INTRODUCTION
Hantaviruses (genus Bunyaviridae) infect rodents worldwide and several Hantavirus species can infect humans and cause illness with varying severity [Reference Plant1]. The predominant species in Western and Northern Europe is Puumala virus (PUUV). It causes nephropathia epidemica (NE), a mild form of haemorrhagic fever with renal syndrome (HFRS) [Reference Zoller2–Reference Ahlm6]. Typical symptoms are fever, headache, muscle pain, nausea and impaired renal function [Reference Settergren7, Reference Vapalahti8]. The main reservoirs of PUUV are bank voles (Myodes glareolus), which can carry the infection persistently [Reference Brummer-Korvenkontio9–Reference Sironen12]. In Western and Central Europe the bank vole's preferred habitat is broad-leaved oak and beech forests as well as densely mixed forests with abundant herb and undergrowth layers [Reference Bujalska, Tamarin, Ostfeld, Pugh and Bujalska13]. The size of bank-vole populations is subject to large fluctuations. Infected bank voles shed the virus with their excreta, which may remain infectious in the environment for up to 12–15 days [Reference Kallio14]. Human infection occurs via inhalation of virus-contaminated particles and symptoms appear after an incubation period of around 2 weeks (1–4 weeks) [Reference Plant1, Reference Schmaljohn and Hjelle15].
Different studies in Europe have revealed various risk factors for PUUV infection, such as observing or trapping rodents, entering potentially rodent-infested rooms, wood cutting, exposure in a forest and being a construction worker [Reference Abu16–Reference Van Loock18].
In Germany, Hantavirus infection has been a notifiable disease since 2001 according to the Protection against Infection Act. About 93% of the notified Hantavirus infections in Germany are caused by the serotype PUUV [19]. The notified incidence from 2002 to 2006 ranged from 0·1 to 0·5/100 000 population [19]. However, some regions in South and West Germany are known to be endemic for PUUV [Reference Zoller2, Reference Essbauer20–Reference Essbauer23]. In the Southwestern state of Baden-Wuerttemberg (area 35 742 km2, population 10·7 million) the notified incidence between 2002 and 2006 ranged from 0·2 to 1·5/100 000 [19]. By the end of March 2007, 87 cases had already been notified in Baden-Wuerttemberg compared to an average of 12 cases in the same time period for the previous 5 years [19].
To investigate this outbreak we started a survey and a case-control study in May 2007. The objective of the survey was to better describe the symptoms and disease burden of PUUV infections in humans. The case-control study was initiated to identify specific risk factors for acquiring PUUV infections in order to promote more targeted preventive measures.
METHODS
Employees of the local public health offices identified case-patients from the notifications of the German surveillance system.
Case definition
For both studies the case definition of the national surveillance system was used. It included clinical symptoms such as fever or impaired renal function or at least two of the following symptoms: headache, muscle pain, nausea, diarrhoea, intermittent blurred vision, coughing, dyspnoea, lung infiltrations and heart failure. All cases had to be laboratory confirmed either with anti-PUUV antibodies (IgM followed by IgG or an increase of IgG level over time) or PCR. We included case-patients from Baden-Wuerttemberg with onset of symptoms from 1 April to 30 June 2007 in the case-control study and from 1 April to 31 December 2007 in the survey.
For all interviews informed consent was obtained and a standardized questionnaire was used. Case-patients were asked about their PUUV infection, e.g. clinical signs and symptoms, hospital stay, haemodialysis and days absent from work. For the case-control study, one control per case was randomly selected from the telephone directory and individually matched for sex, age group (±10 years) and county of residence. Case-patients and controls were both asked about their exposure to risk factors in the 4 weeks before onset of symptoms in the case-patient. Questions about type of residence, entering and cleaning potentially rodent-infested rooms, outdoor activities such as gardening and visiting forests, occupational exposure and contact with rodents or their droppings were included in the questionnaire.
Statistical analysis
For the bivariable and multivariable analysis of the case-control study we used a conditional logistic regression model. All variables with a P value <0·2 in the bivariable analysis were included in the initial model of the multivariable analysis. Starting with the initial model a stepwise backward elimination was carried out, using the likelihood ratio test for the comparison of models at each step. All the analyses were performed with Stata 9 (StataCorp USA).
RESULTS
The notified PUUV incidence in Germany 2007 was 2/100 000 population. With 1089 cases about 65% of the German cases occurred in the federal state of Baden-Wuerttemberg (Fig. 1), where an incidence of 10/100 000 was detected. The incidences in the ten most affected administrative districts were between 12 and 90/100 000. In particular, districts crossed by the Swabian Alb, a low mountain area, had incidences above 30/100 000. The number of notified PUUV infections started to increase from the beginning of 2007. After reaching a peak with 94 cases per week by the end of May the notifications decreased, but remained higher than in the previous years (Fig. 2). No death due to PUUV infection was notified.

Fig. 1. Incidences of notified Puumala virus infections per 100 000 population by administrative district, Germany (right) and Baden-Wuerttemberg (left), 2007.
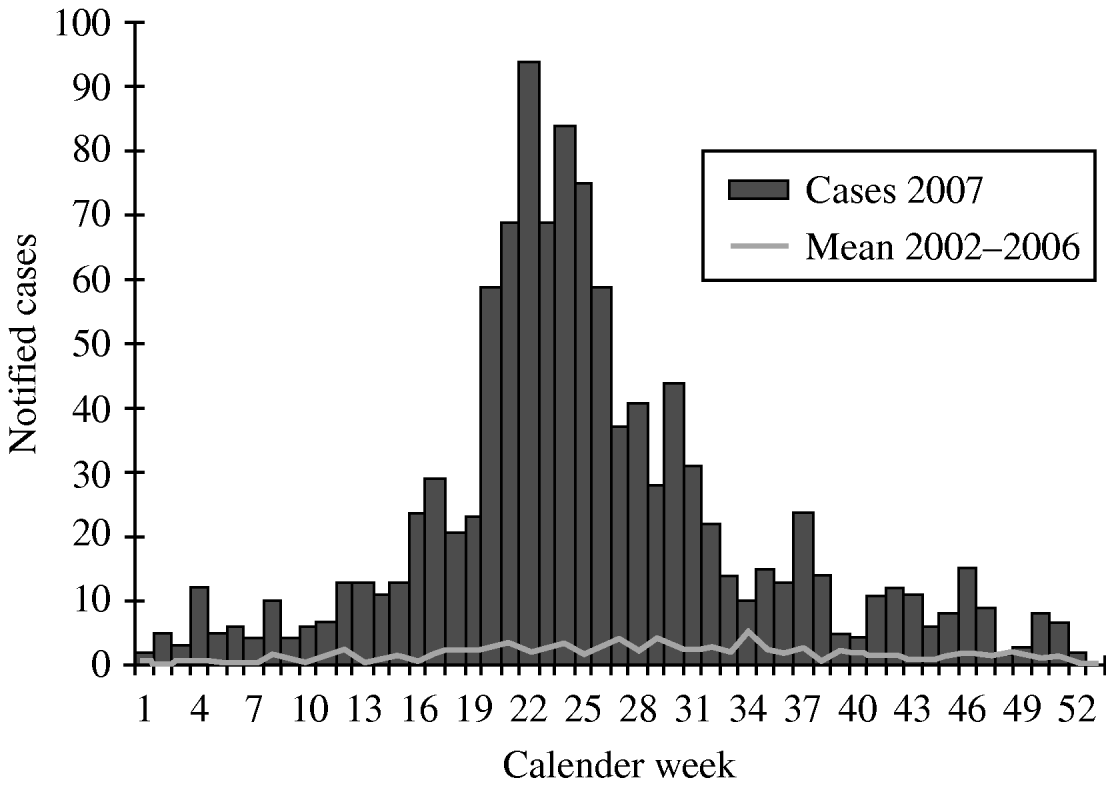
Fig. 2. Notified Puumala virus infections in 2007 compared with the mean number of notifications 2002–2006, by week of notification, Baden-Wuerttemberg.
Survey
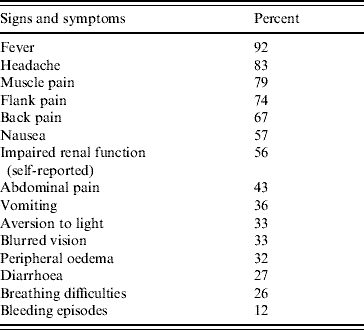
Questionnaires from 496 case-patients were included in the survey. The male/female ratio of case-patients was 2·4 and the median age was 43 years, ranging from 7 to 84 years. Four per cent of interviewees were aged <18 years. Most of the case-patients had influenza-like symptoms such as fever (92%), headache (83%) and muscle pain (79%). Altogether, 276 case-patients suffered from impaired renal function (56%), which was the main cause for hospitalization; in 3·3% the renal impairment was so severe that the patients required haemodialysis. Breathing difficulties were reported from 127 case-patients (26%) and bleeding episodes, e.g. nose bleed and haematuria, during their infection by 58 case-patients (12%) (Table 1). On average the patients were ill for 18·5 days (median 15 days, range 2–70 days). In total 327 of the interviewed case-patients required hospitalization (66%) with a mean hospital stay of 9 days (median 7 days, range 1–51 days). Absence from work in employed case-patients averaged 19 days.
Table 1. Signs and symptoms of 496 patients with Puumala virus infection (survey)
Case-control study
A total of 191 matched case-control pairs were included in the case-control study. The bivariable analysis (Table 2) revealed some potential risk factors, e.g. visiting a forest shelter, cleaning utility rooms, observing small rodents, entering a garden shed, farming and burning wood on an open fire. The majority of cases and controls had visited a forest in the 4-week recall period (71% and 66%, respectively). No occupational risk factor was detected.
Table 2. Bivariable and multivariable analysis for exposure variables for Puumala virus infection, conditional logistic regression (case-control study)
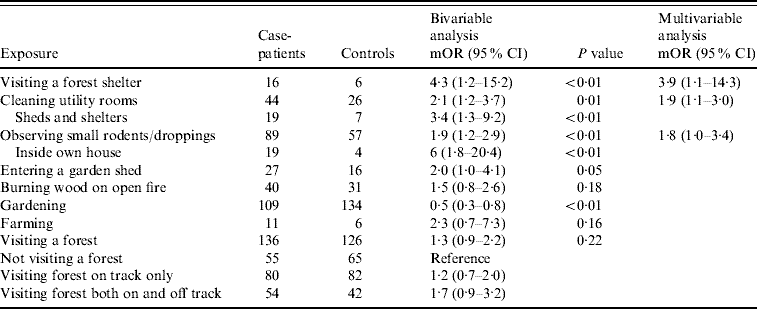
mOR, Matched odds ratio; CI, confidence interval.
The further multivariable analysis demonstrated that case-patients were four times more likely than controls to have visited a forest shelter (mOR 3·9, Table 2). Forest shelters are huts used by forest visitors to make a barbecue or to shelter from the rain. Moreover, case-patients were almost twice as likely to have cleaned utility rooms (mOR 1·9) such as sheds, attics, cellars and garages than controls (Table 2). More specifically, case-patients were involved in the cleaning of rooms, which were more likely to be rodent infested, such as sheds and shelters. Small rodents or their droppings were observed twice more often by case-patients than controls (mOR 1·9), and even more frequently inside the own house (Table 2). The majority of people interviewed in our case-control study referred to having visited a forest. The risk of infection appears to increase when people leave the forest tracks and go deeper into the forest (Table 2).
DISCUSSION
In 2007, Baden-Wuerttemberg faced the largest PUUV epidemic in Western Europe to date. Such high incidences of PUUV infections have only been previously reported from Scandinavian countries and Finland [Reference Brummer-Korvenkontio4, Reference Settergren7, Reference Niklasson24]. Additionally, seroprevalence studies with a human antibody prevalence of about 3% in the epidemic regions of Baden-Wuerttemberg suggest, that only a small fraction (5–10%) of PUUV infections are notified [Reference Zoller2, Reference Kimmig22]. Only a few people have died in the past due to PUUV infection and the case-fatality rate is usually below 0·5% [Reference Brummer-Korvenkontio4, Reference Pettersson25]. From the 1089 case-patients of this outbreak no death was reported.
Our survey showed that PUUV infections cause a lengthy duration of symptoms and can sometimes result in severe disease, with a high proportion of hospitalizations. The proportion of 66% hospitalized cases was lower than in a German study from 2005 (73·4%) [Reference Abu16], but much higher than the 30% in Sweden [Reference Pettersson25]. The reason for the higher proportion of hospitalizations in Germany remains unclear. It could be that Swedish physicians are more aware of PUUV and thus are more likely to test for it, resulting in more frequent diagnosis of less severe cases. In the survey, days of disease duration and absence from work might even be underestimated, because more than one third of patients were still symptomatic at the time of interview. Besides the personal impact for the infected individual, the disease burden had a considerable impact on hospital capacity and costs for the public health system.
As other risk-factor studies have shown, cleaning of sheds and shelters was also associated with a high risk of PUUV infection in our case-control study [Reference Armstrong26]. An explanation could be that sheds and shelters are rarely used and are therefore more likely to be infested with bank voles. Small rodents or their droppings were observed twice as often by case-patients than controls and even six times more often inside their own house. A large proportion of the cases can be explained by this factor, thus in-house rodent control measures play an important role in the prevention strategy and should be recommended strongly in areas where PUUV occurs frequently. In addition, our case-control study shows that there is a significant association between visiting forest shelters and PUUV infections. However, only a small number of cases of this epidemic might be explained by this risk factor.
After favourable feeding conditions with a beech mast year in summer/autumn 2006, followed by a mild winter at 3·8°C above the long-time average, an increase in the bank-vole population was observed in Baden-Wuerttemberg in spring 2007 (A. Gehrke, personal communication, The Forest Research Institute of Baden-Wuerttemberg Freiburg) [27]. This is concordant with other studies, which showed that climatic conditions have a great effect on rodent population dynamics [Reference Korpimäki28, Reference Mills29]. During epidemic times bank voles were trapped from different regions of Baden-Wuerttemberg. Between 20% and 76% of them tested seropositive for PUUV [Reference Ulrich30]. A more effective spread of PUUV can be expected with increasing vole populations because as more voles become newly infected the excretion of virus peaks in the acute phase of infection, i.e. in the faeces at 11–28 days and urine at 14–21 days post-infection, respectively [Reference Hardestam31, Reference Bernshtein32]. Increased bank-vole population densities result in more frequent contacts within the bank-vole population, which increases their likelihood of exposure to PUUV [Reference Olsson33]. In particular, a study from Belgium demonstrated a strong link between mild winter temperature and a high PUUV prevalence in bank voles [Reference Linard34]. Sauvage et al. demonstrated that the dramatic density fluctuations in the vole population over several years can result in simultaneously high numbers of infected voles, with high proportions of them in the acute excretion phase during the population build-up [Reference Sauvage, Langlais and Pontier35]. This would lead to a short peak of very high PUUV concentrations in the environment, and thereby, to increased human exposure [Reference Sauvage, Langlais and Pontier35]. The increase of the bank-vole population together with the increase in their PUUV prevalence seems to be the driving force of the 2007 epidemic in Baden-Wuerttemberg. Comparisons between patient- and bank vole-derived PUUV sequences from the epidemic area in Baden-Wuerttemberg, 2007 confirmed this relationship with a nucleotide identity of more than 98% [Reference Hofmann36].
In addition to a high PUUV prevalence among bank voles, human contact with infested environments seems to be an essential factor for the transmission to humans [Reference Linard37]. For example, almost half of the case-patients in this study observed small rodents or their droppings. Spring started 4 weeks earlier in 2007, and April especially was warm, sunny and dry [27]. This climate influenced the leisure activities of Baden-Wuerttemberg residents, who thus were more likely to be exposed to PUUV-contaminated environments. Our finding that the majority of people interviewed in our case-control study referred to having visited a forest could point to such a change in leisure activities. However, unfortunately no long-term data on leisure activities of Baden-Wuerttemberg residents exist to compare our data with.
Unlike Sweden, where bank voles have been systematically trapped since the 1990s [Reference Olsson11, Reference Ahlm12], no objective data on bank-vole population dynamics exists in Germany to date. Sweden also faced a PUUV epidemic in 2007, which peaked in January. Due to their long-term data on trapping indices it was possible to show the occurrence of a high bank-vole population during the epidemic. However, the population was not higher than in previous peak years and cannot explain the high number of cases alone [Reference Pettersson25]. Additional mild weather conditions and an increased exposure of humans to infected rodent excreta were also considered as the main reasons for the Swedish epidemic. In summary, the reasons for the PUUV epidemic in Baden-Wuerttemberg in 2007 were probably multifactorial. Favourable feeding and climatic conditions led to an increase in the bank-vole population with a higher PUUV infestation, and thus to an increase of PUUV dispersion into the environment [Reference Piechotowski38]. In addition, the dry, warm weather conditions probably resulted in more human contacts with contaminated environments. Further studies on the dynamics of the bank-vole population and their association with human PUUV infections are important for predicting epidemics and the implementation of timely preventive measures.
In PUUV endemic regions, in-house rodent control measures as well as use of protective equipment while cleaning risk locations, such as sheds and shelters, should be considered.
ACKNOWLEDGEMENTS
We thank all the colleagues of the local public health offices in Baden-Wuerttemberg, who actively contributed to the investigation.
DECLARATION OF INTEREST
None.






