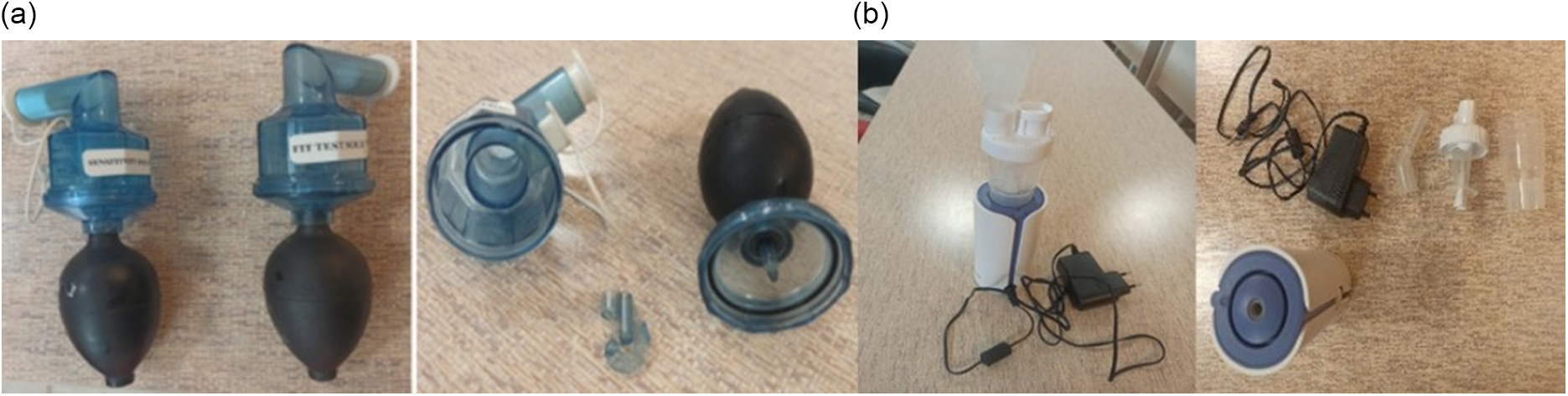
Figure 1. Allegro nebulizer (left 2 panels) and Accumed NF60 nebulizer (right 2 panels) components.
The second one is the handheld and automated medical nebulizer model Accumed NF60 with handheld piston and tubeless design and MMAD ≤ 2.60 μm; Fine Particle Dose (FPD), 75-80%; dimensions of 63.10 mm x 63.90 mm x 153 mm; and average nebulization rate, fully open valve ≥ 0.20 mL/min (0.90% saline solution) and closed valve ≥ 0.08 mL/min. 33 The handheld piston Accumed NF60 nebulizer is made of a nozzle, angled mouthpiece, AC adaptor, On/Off Button, and AC adaptor jack. The aerosol production process of the portable and compressor Accumed NF60 nebulizer is similar to that of the manual Allegro one. The difference is that the Accumed NF60 nebulizer is equipped with a mini pump. When the investigator plugs the nebulizer into the AC adaptor and presses the ON/Off button, the aerosol nebulization process starts and injects into the hole of the fit test hood while the subjects are being fit tested (Figure 1, right 2 panels).
Sensitivity Test Procedure
Two studied nebulizers were randomly used to perform the QLFT procedure according to the OSHA 29 CFR 1910.134 5. The Allegro® Bitrex® solution Part number 2041 (Allegro Industries, Paramount, Calif.) contained 0.0135% denatonium benzoate, 94.9865% water, and 5% sodium chloride. If the participants could not detect the Bitrex®, instead, we used the Allegro® Saccharin fit test solution Part Number 2040 (Allegro Industries, Paramount, Calif.) contained < 1% sodium saccharin and > 99% water. If the participants could not detect the sweet taste of the Saccharin sensitivity solution, they were excluded from the study. Reference Pompeii, Kraft and Brownsword35
To begin the experiment, one of the experienced investigators conducted all the study fit tests on the participants. In the first stage, she added a 1-mL sensitivity test solution into the Allegro nebulizer and squeezed the bulb 3 times to ensure the proper operation. Then, the participants were asked to wear the plastic test hood (12 inches (30.50 cm) in diameter and 14 inches (35.60 cm) tall) without any respirator, position the hood forward about 6 inches (15.25 cm) between their faces and the hood window, then breath through the mouth with an open mouth. Next, the investigator accommodated the nozzle into the hole of the test hood (a-0.75 inches (1.90 cm)) to ensure the test aerosol’s dispersion. In the next step, the investigator requested the participants to report as they characterized the taste of the sensitivity test solution, then the nebulizer bulb was squeezed about 10 times. If they were unable to taste the solution for up to 30 squeezes, it was considered that the participant was not sensitive to the bitter taste of the solution; then, not only did the participants remove the hood, but also they drank plain water and took a 15-min break in order to prevent them from olfactory fatigue.
Overall, if they could correctly detect the taste of the sensitivity test solution, they proceed to the next steps. Regardless of the actual number of squeezes detected by the participants, the taste threshold was classified into high (1-10), medium (11-20), and low (21-30). At last, the study result was recorded in the data collection form. To do the sensitivity test, first, the Accumed NF60 nebulizer was applied for 2 s. If the participants could characterize the taste solution properly using the Accumed NF60 nebulizer, they underwent the QLFT procedure.
User Seal Checks
To do so, the investigator instructed the participants concerning the proper donning and doffing of the respirator. First, they donned the randomly allocated respirators. Second, the participants carried out the user seal checks (USCs) consisting of negative pressure and positive pressure seal checks; for the positive pressure check, subjects were asked to close off the exhalation valve and exhale gently into the facepiece. There was a satisfactory check when the facepiece was slightly pressurized without any evidence of outward air leakage at the sealing surface area. Also, during a negative pressure check, the participants inhaled sharply while closing off the opening inlet of the air by covering it with the palm(s). A successful seal was when the facepiece collapsed slightly. 5
Fit Testing Procedure
First, the investigator asked the participants to hold the same test hood used in the sensitivity test over their heads while donning the respirator. Second, they were instructed to breathe through the mouth with an open mouth. Third, the investigator added the fit test solution into the labeled nebulizer and squeezed it to ensure the capability of generating the aerosols. Fourth, the investigator inserted the Allegro fit test nebulizer nozzle into the hood’s hole and requested the participants to perform 7 fit test exercises, including normal breathing, deep breathing, turning the head side to side, moving the head up and down, talking (reading per the “Rainbow Passage” loud), bending over, and normal breathing, each exercise for 1 min per OSHA 29 CFR 1910. 134. 5 Simultaneously, while carrying out the fit test exercise by the participants, the investigator squeezed the Allegro nebulizer bulb based on the initial number of squeezes detected by the participants in the sensitivity test. Then, the investigator replenished the concentration of the fit test solution based on half of the number of squeezes during the first stage (Figure 2, left).

Figure 2. Performing QLFT procedure using the Allegro nebulizer (left) and the Accumed NF60 nebulizer (right).
Additionally, the qualitative fitting of the randomly allocated respirators was assessed on the participants using the Accumed NF60 nebulizer, too. The same procedures were used while conducting the QLFT procedure using the Accumed NF60 nebulizer (Figure 2, right). During the fit test exercises, the participants were reminded to immediately report if they tasted the fit test agent during the QLFT procedure. If they did not characterize the fit test solution, it was assumed to pass the fit test and vice versa. The results were reported in the data collection form at the end of the test. The facial dimensions of the participants were measured using the digital calipers (0-150-mm, accuracy: 0.01 mm, Model Number HB-101-111, Guanglu® Digital Caliper Manufacturer Co., Ltd, China) according to the ISO 16976-2:2010. 36 37.5%, 46.87%, and 15.63% of the participants had small, medium, and large face sizes based on the NIOSH bivariate fit test panel, respectively.
Statistical Analysis
The descriptive statistics were used to calculate the proportions of passing fit tests of the Accumed NF60 nebulizer compared to the Allegro one. The T-Test was used to check the statistically significant differences between the Allegro and Accumed NF60 nebulizers by the detection time of the test agent during the fit test. The Chi-squared test (χ2) was applied to assess the significant differences between the results of fit tests obtained from both Allegro and Accumed NF60 nebulizers by the studied FFRs. The repeated measurements were taken on the participants, and independent observations were supposed; also, the Fixed Effect Logistic Regression (FELR) model was used to determine the effects of the study variables, including age, sex, respirator brand, nebulizer type on the respirator fitting. A P-value of 0.05 was considered significant. The statistical analysis was conducted using SPSS version 22.
Results
Similar proportions of participants comprised 46.90% males, and 53.10% females participated in this study. The participants ranged from 19 to 39 y, with a mean age of 23.31±3.86 y. The mean of the participants’ face length and face width were 130.34±10.15 mm and 120.05±8.81 mm, respectively.
All the participants could taste the sensitivity test solutions using Allegro and Accumed NF60 nebulizers. There were no significant differences between Allegro and Accumed NF60 nebulizers in terms of the mean number of sprays and taste detection time of sensitivity test solution (6.50±4.82 vs. 4.62±2.87 (P=0.06) and 9.47±5.85 s vs 9.25±5.74 s (P=0.88), respectively) by the T-Test.
Based on Table 2, the D, C, and F respirators had the highest passing rates of all studied FFRs using both Allegro and Accumed NF60 nebulizers (D, 62.50%; C, 59.38%; and F, 56.30%, for the Allegro nebulizer and D, 59.38%; C, 59.38%; and F, 56.30%, for the Accumed NF60 nebulizer, respectively). The chi-squared test determined no statistically significant difference between the fit test results of the Allegro and Accumed NF60 nebulizers during the fit testing procedure (P>0.05).
Table 2. Proportions of passing fit test using the Allegro and Accumed NF60 nebulizers by respirator brands
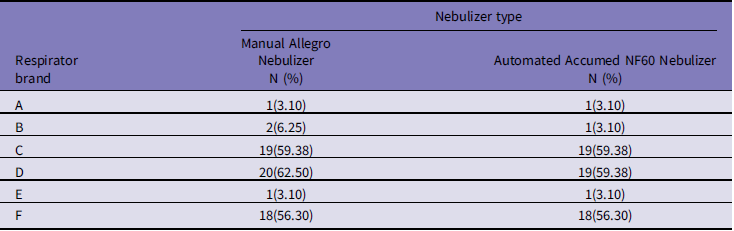
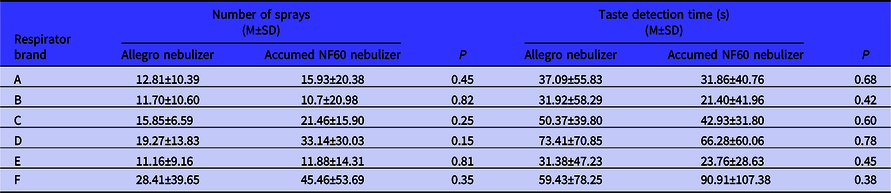
Table 3 compares the results of fit testing by the Accumed NF60 and Allegro nebulizer. As observed, the T-Test noted no statistically significant difference in the number of sprays elicited to detect fit test agent using the Allegro and Accumed NF60 nebulizers by the studied respirator brands. Also, the T-Test determined no statistically significant difference between the Allegro and Accumed NF60 nebulizers by the time of detection during the fit test procedure.
Table 3. Comparison of the Allegro nebulizer to the Accumed NF60 nebulizer by respirator brands
Abbreviation: M±SD, mean ± standard deviation.
Table 4 summarizes the proportions of passing fit tests of the studied respirator brands compared to the D brand (best-fitting respirator) by the FELR model. The model showed no significant difference between the Allegro and Accumed NF60 nebulizers by the fit test passing rates. To do so, the odds ratio (OR) for passing the fit test of the Allegro nebulizer was equal to Accumed NF60 one [OR=1.0, 95% CI (0.58-1.74)]. The OR for fit test passing rates of the A, B, and E brands compared to the D brand were the lowest, whether manual Allegro nebulizer or automated Accumed NF60 one. The odds for passing fit testing of A, B, and E brand respirators were 50 times lower than the odds for that of the D brand respirator.
Table 4. Comparison of the passing fit tests of respirator brands against D brand by nebulizer type
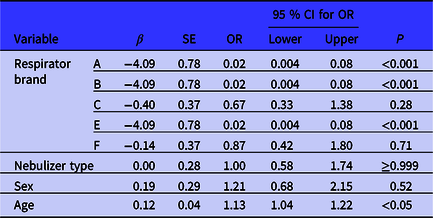
Abbreviation: β: Coefficient; SE: Standard Error; OR: Odds Ratio; CI: Confidence Interval; P: Significant level.
Discussion
This study aimed to evaluate the feasibility of substituting the automated Accumed NF60 nebulizer for the manual Allegro nebulizer in the QLFT procedure. Some valuable findings obtained from this research will be discussed in detail as follows.
In the current study, there were no statistically significant differences between the Accumed NF60 and Allegro nebulizers concerning the mean of sprays and time for detecting the solution’s taste during the sensitivity test procedures. It confirms the similar performance of both nebulizers in the sensitivity tests. The TSI 6-s cycles automated fit tester (Q-fit) were considered equivalent to 5 squeezing of the nebulizer during the QLFT procedure. 37 Lin et al. investigated the fit testing procedure using the automated TSI Q-fit respirator fit tester by applying a 6-s cycle and reported that all the participants tasted the BitrexTM agent during the initial dispersion Reference Lin and Chen17 Moreover, the invention by the SKC Co., a 6-s activations of the automated fit tester were considered equivalent to 5 numbers of squeezing the manual fit test nebulizer. 38
Mullins et al. underlined a test sensitivity of 0.96 between the QLFT and QNFT procedures; then, both methods met the ANSI Z88.10 requirements. Reference Mullins, Danisch and Johnston11 Zhang et al. developed the automated qualitative fit testing system. This invention realized that the automatic qualitative fit tester’s design led to a reduction in hands’ contact stress while repeatedly applying the nebulizer bulbs, cost, and required time for performing the fit tests on more numbers of respirator users compared to the manual one. Reference Zhang, Zanto and Shi39 Mullins et al. invented the automated qualitative fit testing system to simultaneously do fit tests on 2 or more individuals. This invention would result in more easily conducting the QLFT procedure on more subjects during a shorter time, potentially decreasing the cost of administration of fit testing and reducing the fluctuation while manually squeezing the bulbs, Reference Mullins, Martinson and Johnston40 Li et al. assessed the feasibility of substitutions of new fit test kits consisting of new nebulizers using the cost-benefit and available materials for the commercial fit test kits, including the 3M FT-10 and TSI QfitTM. They concluded that users reported no leakage during the QLFT procedures. Reference Li41 Nelson et al. examined the efficiency of the BitrexTM fit testing procedure with 15-s exercises compared to the QNFT procedure. This finding indicated the shorted BitrexTM fit tests were favorably screened for a good respirator fit. Reference Nelson, Janssen and Luinenburg42
In this study, the respirator brands, including the D, C, and F had the highest proportions of passing fit tests of all studied FFRs using both manual Allegro and automated Accumed NF60 nebulizers (D, 62.50%; C, 59.38%; and F, 56.30%, for the Allegro nebulizer and D, 59.38%; C, 59.38; and F, 56.30%, for the Accumed NF60 nebulizer, respectively). The third major finding from this study was that no statistically significant difference was found between the manual Allegro and automated Accumed NF60 nebulizers by the fit test passing rates. On the other hand, the manual Allegro nebulizer could be replaced by the automated Accumed NF60, particularly during severe pandemics like outbreaks of coronavirus disease 2019 (COVID-19). The study by Huang et al. noted that inexpensive medical nebulizers could be considered alternatives for the 3M FT-10 and TSI Q-fit nebulizers during the QLFT procedure. Reference Huang, Hsu, Kuo, Chen and Chen30 Another study by O’Kelly et al. assessed the other options for fit test hoods and nebulizers; therefore, it concluded that aroma diffusers and smaller hoods could be used as alternatives while performing the QLFT procedure. Reference O’Kelly, Arora and Pearson26 It seems necessary to further clarify other alternatives for fit test nebulizers.
Another striking finding from this study was the similar odds ratio for passing the fit test of Allegro and Accumed NF60 nebulizers (OR=1.0). On the other aspect, it seems the Accumed NF60 nebulizer had a similar performance to the Allegro one; thus, it could be an appropriate and pleasant alternative during the QLFT procedures.
In this study, the D brand had the highest odds of passing the fit test than all studied FFRs. The odds for passing the fit test of the C and F brands were 33% and 13% times lower than the odds for the D brand (OR= 0.67 and 0.87, respectively). It means that the D band had a higher chance of passing the fit testthan the C brand. The most significant reason for this finding was that the D, C, and F respirator brands had higher proportions of passing fit tests using manual Allegro and automated Accumed NF60 nebulizers than all studied FFRs; therefore, they obtained higher odds for passing fit test during the QLFT procedures. Consequently, this finding confirmed the similar performance of manual Allegro and automated Accumed NF60 nebulizers.
According to the above mentioned, the Accumed NF60 nebulizer would be a pleasant and appropriate substitution for the manual nebulizer. This is because the Accumed NF60 nebulizer is more cost-effective and available in the Iranian market than manual nebulizers. In addition, it is more convenient and reduces the contact stress in the operators’ hands as it does not require squeezing the nebulizer bulb frequently. This helps to perform fit test procedures more quickly, which is more critical when fit tests are to be performed for many people. Moreover, the Accumed NF60 nebulizer would sufficiently decrease the time investment for performing the QLFT procedures. For example, the required time for connecting the components, cleaning, unblocking the sediments of fit test solutions before fit testing, or reconnecting the manual nebulizer’s compartments due to performing many fit tests or repeatedly manual squeezing the bulbs is about 20 min, while a 5-min time is required for the Accumed NF60 nebulizer. In another aspect, the concentration and size distribution produced by squeezing the bulbs of the manual fit test nebulizer very crucially, while the Accumed NF60 nebulizer could reduce this kind of variation with a handheld piston and tubeless design. Reference Zhang, Zanto and Shi39 As a result, it could be enhanced the comply with the OSHA standard, 29 CFR 19.10.134. 5
Limitations
The main limitation of this research was related to the inherent drawback of QLFT, which relies on the wearer’s subjective response to a test agent. This study is also limited by the small number of recruited subjects which may mask minor differences in nebulizers or respirator brand.
Conclusions
The odds for passing fit testing procedures using the manual Allegro nebulizer were similar to that of the automated Accumed NF60. Therefore, the Accumed NF60 nebulizer could be a cost-benefit option in the long run, particularly during the pandemics of respiratory infections such as COVID-19, in which standard fit test nebulizers would be inaccessible, expensive, or become a shortage. It is more convenient and reduces the contact stress in the operators’ hands as it does not require squeezing the nebulizer bulb frequently. Moreover, the Accumed NF60 nebulizer would sufficiently decrease the time investment for performing the QLFT procedures. For example, the time needed for connecting the components, cleaning, and unblocking the sediments of fit test solutions before fit testing for the OSHA-approved nebulizer is about 20 min, compared to a total of 5-min time for the Accumed NF60 one. Moreover, there was no liquid leakage in the Accumed NF60 nebulizer in contrast to the leakage from the components (bulb and reservoir) of the Allegro one. The test solutions’ preparation process and fit testing procedures on many personnel are more straightforward, faster, more comfortable, efficient, and hygienic than the manual Allegro nebulizer.
Acknowledgments
The authors thank the participants’ contribution to conducting the study tests.
Author contributions
Anahita Fakherpour and Mehdi Jahangiri conceptualized and designed the research. Anahita Fakherpour and Mehdi Jahangiri administrated the project and acquired the data. Mozhgan Seif conducted the statistical analyses and interpreted the data. Anahita Fakherpour and Mehdi Jahangiri prepared the first draft, and validated the accuracy of the work. All authors read and approved the final manuscript.
Funding statement
The present research was financially supported by Shiraz University of Medical Sciences, Shiraz, Iran (Grant No. 98-01-04-20889).
Conflicts of interest
None declared.








