INTRODUCTION
The isolation of Salmonella enterica subsp. enterica serovar Typhi (S. Typhi) is considered the gold standard for the diagnosis of typhoid fever [Reference Bhutta and Mansurali1]. However the isolation rate of S. Typhi from blood cultures of typhoid fever patients is relatively low. Culturing bone marrow aspirates has a much higher sensitivity, but for large population-based studies it is neither appropriate nor feasible to perform on all fever cases. Large studies have to rely on blood cultures which have a sensitivity between 30% and 70% [Reference Bopp and Murray2, Reference Cherian3]. Reasons for reduced S. Typhi detection rates in blood cultures include pre-medication with antibiotics and low bacterial load in the peripheral circulation. The latter point can be partially overcome by incubation of relatively large amounts of blood (10 ml).
Evidence for the relatively low sensitivity of blood cultures in the detection of typhoid fever comes from vaccine probe studies. A Vi polysaccharide (Vi PS) vaccine trial carried out in Guangxi China in 1995 showed similar protection whether cases where detected by blood culture or Widal serology (69% and 68% respectively) [Reference Chongsa-nguan4]. However Widal serology tests (Lanzhou Institute, China) detected 2·6-fold (sum of blood-culture positives and serology positives) more cases than detection based on blood culture alone.
More recently two antibody-based rapid tests, IDL-Tubex® (IDL Biotech AB, Borlange, Sweden) and Typhidot-M® (Malaysian Bio-Diagnostics Research Sdn. Bhd., Selangor, Malaysia), have become available for the detection of typhoid fever in clinical settings [Reference Clegg5–Reference Farooqui8]. We evaluated the use of serological tests to generate a case definition that can identify S. Typhi blood-culture-negative individuals with 100% specificity in Hechi City in preparation of a large Vi PS vaccine trial which may rely on serology in addition to culture for case detection [Reference Hoffman9]. Such a definition, if valid, could be of great benefit when assessing burden of disease or when evaluating public health interventions including vaccines.
METHODS
Study site
The study took place in a typhoid endemic area of southern China, Hechi Prefecture of Guangxi Zhuang Autonomous Region (Guangxi Province). The study site included the two most populous areas in the prefecture Jin Cheng Jiang, an urban area, and Don Jiang, a rural area. Typhoid fever cases were detected through passive surveillance in preparation for a trial with the Vi PS vaccine. All 85 health facilities in the catchment area; five hospitals, two township health centres, 10 factory clinics and 68 private clinics, participated in the surveillance. Patients aged between 5 and 60 years who reported a history of fever for ⩾3 days were eligible to participate in the study. If the patient or a parent/guardian consented a 10 ml blood sample was obtained, of which 8 ml were inoculated in blood culture bottles and sera from the remaining 2 ml blood were used for serology.
Laboratory methods
Blood samples were inoculated immediately after collection into BACTEC® Plus blood culture bottles (Becton, Dickinson & Co., Franklin Lakes, NJ, USA) and cultures were performed by customary methods [Reference House10]. All isolates were confirmed at the Oxford University–Wellcome Trust Research Unit in Ho Chi Min City, Vietnam. Three serological tests, Widal, IDL-Tubex® and Typhidot-M®, were used to detect immune responses to S. Typhi.
Widal
The Widal test was performed with standardized S. Typhi O (TO) and S. Typhi H (TH) antigens and Salmonella enterica subsp. enterica serovar Paratyphi A (S. Paratyphi A) O and H antigens (A) [Reference Coovadia6, Reference Levine11, Reference Olsen12]. Serial tube dilutions were prepared in normal saline and 500 μl of each TO, TH, and A antigens were added to 500 μl of each antiserum dilution. The solutions were incubated at 37°C overnight and checked visually for agglutination. The titre was determined as the highest dilution that showed visible agglutination. The dilutions used were 1:80, 1:160, 1:320 and 1:640.
Typhidot-M®
The Typhidot-M® is a dot enzyme immunosorbent assay that identifies serum immunoglobulin (Ig)M against a 50 kDa outer membrane protein (OMP) from S. Typhi. In total, 15 μl of serum are incubated with 15 μl of IgG inactivation reagent [Reference Clegg5, Reference Espersen7]. The IgG-inactivated serum is incubated with the strips at a final dilution of 1:100. The strips are incubated with the serum for 1 h followed by three washes, and then incubated with HRP-conjugated anti-human IgM for 1 h followed by another three washes. The strips are incubated with the colour reagent and the intensity of the two dots is compared with the intensity of the corresponding dots produced on the positive control strip. The results are qualitative, either negative or positive.
Tubex®
The IDL-Tubex® test is a semi-quantitative competitive agglutination test designed to detect the presence of antibodies directed against S. Typhi O9 antigens [Reference Coovadia6–Reference Farooqui8]. A total of 45 μl of S. Typhi LPS-coated magnetic beads are mixed with 45 μl of serum, then 90 μl of a solution containing monoclonal anti-O9 antibodies, as competitive antibodies, are added. The solution is placed on a magnet. The intensity of the resulting colour is used to assign a score (0, 2, 4, 6, 8, or 10) according to the colour scale provided by the manufacturer.
Analysis
We combined serological tests with the aim of developing a definition of a typhoid fever case with 100% specificity and the highest sensitivity. Sensitivity was defined as the proportion of patients from whose blood S. Typhi was isolated and whose serological tests were positive for S. Typhi. Specificity was defined as the proportion of patients from whom S. Paratyphi A was isolated and whose serological tests were negative for S. Typhi. Among the definitions with the highest specificity, the definition with the highest sensitivity was selected. This approach was applied to Widal and IDL-Tubex® tests which are semi-quantitative tests, for which different cut-offs could be used to calculate the specificity and sensitivity. In contrast, Typhidot-M® is a qualitative test and the results are either positive or negative. The three serological tests were combined utilizing the Boolean operator ‘and’. The analysis was performed using stata version 7.0 software (Stata Corporation, College Station, TX, USA).
Ethical considerations
Ethical considerations and required approvals were obtained for the study following the principles that govern biomedical research involving human subjects. The local ethics committee, the WHO Secretariat Committee on Research Involving Human Subjects, and the International Vaccine Institute (IVI) Institutional Review Board approved the protocol.
RESULTS
From August 2001 to March 2003, a total of 1874 episodes with ⩾3 days of fever were detected in 1861 residents aged 5–60 years attending the Hechi health facilities. All three serological tests, Widal, IDL-Tubex® and Typhidot-M® were performed on specimens collected from 1732 febrile episodes (Fig. 1).

Fig. 1. Assembling a study population for the case definition.
The highest specificity for the Widal test, 95%, was reached by 7 of 12 case definitions (Table 1). Of which the definition ‘TO⩾80 & TH>A’ had the highest sensitivity 62%. A Tubex score of ⩾4 was chosen as the definition of a positive according to the manufacturer's recommendations, which had 95% specificity and 69% sensitivity. The Typhidot-M® test had specificity and sensitivity of 91% and 54% respectively.
Table 1. Sensitivity and specificity of 1732 serological tests

TO, S. Typhi O antigen; TH, S. Typhi H antigen; A, S. Paratyphi A antigens (AO and AH); Tx, Tubex®; Td, Typhidot-M®.
* Sensitivity among 13 S. Typhi positives.
† Specificity among 21 S. Paratyphi positives.
Combining the three individual case definitions increased the number of putative typhoid cases by more than twofold from 13 to 28 cases (Table 2, Fig. 2). The sensitivity of the case definition was 39% while the specificity remained at 100%.

Fig. 2. Sensitivity, specificity and yield of the selected case definition, in all episodes (n=1732) (TH⩾80 & TH>A, Tx⩾4, Td=positive). Wi, Widal test; Tx, Tubex®; Td, Typhidot-M®. A greater than twofold increase in number of cases from 13 culture-confirmed cases to 28 cases by combined serologies.
Table 2. Proposed definition of (Widal, Tubex® and Typhidot-M®) with different cut-off values in 1732 fever episodes
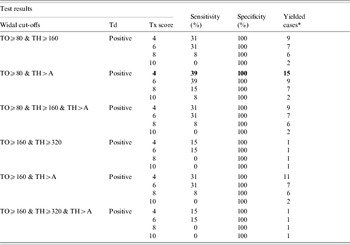
TO, S. Typhi O antigen; TH, S. Typhi H antigen; A, S. Paratyphi A antigens (AO and AH); Tx, Tubex®; Td, Typhidot-M®.
* Yielded cases identified as positive with proposed definition among blood culture negative.
DISCUSSION
A typhoid fever case definition, prolonged fever (⩾3 days of fever) plus a combination of positive serological tests resulted in a twofold increase in case detection retaining 100% specificity. The twofold increase in typhoid fever case detection using three serological tests is within the expected range considering the sensitivity of the blood culture compared to the bone marrow (30–70%). A case definition with specificity close to 100% minimizes misclassification which is paramount in a vaccine trial. A key aspect of our study is to base the specificity on a true S. Typhi culture-negative group, for which we chose the S. Paratyphi A-positive group. Paratyphoid fever has a clinical course indistinguishable to that of typhoid fever. The underlying assumption is that dual infections with S. Typhi and S. Paratyphi are exceedingly rare and the risk that controls are misclassified false positives is therefore much reduced. Although S. Paratyphi A is closely related to S. Typhi, all three serological tests are specific to Typhi antigens (O and H antigens for Widal, O9 antigen for Tubex®, and a S. Typhi-specific OMP for Typhidot-M®), hence the S. Paratyphi A culture-positive group can be used as the comparison group. Additionally, the cut-off values used in the Widal test included the criterion TH>AH, which could mean that the current infection is probably due to S. Typhi rather than S. Paratyphi A.
Nevertheless serology-based detection of typhoid fever cases has to be interpreted with caution. The background levels of agglutinins and antibodies detectable in populations without acute typhoid fever can vary depending on the degree of typhoid fever endemicity [Reference Levine11–Reference Schroeder18]. Although it was not the case with our study (results not shown), there are reports that the prevalence of antibodies increases with age [Reference Olsen12, Reference Senewiratne and Senewiratne19]. Moreover, the level of antibodies can change in areas where typhoid vaccines are widely in use. False positives can be caused by non-typhoid Salmonella; other bacterial species [Reference Somerville20], and with other conditions such as malaria, immunological disorders and chronic liver diseases [Reference Vallenas21]. Furthermore, culture-positive typhoid fever patients may not produce detectable antibody levels resulting in a false-negative serology [Reference Pang and Pothocheary14, Reference Prokopec16, Reference Schroeder18, Reference Yang22, Reference Yang23]. Our findings are based on the acute phase of the febrile illness in which antibody responses may not yet be detectable; hence we may still be underestimating the true number of cases. All these factors can decrease specificity and sensitivity of serological tests in regions where typhoid fever is endemic. Finally, our conclusions are based on relatively small numbers of cases and evaluation in other datasets is required, although typhoid fever phase-III clinical trials are generally powered by small numbers of cases. To achieve a more complete understanding of the antibody responses and serological tests for typhoid fever we are currently comparing and contrasting findings from four additional Asian countries.
The newer serological tests did not offer substantial advantages over the established Widal test. Only the shorter time to generate a result may be of an importance to clinicians. Dutta and co-workers have estimated that 18 h are required for the Widal tube agglutination test, 3 h for Typhidot-M®, and 10 min for the Tubex® test [Reference Dutta24]. Recently it has been reported that the nested PCR-based diagnosis of typhoid could be a more useful tool than either blood culture or Widal test owing to its greater discriminatory ability [Reference Prakash25–Reference Massi27]. It might possibly be of great use in vaccine trials assuming the availability of sophisticated equipment and specialized skills of the laboratory workers.
In summary, case definitions based on combinations of serological tests can detect additional cases while maintaining 100% specificity. These tests were relatively easy to perform and feasible in a small laboratory setting in China. Serology-based case definitions can provide a more accurate picture of the true typhoid fever burden and boost the power of intervention trials.
ACKNOWLEDGMENTS
We thank Yang Honghui, Tang Dongmei, M. John Albert, John Wain, Amanda Walsh, Jeremy Farrar, Yanning Gao, Michael Goon, Eun Young Kim, Sue Kyoung Jo, and the staff of the Guangxi Center for Prevention and Disease Control (CDC), China. This work was supported by the Diseases of the Most Impoverished Program, funded by the Bill and Melinda Gates Foundation. International Vaccine Institute (IVI) is supported by the governments of the Republic of Korea, Sweden, and Kuwait.
DECLARATION OF INTEREST
None.






