INTRODUCTION
The Netherlands is a low endemic country for hepatitis B virus (HBV) and hepatitis C virus (HCV) infections with an estimated prevalence of 0·4% for each infection when migrants are included [Reference Marschall1–Reference Kretzschmar4]. Surveillance data in The Netherlands have shown that 77% of notified patients with chronic hepatitis B were born abroad [Reference Koedijk2]. Nearly 10% of the Dutch population are first-generation migrants (FGM). With regard to hepatitis B, 18% were born in low-endemic countries [prevalence of hepatitis B surface antigen (HBsAg) <2%], 71% in middle endemic countries (prevalence 2–8%, including Turkey) and 11% in high endemic countries (prevalence >8%). The overall HBsAg prevalence of FGM was estimated to be 3·77% and it was estimated that at least 58% of all HBsAg-positive carriers in The Netherlands belong to this group [Reference Marschall1]. Similar calculations have been made for hepatitis C infection rates in FGM in The Netherlands, based on the assumption that the prevalence reflects the prevalence of the country of origin [Reference Kretzschmar4]. Accordingly, the overall HCV prevalence of FGM in The Netherlands was estimated to be 2·16%, accounting for around 60% of all chronic HCV infections in The Netherlands, the majority from four countries: Turkey, Suriname, Morocco and Egypt. However, all the data on seroprevalence of chronic hepatitis B and C infection in the migrant population are still mainly based on assumptions. New data on HBV prevalence in migrants will become available soon from The Pienter-2 study. In that study, a large, random sample of the Dutch population, with oversampling of migrants, was tested for HBV and other pathogens [Reference Van der Klis5].
Patients with chronic HBV and HCV infection are at risk of silently progressing to liver cirrhosis and/or hepatocellular carcinoma in 20–30% of cases. Early detection, contact tracing and eventual treatment are necessary in order to reduce the disease burden for the individual as well as the transmission rate and costs to the health system of dealing with complications of the disease. Effective treatment for both infections has become available in recent years. Long-term antiviral therapy will probably have a positive impact on the mortality and morbidity of active chronic hepatitis B [Reference Toy6]. Moreover, it has been shown that screening and early treatment of migrants for chronic hepatitis B in The Netherlands would be cost-effective [Reference Veldhuijzen7]. Despite this, a screening policy for HBV and HCV in migrants in The Netherlands is still non-existent. In order to assess the prevalence of HBV and HCV in the largest group of FGM in The Netherlands, i.e. inhabitants originating from Turkey, we conducted a screening project in the Turkish community of Arnhem. Being familiar with the stigma regarding hepatitis in that specific group our additional aims were to gather experience with a culturally targeted approach of reaching that population, thereby contributing to a reduction of stigma. Moreover, we hoped that the study results would be helpful for the formulation of a national screening strategy for migrants.
METHODS
We established a project group consisting of Turkish experts from ‘Osmose/Elan’ (an organization specializing in educational issues pertaining to migrants including social, cultural and religious aspects), three general practitioners (GPs) (two of Turkish ethnicity), the municipal health service, the microbiology laboratory, an epidemiologist, and ourselves from the Infectious Disease Unit, Rijnstate Hospital, Arnhem. We wrote a project plan that was approved by the local ethics committee. We divided our project into four phases: (1) the preparation phase; (2) the campaigning phase; (3) the information and screening phase; (4) the post-screening and clinical evaluation phase.
The preparation phase
We developed a brochure, a poster, a video documentary and a website focusing on HBV and HCV in migrants. Existing materials from The Netherlands and Turkey were reviewed. We did not find suitable information in Turkish language that could be applied to our setting. In particularly ‘Dutch information materials’ on hepatitis that were directly translated from Dutch into Turkish were considered inappropriate for our target population as these mostly focused on specific risk groups with an emphasis on sexuality, HIV and drug abuse. Many Turkish people do not recognize themselves in those risk groups. Therefore, another approach was chosen with more attention to lifestyle and other forms of possible transmission, e.g. using the same toothbrush or razors, or perinatal transmission in the past. The final information material such as brochures and posters were thoroughly checked by the four members of the project group of Turkish ethnicity, who were all FGM, for comprehension and medical correctness. Several aspects of hepatitis were outlined in a video documentary Hepatiti Gizli tehlike (Hidden danger) by a GP of Turkish origin whose practice was located in Arnhem. A new interactive Turkish/Dutch website was built with information about hepatitis and the project. A special phone number for Turkish/Dutch information and questions was established and two female Turkish health educators were trained to be ‘ambassadors’ of the hepatitis project. The project was discussed with the association of GPs in Arnhem who were kept well informed throughout the project. All 78 GPs in Arnhem, working in 33 general practices, agreed to collaborate.
The campaigning phase
The ‘ambassadors’ visited Turkish organizations in Arnhem to supply information on the project. Cooperation was established with delegates of mosques, primary schools, social and cultural organizations, community centres, Turkish shops, restaurants and immigrant organizations. Turkish newspapers featured the project, as did the local Dutch newspaper de Gelderlander and websites; moreover, local radio had an item on the project. Since we failed to obtain a complete list of addresses of the Turkish population from the municipal office due to time-consuming legal issues, we distributed the brochures through Turkish shops, barbers and community centres. Much time was spent on reaching a fruitful collaboration with the mosques. Through the mediation of ‘Osmose/Elan’ and of very cooperative imams who considered health education on hepatitis as a task of the mosques we succeeded in organizing educational meetings on HBV/HCV in the mosques for all Turkish migrants, particularly FGM, since we had emphasized in the campaigning phase that FGM have the highest risk of being infected with HBV/HCV.
The information and screening phase
In total, 15 educational meetings about hepatitis were organized in community centres and mosques with 450 participants. Each time the documentary was shown sufficient time was allowed for questions. Thereafter, all participants were offered a blood screening test by our mobile laboratory team including a Turkish laboratory technician. Blood tests were also performed on other occasions such as at a bazaar in the mosque and at a GP's practice.
All participants of the educational meetings received a questionnaire enquiring how they became aware of the meeting, if they liked the meeting and whether they were willing to pass on the new knowledge to family members and friends and to distribute brochures. Whenever persons were tested, they were also asked to complete a consent form and a short questionnaire concerning demographic data (age, sex, country of birth) and risk factors they had been exposed to in the past. The following risk factors were listed: blood transfusion before 1992, drug use, dentist consultation, injection or surgery, circumcision, and tattoo/piercing. All these are highlighted risk factors within HCV campaigns in The Netherlands. We purposely did not include well known risk factors for sexual transmission for hepatitis B infection as explained in the ‘preparation phase’.
Participants were tested for HBsAg, antibodies to hepatitis B core antigen (anti-HBc), and antibodies to hepatitis C virus (anti-HCV). If anti-HBc was positive and HBsAg negative, antibodies to hepatitis B surface antigen (anti-HBs) were determined from the same blood sample; if anti-HBs was negative, the participant was asked to give extra blood for an HBV DNA test, alternatively the GP could test the immunological memory of the participant by giving an extra HBV vaccination and later test for anti-HBs again. Patients with absence of infection (HBsAg and anti-HBc negative) or signs of resolved infection (anti-HBc and anti-HBs positive) were directly informed by a letter with a fax copy sent to their GP. However, patients who were HBsAg positive, or solely anti-HBc positive and/or positive for antibodies against HCV were informed by their GP who received the result from us together with a flow chart on how to proceed further based on the national treatment standard for GPs regarding ‘Viral hepatitis and other liver diseases’ [Reference Bouma8]. Whenever the laboratory found positive results for possible active HBV or HCV, the municipal health service of Arnhem was directly informed to initiate source and contact screening. At the hospital we opened an extra outpatient clinic in order to see all referred patients within 2 weeks.
The post-screening and clinical evaluation phase
The counselling service continued for several months after the screening phase was finished and the interactive website remained open. All patients with a positive test result were offered further outpatient evaluation in the hospital to assess the activity of the hepatitis, complications of the disease and need for treatment.
Prevalence of hepatitis B and C derived from hospital records
Since 1990, our hospital has been the only hospital in Arnhem. All referred patients with viral hepatitis and liver disease are seen by medical specialists as in- or outpatients in that hospital. We were interested to discover how many migrants from Turkey have been registered in our hospital since 1990 with a diagnosis of hepatitis B or C and if there was overlap with the patients found in the screening project. Therefore, we searched all patient records for Turkish migrants with HBV and/or HCV.
RESULTS
In total, 709 persons were tested during our screening project. Since 62 participants did not complete the part of the questionnaire on demographic data and risk factors, a complete dataset was available for 647 persons, of whom 430 (67%) had attended one of the educational meetings. Approximately only 20 (4%) of the 450 persons present at the educational meetings did not go for testing, for reasons unknown. In total, 544 FGM (59% women, mean age 46·96 years) and 103 second-generation migrants (62% women, mean age 22·45 years) were tested. Table 1 illustrates that participation was highest in FGM, the elderly, and in women rather than in men. There was no second-generation participant aged >50 years. Eighteen participants were HBsAg positive, of whom 17 were first- and one second-generation migrants, respectively. We found no infection in the <25 years age group. None of the 25 patients with isolated anti-HBc positivity was found to have active HBV (in 18 patients HBV DNA was not detectable and seven had positive anti-HBs after booster vaccination). We also analysed the prevalence of anti-HBc as a marker for past or current infection in relation to age and generation status (Fig. 1). In FGM the prevalence of anti-HBc was 6·7%, 30·2% and 57·7% for the age groups <25 years, 25–50 years and >50 years, respectively. This was much higher than in second-generation migrants (2·8% and 6·5% for the first two age groups). Only two patients (both FGM, one a 73-year-old male, and one a 42-year-old female) were found to be HCV-positive, both with active infections (HCV RNA positive) but not co-infected with HBV. Only one (50%) of the two patients with HCV infection, and seven (41%) of the 17 HBsAg-positive patients reported one of the listed risk criteria. Of patients with laboratory features of past hepatitis B infection (anti-HBc positive, HBsAg negative) 30% (111/370) had at least one of these risk criteria for transmission and 70% (259/370) had not. This was not significantly different for the total group of participants including those without infection (35·7% reported a risk factor and 64·3% did not). The most frequent risk factors named by the participants were circumcision (23% in total, 58% men), dentist consultation or acupuncture in Turkey (21%), surgery or injection in Turkey (21%), followed by blood transfusion before 1992 (3%), tattoo/piercing (1·2%) and drug use (0·2%).
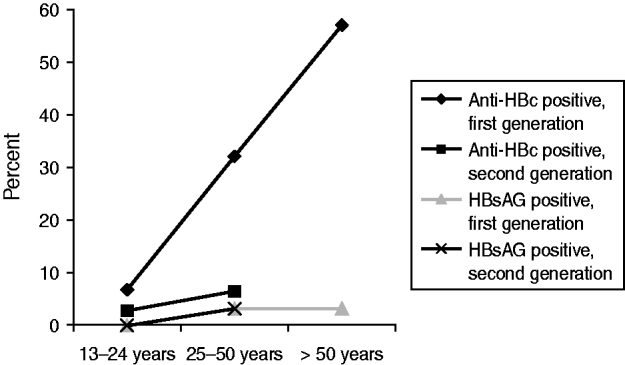
Fig. 1. Anti-HBc and HBsAg positivity rate in relation to age and generation status.
Table 1. Participants (n=647) in relation to the total Turkish population in Arnhem
Source: Arnhem Municipal Office, 2009.
One of the 17 FGM who tested positive for HBV had already tested positively in the past. So, the prevalence rate of newly detected HBV infections in FGM was 2·9% (16/544) related to all age groups and 3·0% (16/529) in those aged >24 years. Further clinical evaluation of the 18 patients with HBV detected through the screening programme revealed that all were HBeAg negative, none had evidence of liver cirrhosis on ultrasound (biopsy or fibroscan not performed) and six out of nine had a positive HBV DNA and are currently being followed up at the outpatient clinic. Nine patients with normal alanine aminotransferase (ALAT) levels had not yet been referred by the GP. One patient with active HCV had liver cirrhosis and treatment is planned. Contact tracing by the municipal health service has not yet revealed any unknown active infections in partners or within the family, but this is hampered by the fact that not all relatives live in the same region.
The exclusion of the one patient (from both the numerator and the denominator) who was in both the hospital and the screening list resulted in a HBV prevalence of 16/528=3·0% (95% CI 1·7–4·9) and in a HCV prevalence of 2/529=0·4% (95% CI 0·1–1·4) in those aged >24 years.
The additional questionnaire was completed by 229/450 (51%) participants with the following results: the majority were made aware of the meeting by family members or friends, the Mosque or brochures (36%, 25%, 17%, respectively); 97% considered the meeting good and understandable with useful information provided, 48% were willing to pass on the new knowledge to family members and friends and 14% to distribute brochures. Orally, they reported back that they were very satisfied regarding the information on hepatitis, the organization of the project, and that they felt they were taken seriously. Although not measured systematically, the impression of our Turkish collaborators of the study was that the stigma around hepatitis decreased during the study just by minimizing the role of sex on the acquisition of infection, since it is well known that hepatitis B in migrants is mainly acquired by vertical transmission or during early childhood.
The review of our hospital patient records established that all patients with HBV or HCV belonged to the FGM group and that all were aged >24 years. The denominator for the hospital prevalence estimate was therefore all Turkish FGM aged >24 years resident in Arnhem; these data was obtained from the municipal database. In Table 2 the numbers of Turkish FGM with active HBV or HCV related to the total Turkish FGM and sex are presented. In total we identified 28 patients with HBV and four others with HCV. All of these were FGM aged >24 years. HBV prevalence in Turkish FGM aged >24 years based on the hospital list was therefore 28/3915=0·7% (95% CI 0·5–1·0) and HCV prevalence was 4/3915=0·1% (95% CI 0·0–0·3).
Table 2. Hospital records from 1990 to 2008: first-generation migrants (FGM) from Turkey with active HBV or HCV in relation to total Turkish FGM

Source: Arnhem municipal database, 2009.
DISCUSSION
In the screening project we demonstrated that 3·4% of participating Turkish FGM aged >24 years in the city of Arnhem have either active HBV (3·0%) or HCV infection (0·4%). In the hospital records from 1990 to 2008 we revealed another 0·7% of FGM from Turkey with HBV and 0·1% with HCV when related to all FGM from Turkey living in Arnhem aged >24 years. All except one patient found in the screening project were new patients. We also found that the transmission routes for HCV that are named in the different HCV campaigns in The Netherlands did not play a role in the majority of patients with active HBV or HCV since only one of the two patients with HCV and 7/17 patients with HBV could remember any of the listed risk criteria. For HBV this is not surprising since we believe that the strongest risk factor for HBV infection for Turkish FGM is vertical transmission and transmission during childhood.
In a population-based study in Amsterdam, a HBsAg seroprevalence rate of 4·8% for FGM from Turkey was found in 2004 [Reference Baaten9]. We are now awaiting the results of the Dutch nationwide seroprevalence Pienter-2 study which has included migrants from Turkey and other countries as well. However, our prevalence data remain an estimation, since only a minority of the migrants were tested and therefore an under- or overestimation cannot be excluded. Moreover, it was not possible to discover how many patients with HBV were already known to GPs without referral to the hospital. The prevalence rates known from Turkey differ considerably due to patient selection and region studied. In recent epidemiological surveys in Turkey in: blood donors in Istanbul, pregnant women in Ankara, elderly residents of nursing homes in Ankara, and in a population-based study in the province of Tokat in the Black Sea region HBsAg seroprevalences of 1·76%, 2·8%, 11·9% and 5·5% and anti-HCV seroprevalences of 0·07%, 0·1%, 2·5% and 2·1%, respectively, were found [Reference Acar10–Reference Yildrim13].
The screening project was greatly appreciated in the Turkish population showing that the cultural, tailored approach of collaborating closely from the beginning with experienced Turkish advisors and GPs was crucial. We believe that the method used of approaching, informing and testing Turkish migrants could be easily applied elsewhere. In Rotterdam, socio-cultural determinants for hepatitis B screening in the Turkish–Dutch population are currently being studied more extensively [Reference van der Veen14]. However, the question arises whether extending this kind of cumbersome screening project to other parts of The Netherlands is really necessary, since it appears unlikely that major regional differences of infection rates among FGM with HBV and/or HCV will be found. The same applies for the other large immigrant groups from countries such as Suriname, Morocco, Indonesia, China, Vietnam, Egypt and sub-Saharan Africa. It is likely, that FGM of these middle or high endemic countries have a similar prevalence of chronic hepatitis B or C infection to that found in their country of origin. We therefore believe that time has already arrived for a general screening policy for all FGM from these countries. Since active double infections of HBV and HCV are rare, screening should always be done for both infections, at least until more prevalence data of migrants are known. Rather than performing more costly and time-consuming screening projects, a national public health strategy should be developed on how general screening for this vulnerable group can be integrated into the health system without creating unnecessary costs. The potential benefit for this strategy is obvious for the individual by preventing liver complications through early detection and if necessary treatment, and for public health by reducing spread of infection and for the economy by reducing costs for hospital care and liver transplantations. Moreover, public health epidemiologists argue for the urgent need of new strategies for secondary prevention of viral hepatitis in migrants [Reference Hahné15]. In the USA, the Center for Disease Control and Prevention (CDC) officially recommends HBsAg testing to identify HBV infection for foreign-born persons from areas with HBsAg prevalence of ⩾2%. In 31 community-based programmes more than 21 817 individuals were screened with an average HBsAg prevalence of 8·1% [Reference Rein16]. As mentioned earlier, a cost-effectiveness analysis for screening and early treatment of migrants for hepatitis B at a given prevalence of 3·35% has shown favourable results [Reference Veldhuijzen7].
ACKNOWLEDGEMENTS
We thank Gilead Sciences, Schering Plough, and the Foundation of Internists and Microbiologists of Rijnstate Hospital for their financial contributions; Dr G. W. M. Haverkamp for helping with the design of the study; all collaborators of Osmose/Elan who participated in the study; T. Berendsen, N. Eker and others from the laboratory of clinical chemistry who assisted with drawing blood samples and S. Hahné for her advice on the analysis and manuscript.
DECLARATION OF INTEREST
None.





