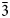Introduction
The palmierite-type structure is characteristic of a wide group of compounds that includes minerals and synthetic materials (Zachariasen, Reference Zachariasen1948; Süsse and Buerger, Reference Süsse and Buerger1970; Sugiyama and Tokonami, Reference Sugiyama and Tokonami1990; Tissot et al., Reference Tissot, Rodriguez, Sipola and Voigt2001; Thompson et al., Reference Thompson, Xie, Zhai, Downs and Yang2013; Tsyrenova et al., Reference Tsyrenova, Pavlova, Solodovnikov, Popova, Kardash, Stefanovich, Gudkova, Solodovnikova and Lazoryak2016; Bismayer et al., Reference Bismayer, Mihailova and Angel2017; Kemp et al., Reference Kemp, Rushton, Horstwood and Nénert2018). In the literature on synthetic compounds with the palmierite-type structure, the general formula is presented in two different ways: M 3(TO4)2 (Grzechnik and McMillan, Reference Grzechnik and McMillan1997; Thompson et al., Reference Thompson, Xie, Zhai, Downs and Yang2013) and M1M22(TO4)2 (Moore, Reference Moore1973; Tsyrenova et al., Reference Tsyrenova, Pavlova, Solodovnikov, Popova, Kardash, Stefanovich, Gudkova, Solodovnikova and Lazoryak2016), where M = Ba2+, Sr2+, Ca2+, Pb2+, Rb+, K+, Na+, NH4+, Tl+ and REE3+, and T = V5+, As5+, P5+, S6+, Cr6+, Se6+, Mo6+ and W6+. Moreover, this type of structure is also noted for materials with the formula A 5R(MoO4)4 (A+ = K, Rb and Tl; R3+ = REE, Y, Bi, Fe and In)(Tsyrenova et al., Reference Tsyrenova, Pavlova, Solodovnikov, Popova, Kardash, Stefanovich, Gudkova, Solodovnikova and Lazoryak2016). Currently, the inorganic crystal structure database (ICSD, https://icsd.products.fiz-karlsruhe.de/) lists ~100 compounds with the same structure type and, depending on the chemical composition, these palmierite-type materials exhibit various optical, luminescence, ferroelectric and catalytic properties (Lagos, Reference Lagos1970; Grzechnik and McMillan, Reference Grzechnik and McMillan1997; Mugavero et al., Reference Mugavero, Bharathy, McAlum and zur Loye2008; Thompson et al., Reference Thompson, Xie, Zhai, Downs and Yang2013; Tong et al., Reference Tong, Liang, Yan, Wang and Li2015). Some compounds, due to the crystal structure flexibility, can accommodate large ion lithophile elements (LILE) and different luminescent ions, mainly rare-earth elements (REE) (Lagos, Reference Lagos1970; Murayama et al., Reference Murayama, Nakai, Kato and Kumazawa1986; Sugiyama and Tokonami, Reference Sugiyama and Tokonami1987; Xie et al., Reference Xie, Minitti, Chen, Mao, Wang, Shu and Fei2004; Thompson et al., Reference Thompson, Xie, Zhai, Downs and Yang2013; Cao et al., Reference Cao, Yu, Sun, Cao and Qiu2014; Tong et al., Reference Tong, Liang, Yan, Wang and Li2015).
Most palmierite-type materials, mainly high-temperature compounds, are trigonal (usually described in a rhombohedral unit cell), isostructural and crystallise in space group R $\bar{3}$![]() m (no. 166). The crystal structure comprises two non-equivalent M-cation sites and a tetrahedrally coordinated T site, which form a three-dimensional anionic framework (Moore, Reference Moore1973; Sugiyama and Tokonami, Reference Sugiyama and Tokonami1987; Azdouz et al., Reference Azdouz, Manoun, Essehli, Azrour, Bih, Benmokhtar, Hou and Lazor2010). However, there are a few monoclinic compounds with С2/c space group, mainly molybdates (Tsyrenova et al., Reference Tsyrenova, Pavlova, Solodovnikov, Popova, Kardash, Stefanovich, Gudkova, Solodovnikova and Lazoryak2016). The symmetry reduction (transition) from trigonal to monoclinic is related to the structure distortion, mostly noticed through the shifts of M-cations and (PO4)3- anions from the special position on three-fold axes (Tsyrenova et al., Reference Tsyrenova, Pavlova, Solodovnikov, Popova, Kardash, Stefanovich, Gudkova, Solodovnikova and Lazoryak2016).
m (no. 166). The crystal structure comprises two non-equivalent M-cation sites and a tetrahedrally coordinated T site, which form a three-dimensional anionic framework (Moore, Reference Moore1973; Sugiyama and Tokonami, Reference Sugiyama and Tokonami1987; Azdouz et al., Reference Azdouz, Manoun, Essehli, Azrour, Bih, Benmokhtar, Hou and Lazor2010). However, there are a few monoclinic compounds with С2/c space group, mainly molybdates (Tsyrenova et al., Reference Tsyrenova, Pavlova, Solodovnikov, Popova, Kardash, Stefanovich, Gudkova, Solodovnikova and Lazoryak2016). The symmetry reduction (transition) from trigonal to monoclinic is related to the structure distortion, mostly noticed through the shifts of M-cations and (PO4)3- anions from the special position on three-fold axes (Tsyrenova et al., Reference Tsyrenova, Pavlova, Solodovnikov, Popova, Kardash, Stefanovich, Gudkova, Solodovnikova and Lazoryak2016).
The present work describes the nomenclature and classification of the palmierite supergroup approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA-CNMNC Proposal 22-L, Bosi et al., Reference Bosi, Hatert, Pasero and Mills2023). The proposal to the IMA and the requirement for developing the palmierite supergroup classification are related to the discovery of a new mineral, mazorite Ba3(PO4)2 (IMA2022–022; Juroszek et al., Reference Juroszek, Galuskina, Krüger, Krüger, Vapnik, Kahlenberg and Galuskin2023). The approval of the nomenclature and the classification of the palmierite supergroup allows us to not only classify the five minerals into one structural supergroup, but also to formally use the name already firmly established in the literature.
Nomenclature and classification of the palmierite supergroup
The general crystal-chemical formula for the palmierite-supergroup minerals is XIIM1XM22(IVTO4)2 (Z = 3), where the left superscripts (Roman numerals) indicate the ideal coordination numbers. The M sites can be occupied by K, Na, Ca, Sr, Ba, Sr, Pb, Rb, Zn, Tl, Cs, Bi, NH4 and REE with 1+, 2+ and 3+ charges. In turn, the T site can be occupied by Si, P, V, As, S, Se, Mo, Cr and W with 4+, 5+ and 6+ charges. The palmierite supergroup includes five mineral species, defined chemically as sulfates, phosphates and vanadates (Table 1). According to the data on synthetic analogues and their structure type, this supergroup may also include chromates, molybdates, selenates, arsenates and wolframates (Morris et al., Reference Morris, McMurdie, Evans, Paretzkin, de Groot, Newberry, Hubbard and Carmel1977; Chance and Loye, Reference Chance and Loye2013; Tsyrenova et al., Reference Tsyrenova, Pavlova, Solodovnikov, Popova, Kardash, Stefanovich, Gudkova, Solodovnikova and Lazoryak2016; Bismayer et al., Reference Bismayer, Mihailova and Angel2017; Smith et al., Reference Smith, de Zoete, Rutten, van Eijck, Griveau and Colineau2020).
Table 1. Minerals of the palmierite supergroup and their synthetic counterparts.

The heterovalent isomorphic substitution scheme M ++T 6+ ↔ M 2++T 5+ implies many possible combinations of different ions and, thus, of potentially new mineral species in the palmierite supergroup. Moreover, such a scheme allows us to distinguish two groups on the basis of crystal-chemical arguments: the palmierite group M12+M22+(T 6+O4)2 for the sulfates; and the tuite group M12+M222+(T 5+O4)2 for phosphates/vanadates. At this moment, the palmierite supergroup includes five mineral species: palmierite K2Pb(SO4)2, kalistrontite K2Sr(SO4)2, tuite Ca3(PO4)2, gurimite Ba3(VO4)2 and mazorite Ba3(PO4)2.
According to the IMA-CNMNC rules (Mills et al., Reference Mills, Hatert, Nickel and Ferraris2009), the name of the established mineral supergroup should originate from palmierite, which was described as the first mineral species in this supergroup (Lacroix, Reference Lacroix1907; Zambonini, Reference Zambonini1921). Moreover, the term 'palmierite-type structure' is commonly used within the literature on various synthetic compounds (Thompson et al., Reference Thompson, Xie, Zhai, Downs and Yang2013; Tsyrenova et al., Reference Tsyrenova, Pavlova, Solodovnikov, Popova, Kardash, Stefanovich, Gudkova, Solodovnikova and Lazoryak2016; Bismayer et al., Reference Bismayer, Mihailova and Angel2017).
Background information of the palmierite-supergroup members
Palmierite group:
Palmierite K2Pb(SO4)2, is a rare fumarolic mineral that occurs as a result of volcanic eruptions, described for the first time by Lacroix (Reference Lacroix1907) at Mount Vesuvius in Italy. Using synthetic crystals, Zambonini (Reference Zambonini1921) performed more detailed analyses and redefined the composition of palmierite. The same material was also used for microscopic and X-ray studies (Bellanca, Reference Bellanca1946). Later, palmierite was synthesised using thermal and aqueous methods (von Schwarz, Reference Von Schwarz1966). Morris et al. (Reference Morris, McMurdie, Evans, Paretzkin, de Groot, Newberry, Hubbard and Carmel1977) and Tissot et al. (Reference Tissot, Rodriguez, Sipola and Voigt2001) obtained the experimental powder X-ray diffraction patterns. To the best of our knowledge, there is no structural data for natural palmierite. As a rare mineral, palmierite has been found in only a few localities around the world. At the Satsuma-Iwojima volcano in Japan, it occurs as a natural fumarolic sublimate (Africano et al., Reference Africano, Van Rompaey, Bernard and Le Guern2002). At the Tolbachik volcano (Kamchatka Peninsula, Russia), it was detected in association with the new mineral cupromolybdite Cu3O(MoO4)2 (Zelenski et al., Reference Zelenski, Zubkova, Pekov, Polekhovsky and Pushcharovsky2012).
Kalistrontite K2Sr(SO4)2, the Sr-analogue of palmierite, was first described from the Lower Permian evaporites near the village of Alshtan in Bashkiria, Russia, where it formed as a result of the reaction of sylvite from anhydrite layers with Sr-bearing solutions (Voronova, Reference Voronova1962). Worldwide occurrences of kalistrontite are related to the Permo–Triassic, Neogene, or younger sedimentary evaporite deposits in Germany, Ukraine, China, Namibia, Israel and Turkey (Bader and Boehm, Reference Bader and Boehm1966; Griniv et al., Reference Griniv, Iorysh and Skul'skaya1986; Min, Reference Min1987; Mees, Reference Mees1999; García-Veigas et al., Reference García-Veigas, Rosell, Zak, Playà, Ayora and Starinsky2009, Reference García-Veigas, Rosell, Ortí, Gündoğan and Helvacı2011). In contrast to the mentioned localities, in Italy kalistrontite occurs within the Pleistocene geothermal field at Latium (Maras, Reference Maras1979). Kalistrontite usually forms due to alteration of primary evaporite minerals or as an early diagenetic precipitate from high K- and SO4-rich brines (Kemp et al., Reference Kemp, Rushton, Horstwood and Nénert2018). Except for the first description, the most detailed mineralogical characterisation of kalistrontite was done on a sample from the Permian polyhalite-bearing evaporite deposits in North Yorkshire, UK (Kemp et al., Reference Kemp, Rushton, Horstwood and Nénert2018). These authors provided results of the structure analyses from powder X-ray diffraction, as well as chemical, thermal and isotopic data. Moreover, the Raman spectrum of kalistrontite was presented in this work for the first time.
Tuite group:
Tuite γ-Ca3(PO4)2, is a high-pressure polymorph of β-Ca3(PO4)2 (Xie et al., Reference Xie, Minitti, Chen, Mao, Wang, Shu and Fei2004). The transition from the β to γ phase occurs at 1000°C and 25 GPa (Murayama et al., Reference Murayama, Nakai, Kato and Kumazawa1986). Additionally, two more polymorphs of Ca3(PO4)2 exist, α and α', which are stable at high-temperature conditions (Sugiyama and Tokonami, Reference Sugiyama and Tokonami1987; Xie et al., Reference Xie, Minitti, Chen, Mao, Wang, Shu and Fei2004; Thompson et al., Reference Thompson, Xie, Zhai, Downs and Yang2013; Zhai et al., Reference Zhai, Yamazaki, Xue, Ye, Xu, Shan, Ito, Yoneda, Yoshino, Guo, Shimojuku, Tsujino and Funakoshi2013). The γ-Ca3(PO4)2 phase was first obtained as a product of apatite decomposition (Murayama et al., Reference Murayama, Nakai, Kato and Kumazawa1986), and such a process is usually used to obtain tuite in synthesis experiments (Zhai et al., Reference Zhai, Yamazaki, Xue, Ye, Xu, Shan, Ito, Yoneda, Yoshino, Guo, Shimojuku, Tsujino and Funakoshi2013). Natural tuite was discovered in association with ringwoodite, majorite and hollandite in a shocked vein of the Suizhou L6 chondrite (Xie et al., Reference Xie, Minitti, Chen, Mao, Wang, Shu and Fei2002, Reference Xie, Minitti, Chen, Mao, Wang, Shu and Fei2004). Its formation conditions were specified to be up to 23 GPa and 2000°C (Xie et al., Reference Xie, Minitti, Chen, Mao, Wang, Shu and Fei2004). So far, tuite has been detected only in extraterrestrial rock samples (meteorites). The γ-Ca3(PO4)2 phase, due to the crystal structure and the presence of large, high-coordination cation sites, can accommodate REE and LILE elements, such as Sr and Ba, under P–T conditions of the upper mantle (Murayama et al., Reference Murayama, Nakai, Kato and Kumazawa1986; Sugiyama and Tokonami, Reference Sugiyama and Tokonami1987; Xie et al., Reference Xie, Minitti, Chen, Mao, Wang, Shu and Fei2004; Zhai et al., Reference Zhai, Yamazaki, Xue, Ye, Xu, Shan, Ito, Yoneda, Yoshino, Guo, Shimojuku, Tsujino and Funakoshi2013; Skelton and Walker, Reference Skelton and Walker2017).
Gurimite Ba3(VO4)2, is an alkaline earth metal orthovanadate found only in natural outcrops in the pyrometamorphic rock of the Hatrurim Complex in Israel (Galuskina et al., Reference Galuskina, Galuskin, Vapnik, Prusik, Stasiak, Dzierżanowski and Murashko2017; Krzątała et al., Reference Krzątała, Krüger, Galuskina, Vapnik and Galuskin2020). Its formation is related to the crystallisation from residual melt enriched in incompatible elements that fill interstices between the main rock-forming minerals of paralava (Galuskina et al., Reference Galuskina, Galuskin, Vapnik, Prusik, Stasiak, Dzierżanowski and Murashko2017). The crystal structure of synthetic Ba orthovanadate was investigated in detail (Süsse and Buerger, Reference Süsse and Buerger1970; Morris et al., Reference Morris, McMurdie, Evans, Paretzkin, de Groot, Newberry, Hubbard and Carmel1977; Mugavero et al., Reference Mugavero, Bharathy, McAlum and zur Loye2008; Azdouz et al., Reference Azdouz, Manoun, Essehli, Azrour, Bih, Benmokhtar, Hou and Lazor2010), whereas for its natural counterpart enriched in P5+, the single-crystal X-ray diffraction investigation was carried out only recently (Juroszek et al., Reference Juroszek, Galuskina, Krüger, Krüger, Vapnik, Kahlenberg and Galuskin2023). Previously, only electron back-scatter diffraction (EBSD) data were available (Galuskina et al., Reference Galuskina, Galuskin, Vapnik, Prusik, Stasiak, Dzierżanowski and Murashko2017). Synthetic Ba3(VO4)2 and their Sr-analogue have been analysed extensively due to their optical and ferroelectric properties, which indicated that such compounds could be used as luminophores, host material for lasers or television tubes (Merkle et al., Reference Merkle, Pinto, Verdún and McIntosh1992; Grzechnik and McMillan, Reference Grzechnik and McMillan1997; Mugavero et al., Reference Mugavero, Bharathy, McAlum and zur Loye2008; Azdouz et al., Reference Azdouz, Manoun, Essehli, Azrour, Bih, Benmokhtar, Hou and Lazor2010).
Mazorite Ba3(PO4)2, the P-analogue of gurimite, is a new mineral that was recently found as an accessory phase in coarse-grained gehlenite–rankinite paralava in the Hatrurim Complex in Israel (IMA2022–022; Juroszek et al., Reference Juroszek, Galuskina, Krüger, Krüger, Vapnik, Kahlenberg and Galuskin2023). The similarity of crystallisation conditions between mazorite and gurimite, as well as the relationship with the associated Ba-bearing minerals like celsian, hexacelsian, walstromite, fresnoite, zadovite and barioferrite, in both cases, confirm the high-temperature formation of this phase. Moreover, mazorite was also detected in a carbonate–silicate xenolith from the Bellerberg volcano area in Germany, where it occurs as a small inclusion (<15 μm) inside the bennesherite crystal (Juroszek and Ternes, Reference Juroszek and Ternes2022). Synthetic mazorite doped with various metals and rare earth elements is an important phosphor material characterised by colour purity and good luminescence efficiency (Tāle et al., Reference Tāle, Kūlis and Kronghauz1979; Mu and He, Reference Mu and He2012; Tong et al., Reference Tong, Liang, Yan, Wang and Li2015).
Crystal structure of palmierite-supergroup minerals
Minerals and synthetic compounds with the palmierite-type structure crystallise in space group R $\bar{3}$![]() m (no. 166) (Table 1). Generally, the crystal structure of the palmierite-supergroup minerals consists of a three-dimensional framework constructed of cations at M1, M2 and T sites (Fig. 1a). Two large metal and symmetrically non-equivalent M sites are distinguished within the palmierite-type structure. The M1 atom, located at a 3a Wyckoff position with $\bar{3}$
m (no. 166) (Table 1). Generally, the crystal structure of the palmierite-supergroup minerals consists of a three-dimensional framework constructed of cations at M1, M2 and T sites (Fig. 1a). Two large metal and symmetrically non-equivalent M sites are distinguished within the palmierite-type structure. The M1 atom, located at a 3a Wyckoff position with $\bar{3}$![]() m site symmetry, is coordinated by twelve oxygen atoms. These M1O12 polyhedra are linked by edges to each other and form layers perpendicular to c (Fig. 1b). In turn, the M2 atom placed at a 6m Wyckoff position with 3m point symmetry is ten-coordinated. The M2O10 polyhedra share square faces and corners and form layers perpendicular to c (Fig. 1c). The T atom in the palmierite-type structure is tetrahedrally coordinated by oxygen atoms. The TO4 tetrahedra share edges and corners with M1O12 and M2O10 polyhedra (Fig. 1a–c).
m site symmetry, is coordinated by twelve oxygen atoms. These M1O12 polyhedra are linked by edges to each other and form layers perpendicular to c (Fig. 1b). In turn, the M2 atom placed at a 6m Wyckoff position with 3m point symmetry is ten-coordinated. The M2O10 polyhedra share square faces and corners and form layers perpendicular to c (Fig. 1c). The T atom in the palmierite-type structure is tetrahedrally coordinated by oxygen atoms. The TO4 tetrahedra share edges and corners with M1O12 and M2O10 polyhedra (Fig. 1a–c).

Figure 1. (a) The general view of the palmierite-type structure along (010) consists of a three-dimensional framework constructed of cations at M1, M2 and T sites. (b) The M1O12 polyhedra are linked by edges to each other and form layers along (010). (c) The M2O10 polyhedra share square faces and corners and form a layer arrangement along the b axis. (d) The interconnected polyhedral sequence TO4–M2O10–M1O12–M2O10–TO4 present in the palmierite-type structure and the linkage scheme of polyhedral cations and surrounded oxygen atoms.
A characteristic feature of the palmierite-type structure is the translationally interconnected sequence of polyhedra TO4–M2O10–M1O12–M2O10–TO4 along the direction of the c axis (Fig. 1d) (Moore, Reference Moore1973; Sugiyama and Tokonami, Reference Sugiyama and Tokonami1987). This so-called ‘columnar arrangement’ shows that the M1O12 polyhedron shares two triangular faces, formed via O2 oxygen atoms from both sides, with two M2O10 polyhedra (up and down). The remaining six O1 atoms are located in the same plane around M1. It should be emphasised that the six M1–O1 bond lengths are notably longer than the other six M1–O2 bonds (Fig. 1d). In the M2O10 polyhedra, the six M2–O2 bonds form a hexagonal arrangement in the plane around the M2 atom, three M2–O2 derive from the triangular faces shared with M1O12 polyhedra, and one M2–O1 is a bridging oxygen between M2O10 polyhedra and TO4 tetrahedra (Fig. 1d). The TO4 tetrahedra show one shorter T–O1 and three longer T–O2 bond lengths.
Comment on the palmierite supergroup
Minerals of the palmierite supergroup with the approved general crystal-chemical formula XIIM1XM22(IVTO4)2 are divided into two groups based mainly on the heterovalent isomorphic substitution scheme M ++T 6+ ↔ M 2++T 5+. The palmierite group M12+M22+(T 6+O4)2 includes trigonal sulfates, which contain different prevailing (species-defining) cations at the M1 and M2 sites. In turn, the tuite group M12+M222+(T 5+O4)2 comprises trigonal phosphates and vanadates characterised by the same prevailing (species-defining) cations at both the M1 and M2 sites. The overall cation charge based on possible cation site occupancies equals 16 in both groups. However, there is no principle rule concerning the occupation of these sites by specific cations. This may trigger problems determining the crystal-chemical formulae of possible new supergroup members. The following cations, S6+, Mo6+, Cr6+, Se6+, W6+, P5+, V5+, As5+ and Si4+, are expected to be allocated at the tetrahedrally coordinated T site. All remaining cations will occupy the polyhedral M sites. According to the general crystal-chemical consideration and relation to other mineral supergroups, e.g. the apatite supergroup (Pasero et al., Reference Pasero, Kampf, Ferraris, Pekov, Rakovan and White2010), we assume that the M sites will be filled with cations in order of increasing ionic radius, with smaller cations such as Ca2+ at the M1 site, and larger cations such us Ba2+ and K+ at the M2 site. This assumption is valid for the members of the palmierite group. In the tuite group, the same cation may occupy both M sites. This indicates that the precise evaluation of the electron density at each site is required, and a structural investigation should be mandatory.
To summarise, there is a need to perform a structural study of potential new members of the palmierite supergroup because the abundance of different ions, which may be hosted primarily at the M1, M2, and also T crystal sites, and the isostructural relation between the natural samples and synthetic materials, suggests that many more minerals and isomorphic series could exist within this supergroup.
Acknowledgements
The authors thank three anonymous reviewers for their helpful and constructive comments, which allowed them to improve a previous version of the manuscript.
Competing interests
The authors declare none.