Introduction
Adolescents with disruptive behavior disorders (DBD) show increased antisocial and aggressive behavior, emotional dysregulation, and risky decision-making. DBD is associated with high societal impact and public health costs, due to their aggregate burden on the perpetrators themselves, victims, their families, and communities (Foster & Jones, Reference Foster and Jones2005; Rivenbark et al., Reference Rivenbark, Odgers, Caspi, Harrington, Hogan, Houts and Moffitt2018). Despite the significant impairments associated with DBD, the underlying mechanisms of antisocial development are only partly understood, necessitating further research to improve the effectiveness of prevention and intervention strategies (Epstein, Fonnesbeck, Potter, Rizzone, & McPheeters, Reference Epstein, Fonnesbeck, Potter, Rizzone and McPheeters2015; Eyberg, Nelson, & Boggs, Reference Eyberg, Nelson and Boggs2008). A wide range of family and twin studies have shown that about half of the variance in antisocial behavior can be explained by environmental factors, whereas the other half is explained by genetic influences (Polderman et al., Reference Polderman, Benyamin, de Leeuw, Sullivan, van Bochoven, Visscher and Posthuma2015). Over the past few decades, the majority of research designs addressing the etiology of ASB have included explanatory models integrating either environmental factors or biological factors. In the present study, we test for the presence of an interaction effect by examining brain correlates of reward sensitivity and delinquent peer affiliation (DPA) in relation to DBD symptom scores.
Delinquent peer affiliation and DBD
An important environmental risk factor associated with DBD is the exposure to delinquent peers. For instance, a longitudinal study of Simonoff et al. (Reference Simonoff, Elander, Holmshaw, Pickles, Murray and Rutter2004) demonstrated that adolescents with antisocial peers are five times more likely to commit a crime during adolescence and ten times more likely to be diagnosed with antisocial personality disorder in adulthood than adolescents without delinquent peers (Simonoff et al., Reference Simonoff, Elander, Holmshaw, Pickles, Murray and Rutter2004). Despite the well-supported association between DPA and DBD, the nature of this relationship has been debated. Scholars have proposed two general mechanisms explaining the association: (A) causal socializing effects of DPA on DBD, such that affiliating with deviant peers leads to more engagement in disruptive behavior or (B) non-causal selection processes, such that disruptive individuals choose to affiliate with disruptive peers (Fergusson, Woodward, & Horwood, Reference Fergusson, Woodward and Horwood1999; Moffitt, Reference Moffitt1993). Fergusson, Swain-Campbell, and Horwood (Reference Fergusson, Swain-Campbell and Horwood2002) found support for both processes being complementary and reported that after controlling for selection, the strength of the association between DPA and disruptive outcome measures was reduced, yet still present (Fergusson et al., Reference Fergusson, Swain-Campbell and Horwood2002). The relative influence of these processes has been shown to vary over age, with stronger selection effects in middle adolescence, and more powerful socialization effects in late adolescence (Monahan, Steinberg, & Cauffman, Reference Monahan, Steinberg and Cauffman2009). Although individuals with deviant peers are more likely to engage in antisocial behavior, not all individuals are equally influenced by their friends. A number of studies have explored which factors underlie these individual differences in vulnerability to peer influences. For example, the relationship between DPA and DBD in adolescents was found to depend on the presence of callous and unemotional traits (CU-traits), such that DBD adolescents with CU-traits displayed higher levels of DPA (Kimonis, Frick, & Barry, Reference Kimonis, Frick and Barry2004). In contrast, another study found that high prosocial involvement was a protective factor against the contagion effects of DPA (Kaufmann, Wyman, Forbes-Jones, & Barry, Reference Kaufmann, Wyman, Forbes-Jones and Barry2007). The individual variability in susceptibility to environmental influences may in turn be explained by the level of resistance to peer influences. Monahan et al. (Reference Monahan, Steinberg and Cauffman2009) found that socialization effects on self-reported antisocial behavior were moderated by a set of personality characteristics referred to as peer influence susceptibility, such that socialization processes were stronger among adolescents with low resistance compared to those with a high resistance to peer influence (Monahan et al., Reference Monahan, Steinberg and Cauffman2009). Moreover, studies examining general peer-relationship processes have also demonstrated sex-differences in how peers contribute to emotional and behavioral development. In their review, (Rose & Rudolph, Reference Rose and Rudolph2006) found that female-linked peer relationships generally inhibit antisocial behavior, whereas male-linked relationships generally contribute to behavioral problems.
Adolescence and reward processing
Adolescence is characterized by increased social interactions, risk taking, impulsivity, and reward sensitivity (Casey, Jones, & Hare, Reference Casey, Jones and Hare2008). This crucial transitional developmental period is also marked by drastic neurobiological and hormonal changes. Preclinical studies have shown that various components of the dopamine system go through extensive structural changes during adolescence, i.e. there is a peak in dopamine transporter and D1-like receptor density in the rat striatum (Moll et al., Reference Moll, Mehnert, Wicker, Bock, Rothenberger, Rüther and Huether2000; Tarazi, Tomasini, & Baldessarini, Reference Tarazi, Tomasini and Baldessarini1999), and ventral tegmental area (VTA) dopamine neurons in adolescent rats fire faster than in adult rats (McCutcheon et al., Reference McCutcheon, Conrad, Carr, Ford, McGehee and Marinelli2012). Neuroimaging work strongly supports the notion that the greater reward-seeking behavior during adolescence is reflected by a hyper-responsive reward system in the brain (Galvan, Reference Galvan2010). In addition, developmental cognitive neuroscience studies have shown adolescent-specific influences on reward system function, with elevated recruitment of subcortical limbic systems relative to top-down (cortical) control systems during adolescence compared to childhood and adulthood (Galvan et al., Reference Galvan, Hare, Parra, Penn, Voss, Glover and Casey2006; Steinberg et al., Reference Steinberg, Albert, Cauffman, Banich, Graham and Woolard2008). Casey, Jones, and Somerville (Reference Casey, Jones and Somerville2011) have proposed a neurobiological model of adolescent development describing the imbalance between an accelerated responsiveness to motivational cues and immaturity in cognitive control (Casey et al., Reference Casey, Jones and Somerville2011).
These findings suggest that structural and functional changes in corticosubcortical circuitry during this developmental period may predispose adolescents to a higher sensitivity to reward-related cues, such as cooperative peer-interaction.
Susceptibility to peer influence, brain function, and risk taking behavior
Neuroimaging studies have shown activation of the mesolimbic dopamine reward system in response to peer-interactions, particularly cooperative and socially shared context as well as emotional (positive and negative) experiences recruit the VS (Schilbach et al., Reference Schilbach, Wilms, Eickhoff, Romanzetti, Tepest, Bente and Vogeley2010; Tabibnia & Lieberman, Reference Tabibnia and Lieberman2007; Wagner et al., Reference Wagner, Galli, Schott, Wold, van der Schalk, Manstead and Walter2015). Likewise, other human brain imaging studies revealed individual differences in dopaminergic neurotransmission in reward-related circuits, which may lead to different responses to environmental cues, such as peer influences. Chein and others (Reference Chein, Albert, O'Brien, Uckert and Steinberg2011) demonstrated that, in the awareness of their peers, adolescents showed an amplified activity in reward-related brain regions, including the VS, which had a subsequent risk-promoting effect (Chein, Albert, O'Brien, Uckert, & Steinberg, Reference Chein, Albert, O'Brien, Uckert and Steinberg2011). The adolescent brain's incentive processing system would thus differentially respond to the potential reward of risky choices, dependent on the presence or absence of peers.
Neuroimaging studies have also reported significant group differences between antisocial adolescents and healthy controls concerning reward-sensitivity (Cohn et al., Reference Cohn, Veltman, Pape, van Lith, Vermeiren, van den Brink and Popma2015; Gatzke-Kopp et al., Reference Gatzke-Kopp, Beauchaine, Shannon, Chipman, Fleming, Crowell and Aylward2009; Matthys, Vanderschuren, Schutter, & Lochman, Reference Matthys, Vanderschuren, Schutter and Lochman2012). Buckholtz and others demonstrated that individuals displaying impulsive-antisocial behavior show heightened dopamine release in the nucleus accumbens during monetary reinforcement, compared to healthy controls (Buckholtz et al., Reference Buckholtz, Treadway, Cowan, Woodward, Benning and Li2010). In addition, a multi-modality functional imaging study found that maladaptive decision-making in incarcerated offenders was associated with a disrupted regulation in the cortico-striatal circuit (Hosking et al., Reference Hosking, Kastman, Dorfman, Samanez-Larkin, Baskin-Sommers, Kiehl and Buckholtz2017). Neuropsychological and physiological studies have shed light on the amygdala's multifaceted role in reward processing, expanding beyond its well-known association with fear conditioning and the processing of negative emotions (Morrison & Salzman, Reference Morrison and Salzman2010). The amygdala has been shown to play a key role in learning about the positive value of stimuli, demonstrating its involvement in reward representation and stimulus-reward learning (Baxter & Murray, Reference Baxter and Murray2002). Lastly, extensive preclinical and human research emphasizes the critical role of the medial prefrontal cortex (mPFC) in incentive processing (Tzschentke, Reference Tzschentke2000; Xue et al., Reference Xue, Lu, Levin, Weller, Li and Bechara2009). Notably, previous fMRI studies have implicated the mPFC as a pivotal region mediating the effects of stress on reward sensitivity (Ironside, Kumar, Kang, & Pizzagalli, Reference Ironside, Kumar, Kang and Pizzagalli2018; Treadway, Buckholtz, & Zald, Reference Treadway, Buckholtz and Zald2013). Furthermore, the mPFC is instrumental in integrating sensory information with learned emotional values and exerting cortical control over reward-based behavioral output (Pastor & Medina, Reference Pastor and Medina2021).
In light of these findings, the present study explored whether the strength of the association between DPA and DBD could be linked to the responsiveness of reward-circuitries. Neuroimaging research has identified the ventral striatum, amygdala, and mPFC as crucial brain areas involved in reward processing (Baxter & Murray, Reference Baxter and Murray2002; Haber, Reference Haber and Gottfried2011; Murray, Reference Murray2007; O'Doherty, Reference O'Doherty2004). To assess sensitivity to reward, neural activity in the VS, amygdala, and mPFC during the fMRI-based monetary incentive delay (MID) task, which involves reward anticipation and outcome response, was employed as an intermediate phenotype. While the MID paradigm serves as a well-established and controlled measure of reward sensitivity, it is crucial to acknowledge that social rewards may entail more intricate and nuanced processes that extend beyond simple monetary incentives. Prior research has demonstrated that tangible and quantitative social rewards hold greater incentive power among children and adolescents compared to monetary rewards (Wang, Liu, & Shi, Reference Wang, Liu and Shi2017). Interestingly, although studies have shown that the neural basis for anticipating social approval is similar to that of anticipating monetary rewards, men exhibit a wider network of mesolimbic brain regions activated during the prospect of monetary rewards, whereas limited activation is observed for social rewards (Spreckelmeyer et al., Reference Spreckelmeyer, Krach, Kohls, Rademacher, Irmak, Konrad and Gründer2009). We hypothesized that increased reward-sensitivity is related to DBD symptoms, particularly in the context of DPA.
Materials and methods
Subjects
The present study makes use of phenotypic and neuroimaging data collected during May 2011 – June 2012 in a subsample of childhood onset offenders at the mean age of 17.7 (s.d. 1.6) years, of which 85% were males (Cohn et al., Reference Cohn, Veltman, Pape, van Lith, Vermeiren, van den Brink and Popma2015). Subjects were drawn from a cohort of 364 first-time arrestees recruited in three different police regions in the Netherlands during the period of July 2003 – December 2005 (van Domburgh, Vermeiren, Blokland, & Doreleijers, Reference van Domburgh, Vermeiren, Blokland and Doreleijers2009). The participants at that time were all younger than 12 years, which is the age of criminal responsibility for Dutch law. This subsample was selected to represent the entire severity spectrum of antisocial behavior, ranging from low-risk to high-risk, based on previous waves’ DBD symptom counts (NIMH Diagnostic Interview Schedule, DISC-IV (Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, Reference Shaffer, Fisher, Lucas, Dulcan and Schwab-Stone2000)), aggression scores (Reactive Proactive Aggression Questionnaire, RPQ (Raine et al., Reference Raine, Dodge, Loeber, Gatzke-Kopp, Lynam, Reynolds and Liu2006); see below) and psychopathic traits scores (The Youth Psychopathic traits Inventory, YPI (Andershed, Hodgins, & Tengström, Reference Andershed, Hodgins and Tengström2007)). We employed the same exclusion criteria as Cohn et al. (Reference Cohn, Veltman, Pape, van Lith, Vermeiren, van den Brink and Popma2015) and added missing DPA data as an extra criterion, resulting in the exclusion of 24 out of the 150 participants on the basis of invalid or missing MRI data (n = 10), missing DPA data (n = 2), task performance rates on the MID task deviating more than 3 s.d. from the mean (n = 9) or drug use in the last 24 h before scanning (n = 3). The excluded group (n = 24) and the study sample (n = 126) were similar regarding levels of antisocial behavior, aggression, psychopathic traits, age, IQ, sex, ethnicity, or socioeconomic status. Comparing the neuroimaging subsample to the remainder of the childhood arrestees cohort, we found that the study sample had higher initial DBD symptom scores. For a complete description of the recruitment strategy, see Cohn et al. (Reference Cohn, Veltman, Pape, van Lith, Vermeiren, van den Brink and Popma2015).
Measurements and procedure
Delinquent peer affiliation
DPA was assessed by a self-report questionnaire developed by the RADAR study (Research on Adolescent Development and Relationships; (37)). This questionnaire consists of eleven items, scored on a three-point scale, of which the first half measured the peer delinquency, whereas the second half measured the level of peer affiliation. The first six items were summed to index ‘crime level peers’ and were scored using a three-choice response format (0 = none of my friends are like that, 1 = just a few of my friends are like that, 2 = most of my friends are like that). The last five items were summed in the same way to index peer affiliation. The DPA score (ranging from 0 to 120) was displayed by multiplying crime level peers score with the peer affiliation score. At baseline interview, the internal consistency of the two subscales was adequate (peer delinquency, Cronbachs α = 0.83; peer affiliation, α = 0.74).
Disruptive behavior disorder symptoms
The VU University Medical Ethics Committee and the Ministry of Justice approved the phenotypic and neuroimaging data collection. Informed written consent was obtained from all participants and from their parents or guardians (in case participants were below 18 years of age). Behavioral data on DBD were collected retrospectively using a structured psychiatric interview (DISC-IV, child and parent version) (Knutson, Adams, Fong, & Hommer, Reference Knutson, Adams, Fong and Hommer2001a), administered at home. The DISC-IV assessment evaluates DBD symptomatology within the past twelve months. Therefore, DBD symptoms related to their initial offense, which may have led to their arrest, were not included in their DBD-score at the age of 18. DBD-score was computed as a continuous measure based on whether symptoms were scored for CD and ODD on the child version of the DISC.
MID task
We used the MID paradigm as described by Cohn and others that consisted of 72 six second trials (Knutson, Fong, Adams, Varner, & Hommer, Reference Knutson, Fong, Adams, Varner and Hommer2001b). During the MID-task subjects anticipated on a potential monetary reward, punishment or no consequences depending on the type of cue. Cues signaling the potential of winning €0.50 were denoted by circles, the potential of losing €0.50 were denoted by squares and no monetary outcome was denoted by triangles. Within each trial, participants saw one of three cue shapes (cue, 2000 msec), fixated on a crosshair as they waited during a variable interval (delay, 2000–2500 msec), and then responded to a white target square that appeared for a variable length of time (target 160–260 msec) with a button press. After target presentation, the outcome (outcome, 1650 msec), which followed the disappearance of the target, notified participants whether they had won or lost money during that trial and indicated their cumulative total at that point. The MID paradigm has been tested extensively and successfully recruits the reward regions of the brain, which is also reflected in the expected activation in the limbic and frontostriatal neurocircuitry in the present study (Cohn et al., Reference Cohn, Veltman, Pape, van Lith, Vermeiren, van den Brink and Popma2015; Knutson et al., Reference Knutson, Adams, Fong and Hommer2001a; Knutson et al., Reference Knutson, Fong, Adams, Varner and Hommer2001b).
Our study focused on reward sensitivity by observing the reward trials. The outcome of these reward trials could be either (1) ‘reward outcome’ if the participant pressed the button fast enough during target presentation or (2) ‘no reward outcome’ if the participant was too slow. Trial types were pseudo randomly ordered within each session. Task difficulty, based on reaction times collected during the practice session before scanning, was set such that each participant should succeed on 66% of his or her target responses (Knutson et al., Reference Knutson, Adams, Fong and Hommer2001a).
fMRI acquisition
Functional MRI data were obtained on a second occasion, during which participants were scanned in a Philips 3 T Intera MRI-scanner in the Academic Medical Center Amsterdam. Neuroimaging generally took place within 1.5 months after the behavioral session, with a few exceptions where the scan was performed after 2 months. During this scan protocol, T1 weighted anatomical scans were acquired using a 8-channel SENSE head-coil and consisted of 180 sagittal 1 mm thickness slices, with an in-plane resolution of 1 × 1 mm (FOV 256X256X180 mm, TR 9.0 ms, TE 3.5 ms). Furthermore, 204 T2* weighted echo planar images (EPI) were acquired, each volume consisting of 38 ascending slices of 3 mm thickness and 2.29 × 2.29 in-plane resolution, parallel to the anterior commissure – posterior commissure line (FOV 220 × 220 mm, TR 2300, TE 30 ms).
fMRI analysis
Functional MRI data were processed using SPM8 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, London, UK) in MATLAB (Mathworks, Inc., Sherborn, MA). Analyses focused on changes in blood oxygen level-dependent (BOLD) contrast that occurred during anticipation and outcome in the monetary reward condition of the MID task. Preprocessing included spatial realignment, unwarping, slice-time correction, spatial normalization into standard Montreal Neurological Institute space based on the segmented anatomical scan and spatially smoothening with an 8 mm full width half-maximum Gaussian kernel.
First and second level analyses
Changes in the BOLD signal for each subject (i.e. ‘activation’) were tested on the basis of linear combinations of estimated GLM parameters (beta values). These changes, displayed by individual contrast images, correspond to the percent signal change (effect size). First level analyses were performed by modeling two separate reward regressors for each trial type: anticipation period and outcome period (40). Realignment parameters were included in the statistical model as additional regressors to correct for motion effects. Then, contrast images were calculated to assess reward anticipation (reward trial anticipation > neutral trial anticipation) and reward outcome (reward trial hit > reward trial miss). Next, to compute group-level statistics, these individual contrast images were entered into second-level analyses. The second-level data were then extracted from MATLAB and used to perform multiple regression analyses in SPSS.
Statistical analyses
Power calculations were conducted using G-Power software (Faul, Erdfelder, Lang, & Buchner, Reference Faul, Erdfelder, Lang and Buchner2007) (Version 3.1.9.7). For the multiple linear regression analysis, we considered small (F 2 = 0.02), medium (F 2 = 0.15), and large (F 2 = 0.35) effect sizes, accounting for 12 predictors and a corrected alpha level of 0.008. The estimated power was 0.03 for small effect sizes, 0.54 for medium effect sizes, and 0.98 for large effect sizes, respectively. Zero-order correlations were calculated to inspect the relationship between the variables. These indicated that the neural activity during the tasks in the VS, amygdala and mPFC was highly correlated across hemispheres (see correlation matrix, online Supplementary Table S1; for main effects of the MID task, see online Supplementary Figure S1). In our analyses, we therefore utilized the average activation of regions of interest (i.e. total VS) to examine the functional brain correlates. Then, to reduce structural multicollinearity between the main effects of the predictors and their interaction terms and to facilitate interpretation of our parameter estimates, we mean-centered all predictors. Second, for all predictors and for the DBD outcome measure, outliers were defined as the scores deviating more than three s.d. from the mean. These outliers (N = 6) were constrained at their lower and upper boundaries (mean + /- three s.d.), so that no extreme score carried excessive weight. We applied a square root transformation of the DBD variable, to obtain a non-significant departure from normality (see online Supplementary Figure SI). We observed only minor differences between the outcomes before and after transformation, therefore, for the purpose of interpretation, we report the standardized regression coefficients and p-values for the untransformed DBD score throughout this study. We then ran a multiple linear regression model to test for the interaction effect of DPA and reward sensitivity on DBD outcome, correcting for the possible confounders sex, SES, age, and the covariate interaction terms. Since reward sensitivity was indexed by the neural response in three brain regions (striatum, amygdala, and mPFC), during two conditions (reward anticipation and outcome), six separate multiple linear regression analyses – each including twelve predictors – were run. In order to account for multiple testing, the threshold for significance of the interaction terms was corrected for 6 independent tests and hence chosen as p < 0.00833. Lastly, in case of nominally significant (p < 0.05) interaction of a covariate and DPA on DBD, we performed separate follow-up analyses in strata defined by the covariate to further explore the relationship between DPA and DBD.
Results
In this cross-sectional study, we observed a significant positive correlation between DPA and DBD (rs = 0.55, p < 0.001), but not between any of the ROI's reward-related neural activity and DPA or DBD (p > 0.05).
Linear regression analyses showed a significant interaction effect of DPA with VS responsiveness on DBD during reward outcome (β = 0.314, p < 0.001). In addition, our final model revealed a significant interaction effect of DPA with age (β = −0.267, p = 0.006). No interaction effect was found between DPA and VS responsiveness during reward anticipation (p > 0.05, see online Supplementary Table S2). Moreover, additional testing did not reveal evidence for an interaction effect of DPA with amygdala and mPFC responsiveness on DBD during reward anticipation and outcome (p > 0.05, see online Supplementary Table S3–S6) (Table 1).
Table 1. Final model of the linear regression analysis of 126 individuals examining the interaction effect of DPA and VS responsiveness during reward outcome on DBD
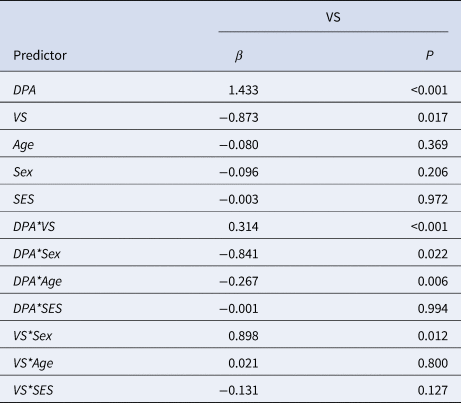
β, standardized beta; P, P-value; VS, ventral striatum. DBD. disruptive behavior disorder; DPA, delinquent peer affiliation.
To further investigate the interactions, participants were categorized into three groups based on their neural activity in the ventral striatum (VS) during the reward outcome phase. The group division was determined using quartiles, resulting in a low VS group (N = 31), a middle VS group (N = 64), and a high VS group (N = 31). Subsequent post-hoc analyses revealed a strengthening association between DPA and DBD as VS responsiveness increased: lowest quartile VS group correlation (r) = 0.49, p = 0.005), middle quartile VS group (r = 0.56, p < 0.001) high quartile VS group (r = 0.64, p < 0.001).
Sex and age-specific effects on the DPA-DBD relationship
Sex-specific analyses revealed a significant positive association between DPA and DBD in males (r(105) = 0.59, p < 0.001), while no such association was observed in females (r(17) = 0.28, p = 0.25). Age-specific analyses indicated a diminishing association with increasing age: the lowest age group (12–16) exhibited a strong correlation (r(36) = 0.72, p < 0.001), followed by the middle age group (17–18) with a moderate correlation (r(59) = 0.57, p < 0.001), and the highest age group (19–20) showing a weaker correlation (r(25) = 0.30, p = 0.13).
Discussion
To our knowledge, this study presents the first evidence of an interaction effect between a brain mechanism and an environmental factor in relation to DBD symptom severity. We found that the link between affiliation with delinquent peers and DBD symptom scores was associated with reward sensitivity in the VS (i.e. striatal reward responsiveness), with the association being stronger in individuals displaying increased VS response to reward compared to those displaying a low response.
The present study found a significant positive correlation between DPA and DBD, which is in line with developmental theories proposing that affiliation with delinquent peers and susceptibility to peer influence are important contributors to adolescent delinquency. Although prior studies have reported that genetic variation, such as MAOA (Lu & Menard, Reference Lu and Menard2017) and GABRA2 (Villafuerte, Trucco, Heitzeg, Burmeister, & Zucker, Reference Villafuerte, Trucco, Heitzeg, Burmeister and Zucker2014), could moderate peer influences on externalizing or antisocial behavior, such candidate gene studies have not been replicated well (Dick et al., Reference Dick, Agrawal, Keller, Adkins, Aliev, Monroe and Sher2015; Duncan & Keller, Reference Duncan and Keller2011). Burt and Klump (Reference Burt and Klump2013) found that DPA was an etiological moderator of childhood delinquency and demonstrated that not shared genetic, but shared environmental influences on delinquency were larger in higher levels of DPA as compared to those with lower levels of DPA, indicating socialization effects are stronger than selection effects (Burt & Klump, Reference Burt and Klump2013). The main effect of DPA and its interaction effect with reward sensitivity in this study were only present in males. It should be noted however, that the power to detect an interaction effect was limited in the female-specific sample (N = 19). A previous meta-analysis in 277 studies did not find evidence (d = 0.01) for sex-differences in reward sensitivity (Cross, Copping, & Campbell, Reference Cross, Copping and Campbell2011). Still, others have found higher scores in males on antisocial peer conformity vignettes, but no sex-differences on the neutral peer pressure subscales, indicating that the absence of an interaction effect in females could be due to differences in sensitivity specifically towards antisocial peer affiliation mechanisms (Santor, Messervey, & Kusumakar, Reference Santor, Messervey and Kusumakarn.d.).
The present study did not find a significant association between DBD symptom scores and reward-related neural activity. In an earlier study, carried out in the same sample, Cohn et al. (Reference Cohn, Veltman, Pape, van Lith, Vermeiren, van den Brink and Popma2015) found that persistent DBD subjects were associated with lower neural responses in the VS compared to desisting DBD and healthy subjects. This finding suggests that persistent DBD subjects may be less sensitive to the socializing effects of its peers and selection mechanisms might have a stronger effect on the association between DPA and DBD in this distinct group.
We only found evidence of an interaction effect for responsiveness of the VS to reward and not for reward anticipation. Previous studies have reported associations between VS response and antisocial behaviors, including increased activation of the VS already during the anticipation phase (Huettel, Stowe, Gordon, Warner, & Platt, Reference Huettel, Stowe, Gordon, Warner and Platt2006; Knutson et al., Reference Knutson, Adams, Fong and Hommer2001a). Van Leijenhorst et al. (Reference Van Leijenhorst, Moor, Op de Macks, Rombouts, Westenberg and Crone2010) have argued that in those experiments, since the participants can to some extent predict that a reward will follow, the neural activity is merely an early excitement response rather than anticipating a possible reward (Van Leijenhorst et al., Reference Van Leijenhorst, Moor, Op de Macks, Rombouts, Westenberg and Crone2010). In their study, using a task design that did not allow for reward prediction, the peak in VS activation was only observed during the actual delivery of rewards. Glenn and Yang (Reference Glenn and Yang2012) proposed that the increased VS response observed in antisocial and psychopathic individuals may not necessarily indicate hypersensitivity in reward processing, but a failure to signal the absence of a reward, which in turn may lead to enduring maladaptive behaviors (Glenn & Yang, Reference Glenn and Yang2012).
Our findings indicate that individual differences in neural reward processing may be linked to the sensitivity towards peer affiliation, when examining their effects on DBD. Therapeutic interventions focusing on hypersensitive adolescents may thus specifically target peer affiliation to modify the environment by withholding adolescents from negative peer influences and promoting positive, healthy peer interactions.
Limitations and future directions
Our results underscore the multifaceted nature of brain-behavior relationships and the importance of considering neural specificity, individual variability, and sample characteristics when interpreting research outcomes. We did not find evidence for an interaction effect between DPA and amygdala or mPFC responsiveness during reward anticipation and outcome. It is plausible that the association between DPA and disruptive behavior may primarily involve the VS, as this region is more closely tied to social reward sensitivity and reinforcement learning relevant to affiliating with delinquent peers. In contrast, the amygdala and mPFC may not be as directly involved in the specific social reward aspects related to DPA in this context, which could explain the absence of an interaction effect with these regions. At the same time, brain-behavior relationships often involve multiple factors, and it is possible that other unmeasured variables or individual differences contributed to the lack of an observed interaction with the amygdala and mPFC. Further research with larger samples and targeted paradigms could enhance statistical power and sensitivity to detect potential interactions with the amygdala and mPFC. Moreover, in future studies, comparing brain responses of delinquent adolescents with a control group will reveal if VS interactions are specific to this population or generalize to others without delinquent affiliations. Even though our study finds strong support for a biosocial mechanism, the proportion of explained variance is limited, which is expected given the variety of other factors that play a role. In addition, research in twins has demonstrated shared genetic liability of DPA and conduct problems, indicating a gene-environment correlation (Button et al., Reference Button, Corley, Rhee, Hewitt, Young and Stallings2007). Strikingly, the genetic correlation between DPA and conduct problems was found to be context-dependent in this study, such that the correlation increased with higher DPA scores. This GxE correlation offers the possibility of selection mechanisms explaining the correlation between DPA and DBD and can potentially confound the estimated interaction effect in our study, by increasing the type I error rate. Future studies examining the interplay between DPA and DBD should thus consider both plausible mechanisms to inform prevention strategies. We acknowledge the potential influence of substance use on our findings, given the literature linking reward sensitivity to substance use during adolescence (Obando, Trujillo, & Trujillo, Reference Obando, Trujillo and Trujillo2014) and its common co-occurrence with antisocial peers (Andrews, Tildesley, Hops, & Li, Reference Andrews, Tildesley, Hops and Li2002) and DBD (Bukstein, Reference Bukstein2000). While we were unable to directly control for substance use in our analyses due to data limitations, we recognize its potential significance in shaping the observed relationships. Substance use may act as a mediating or moderating factor, influencing the neurobiological processes underlying disruptive behavior (Andrews & Hops, Reference Andrews, Hops and Scheier2010). Given this complexity, future research should incorporate substance use data into investigations exploring the interplay between antisocial peers, reward sensitivity, and DBD symptoms.
Moreover, to reliably capture the concept of reward-sensitivity, it should ideally be examined in different modalities, both behaviorally (through questionnaires and cognitive tasks) and biologically (genetic risk score). Our study was not designed to formally test the differentially susceptibility theory, which proposes that neurobiological characteristics may determine who will benefit disproportionally from positive environmental factors, while being also more vulnerable to negative environments (Belsky & Pluess, Reference Belsky and Pluess2009). We thus encourage future research to examine whether individuals differ in their susceptibility to both the antisocial and prosocial effects of peer affiliation on DBD symptom severity. Moreover, longitudinal studies could explore striatal reactivity to reward and sensitivity to DPA during pre-adolescence and then follow-up with DBD at middle and late adolescence. Future studies could also examine differences in the neural underpinnings of social and monetary incentive processing (Greimel et al., Reference Greimel, Bakos, Landes, Töllner, Bartling, Kohls and Schulte-Körne2018). Ideally, since reward-sensitivity is a rather broad and indirect measure of susceptibility, future studies could focus on peer susceptibility instead, to achieve a more ecologically valid interaction design. Additionally, despite successfully obtaining unique fMRI data from a relatively large at-risk sample, power analyses demonstrated that detecting small or subtle effects remains a challenge, also due to the number of predictors, including interaction variables. Lastly, we recognize the broader challenges in fMRI studies as highlighted by recent literature (Elliott et al., Reference Elliott, Knodt, Ireland, Morris, Poulton, Ramrakha and Hariri2020). Task fMRI measures have demonstrated poor reliability and limited test-retest reliabilities in a priori regions of interest. We therefore remain cautious in our interpretations and recognize the need for replication and validation in independent samples to strengthen the generalizability of our findings.
To conclude, our results demonstrate the potential of brain-environment interaction studies (with careful methodological designs), as a powerful tool to advance our understanding of adolescent risk-taking and deviant behaviors. Intervention studies aimed at enriching peer-to-peer interactions may serve as a buffering strategy against disruptive behaviors, particularly for reward-sensitive adolescents.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723003380.
Competing interests
None.




