Introduction
Artemia (brine shrimp, “sea monkeys”) is a generic group of small marine crustaceans including several species of similar size and morphology [Reference Abatzopoulos1]. Artemia salina is the most popular species of this sort, first found on the coast of England and described by Carl Linnaeus in his Systema Naturae in 1758 [website a]. In the USA, two species are ubiquitous: Artemia franciscana [website b] and Artemia gracilis (so-called “New England brine shrimp”) [Reference Abatzopoulos1]. These organisms are industrially cultivated and exported all over the world; they are used for feeding fish and shrimp in aquaculture farms and are distributed in pet shops and toy shops. In the latter case, Artemia is provided as a study object in experiment sets for children. Figure 1 is typical of such science-based toys.

Figure 1 Typical outfits of commercial Artemia sets are available as experimental toys for young students. German slogans are translated into English.
This article provides instructions for culturing Artemia, followed by an overview of the various phases of development that can be observed in Artemia franciscana as obtained from a commercial experiment “toy” set. As Artemia is sized from 0.5 mm up to 15 mm, it is well suited for both macroscopic and microscopic observation, and successive growth processes and morphological differentiation can be demonstrated easily to young students. Finally, some experiments are suggested that can be used to introduce young people to elementary environmental and ecological research and to the principles of statistics and scientific workflows.
Material and methods
Commercial experiment sets, such as the “brine shrimp farm” distributed by the “Franckh-Kosmos” Company in Germany and “Thames & Kosmos, LLC” in the USA (Figure 1), are readily available worldwide. In the work presented here, the “Komos” set containing Artemia franciscana was used for daily observations of living Artemia over a period of 35 days, from the first day of hatching up to the day of the last surviving animal’s death. Larvae and adult individuals were observed and photographed in bright-field and dark-field light microscopy, and written notes were taken describing daily observations of the Artemia development. All photomicrographs and written notes were taken by an eleven-year-old boy as part of a middle school class, thus showing that commercial sets for breeding and examining Artemia can be managed well even by rather young children who are interested in exploring nature.
Breeding Artemia
Commercial sets for “young explorers and scientists” contain all the equipment needed for a start-up culture: a small aquarium holding about 250 ml, an appropriate portion of sea salt, dried algae for feeding, a little bit of sand or small stones, and a supply of Artemia cysts. Of course, one could purchase these components from pet shops instead of using a starter set sold as a toy.
The aquarium should be illuminated with a 60-watt incandescent bulb (about 800 lumens) or an equivalent LED (9–12 watt, 800 lumens, 2700 K). The water temperature should be near 25 °C (77 °F), while the pH-value can range from 6.5–8.0 [Reference Dhont and Lavens2]. About 30 g sea salt (or common salt) should be added to one liter of fresh water so that the resulting density is about 1.022 g/cm3. The bottom of the aquarium should be covered with a thin layer of sand or small stones.
When Artemia cysts are put into the salt water, young larvae (nauplii) will hatch out within 1–2 days and are immediately visible with the naked eye as small motile “dots.” For the first 3 days the nauplii can live off their yolk sac. After this period dried algae (Spirulina powder, for instance) should be given at 2-day intervals, and small portions of baker´s yeast can be added once per week. Artemia is sensitive to poor water quality, so water exchanges are necessary if the water becomes cloudy.
Reproduction and maturation of Artemia
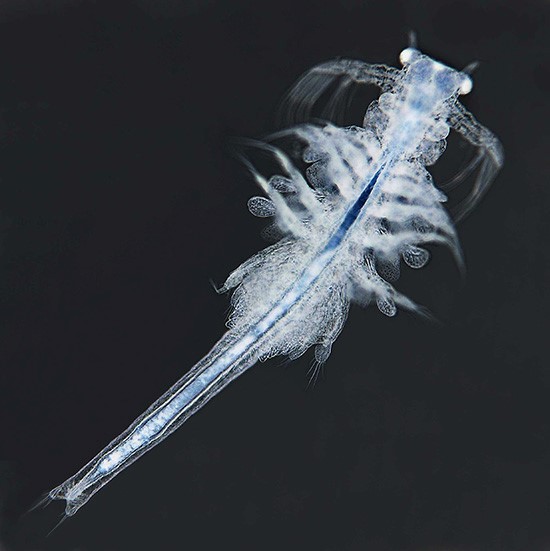
Artemia propagates by parthenogenesis or sexual reproduction. In both cases, embryos remain within the adult female brood sac for a period of about 14 days. With proper environmental conditions, young larvae leave their brood sac and grow to adult individuals within 4–5 weeks. In poor environmental conditions, however, embryos become encapsulated and can survive within cysts through long periods of dryness and heat. When Artemia cysts are surrounded by salt water, young larvae will appear within 1–2 days; the length of these nauplii is typically 0.5 mm (phase 1 of development). For the first 3 days, while living off their yolk sac, fresh nauplii grow to a size of about 1.2 mm (phase 2, metanauplius stage). Over the next 10 days rudimentary thoracopods and primordial bilateral compound eyes develop (see Figure 2); the body size grows to 4–5 mm (phase 3, zoea stage). At the end of the third week, larvae have grown to 6–8 mm, and their shape is similar to adult animals (phase 4, pre-adult stage). Maturation is finished within 4–5 weeks; at this stage young adult individuals are about 10 mm and capable of reproduction (phase 5, adult stage). Within the following days or weeks body length can increase further up to a final size of 15 or 20 mm. In the final stage, Artemia shows a complex anatomy (Figure 2, modified from Ruppert et al., 2006 [Reference Ruppert3] and website [c]).
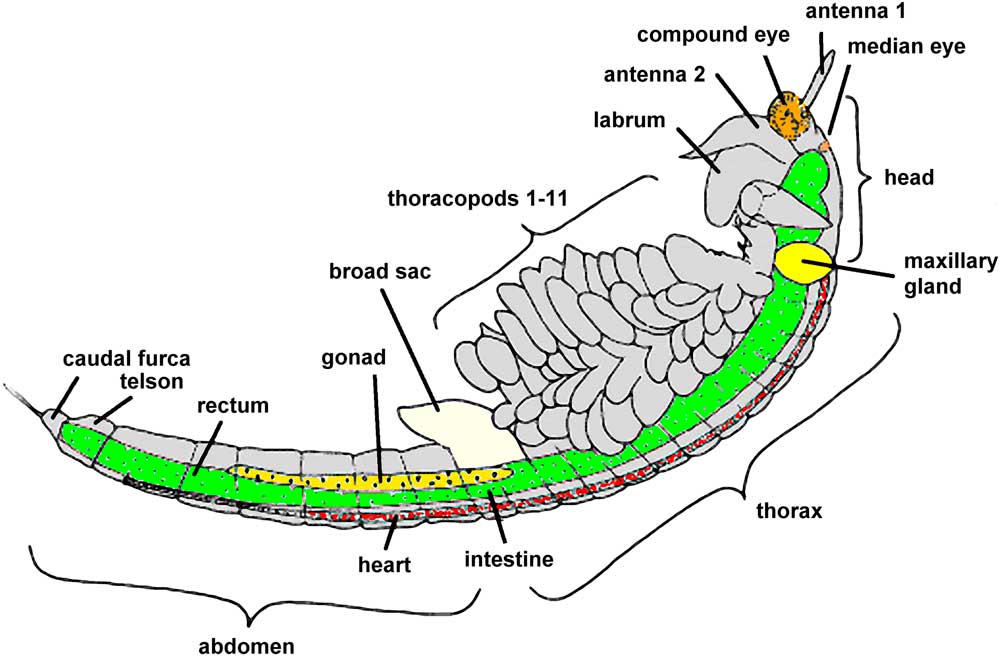
Figure 2 Anatomy of mature Artemia franciscana (modified from [Reference Ruppert3] and website [a]).
Observing Artemia
In all phases of differentiation and maturation, Artemia can be observed by placing the organisms on a slide with an adequate amount of salt water. Special microscope depression slides for examination of water organisms should be used; these have a cavity so that specimens can be observed within greater volumes of water than achievable with standard flat slides. Examinations have to be carried out without a cover slip so that the specimens can survive imaging procedures and returned without damage to the aquarium. A suitable student microscope should be configured for bright-field illumination and preferably be fitted with additional equipment for dark-field illumination. Instead of using a special dark-field condenser, an appropriately sized circular black plate can be mounted with the bright-field condenser or put onto the light outlet of the microscope´s stand so that the central part of the illuminating light beam is blocked. Alternatively, some bright-field condensers can be equipped with special light masks for dark-field mounted on sliders. Detailed instructions for upgrading student microscopes for darkfield (and phase contrast) are given in a previous article [Reference Piper4]. In all stages Artemia moves very quickly in horizontal and vertical directions so that an appropriate field of view and a large depth of field are the primary imaging requirements, whereas the numerical aperture (NA) and power of the objective lens are of less importance. Thus, low-magnification objective lenses (range of magnification: 2× to 4×) should be used. All microscope objectives used must be designed for long working distances.
Photgraphing Artemia
Photomicrographs must be taken with a flash in order to avoid blurring from specimen motion. Some flash devices are capable of automatic exposure by detecting the light through the lens, whereas in other models exposure can be regulated with a set of neutral gray filters or a couple of rotatable polarizing filters. Alternatively, video clips can be made with a digital camera, and still images can be extracted from the video files. Most student microscopes are not fitted with an integrated digital camera, so consumer cameras need to be adapted to the microscope. Several practical photographic hints, including handling of a flash, are given in a previous publication [Reference Piper5]. Figures 3a to 3d show practical examples of coupling of cameras to microscopes. Even smartphones or iPhones can be mounted to a monocular or binocular tube (Figure 3e). Smartphones are attractive tools for introducing photomicrography and cinematography since students are already familiar with the handling of such equipment.

Figure 3 Examples of camera-microscope setups. (a) Digital SLR camera fitted with a suitable lens adapted to a trinocular photo-tube with a photo eyepiece 10×. (b) Digital bridge camera with an integrated zoom lens mounted with a vario (zoom) photo ocular 5× to 12.5×. (c) Special microscope camera mounted with a photo eyepiece 10×. (d) Compact digital camera mounted with a monocular tube. (e) Smartphone-adapter (UNIKOP, manufactured by Immunocons Co., Germany) mounted with a stereo microscope. This adapter consists of a variable holder so that smartphones of different sizes can be used.
Microscopy studies of Artemia do not require special sophisticated equipment; even low-cost microscopes can be used for the first steps. By successive observations of growth and maturation, ideally from day to day, young students can comprehend all phases of “metamorphosis” from young larvae to adult individuals. Along with morphologic development, young “researchers” can also study the increasing variation and differentiation of locomotion patterns.
Results
Most of the Artemia cysts from the commercial experiment set were unimpaired, and the young nauplii hatched within about 36 hours. All individuals showed a high synchronicity of development and maturation so that their morphological appearance was rather uniform. Figure 4 shows the entire 35 days of maturation and morphological differentiation that was observed and photographed in the classroom.

Figure 4 Morphological differentiation of Artemia franciscana, from day 1 through day 35. The labeling of the single images indicates the age of the individual animals in days. Dark-field imaging with a low magnification lens (Pl 2.5/0.08), a zoom-ocular 5×–12.5×, and flash illumination (according to the arrangement shown in Figure 3b). The width of field changes as the organism grows. See Figure 10 for typical organism lengths through day 14. Colorization is modulated from bright gray to yellowish to reddish as the concentration of salt increases in the water.
The natural color of individuals is influenced by the quality of water. When the salt concentration is low, the utilization of oxygen is most effective. Over the course of several days some water may evaporate and the concentration of salt will increase in the remaining volume of water. At that point the utilization of oxygen is reduced so that the production of hemoglobin is intensified and the colorization of Artemia is shifted to red. Thus, red colorization is an indicator of hypoxemia. In this case an adequate proportion of fresh water should be added. These color shifts from gray to bronze to reddish can be seen in the image sequences of Figure 4.
Young larvae showed only a stereotypical movement similar to breast-stroke swimming (Figure 5a). At the beginning of the third phase of development (days 4–6), additional asynchrony patterns could be seen comparable with swimming the crawl (Figure 5b). In the course of phase 4, some larvae could be observed swimming as a couple showing synchronized formations of movement (Figure 6). Some days later, even looping, forward rolls, and other complex movement patterns could be observed (Figure 7). After a period of four weeks, only a few individuals survived, and on the 35th day of observation the last animal expired.
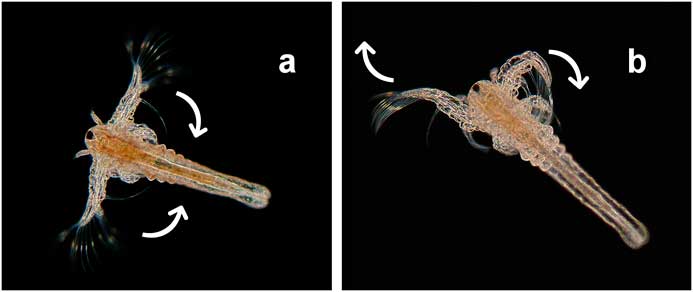
Figure 5 Synchronic movement patterns (a) similar to breast-stroke swimming (day 4), (b) asynchrony movement patterns comparable with swimming the crawl (day 6). Dark-field, objective Pl 2.5/0.08, flash.

Figure 6 Synchronized movements like couple swimming (day 19). Bright-field, objective Pl 2.5/0.08, flash.

Figure 7 Examples of various movement patterns in elderly individuals (days 26–32). Same equipment as Figure 4.
Digital image processing
Photomicrographs taken from Artemia were also used for introductions to digital image processing. In particular, bright-field images were digitally inverted by use of standard imaging software so that digital “dark-field” images could be obtained revealing fine details with an improved clarity. The appearance and fascinating appeal of such inverted images was similar to X-ray images (Figure 8). The appearance of an inverted bright-field image was strongly influenced by the exposure and the range of contrast between specimen and background. When photomicrographs were taken in brightfield and properly exposed, the background was whitish, and high-density specimens appeared rather dark, leading to a high range of contrast (Figure 8).

Figure 8 Digital inversion of bright-field images. Original photomicrographs taken in brightfield with a flash (a, c) and inverted to “digital darkfield” (b, d) on day 8 (a, b) and day 28 (c, d).
In underexposed bright-field images the range of contrast was reduced significantly: the background showed a lower brightness and a yellowish or orange tint. Thus, the background was no longer black or dark when a digital inversion was carried out. In such images fine nuances of density could be revealed such that the appearance of the inverted image (Figure 9) seemed to be similar to (negative) phase contrast.

Figure 9 Digital inversion of an underexposed bright-field image with flash, original photomicrograph (left), “digital phase contrast” (right), day 8. Specimen and equipment same as Figure 8.
In comparison to real darkfield, digital “dark-field” images did not show the specimens in their natural color, but they were free from typical artifacts often seen in normal dark-field images, such as ultra-bright contours (marginal irradiation) and other effects of reflection caused by a very bright illuminating light, such as blooming and scattering. Similarly, digital “phase-contrast” images were free from typical artifacts associated with true phase-contrast imaging such as haloing and shade-off (further explanations in web source [d]).
Suggestions of further experiments
Observations of growth and locomotion can be made with naked eyes even by very young children so that children´s inherent interest in nature can be stimulated when Artemia is presented in classrooms and integrated into lessons (web source [e]).
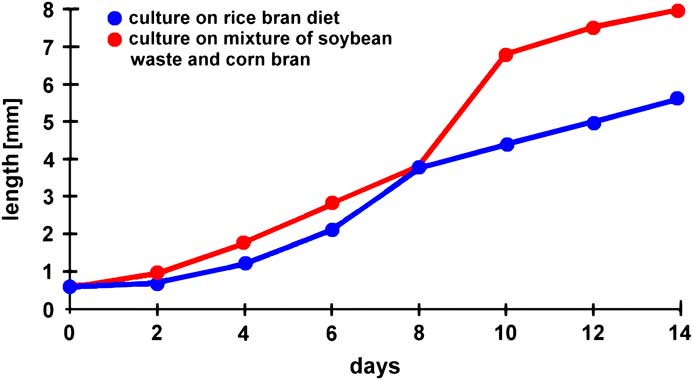
Through successive microscopic observations of Artemia, young students can be introduced to several principles of scientific work. For instance, larvae can be photographed daily, and their body length measured. Since there is a well-defined number of individuals, the body length can be averaged for each day of observation, and corresponding standard deviations can be calculated. Based on these data, kinetics of growth can be graphically demonstrated and mathematically described (Figure 10).

Figure 10 Influence of feeding protocols on the growth kinetics of Artemia (modified from [Reference Dhont6]).
In other experiments several environmental parameters could be modified, such as the concentration of salt, pH-value, water temperature, materials for feeding, or the population density. Correlations of such parameters with growth kinetics and/or the maximal duration of survival could be undertaken. The results derived from such experiments could be compared with published data compiled in the literature. Figure 10 (modified from [Reference Dhont6]) and Figure 11 (modified from [Reference Evjemo and Olsen7]) give examples of published findings with regard to different feeding protocols and varying population densities. These experiments reveal that feeding soybean waste and corn bran is more efficient than a rice bran diet and that the growth rate is significantly reduced when the population density increases. In other experiments the influence of environmental parameters on the hatching rate of Artemia cysts can be evaluated (example reported in web source [f]). Thus, Artemia could be used for introductory pedagogic projects focused on repetitive measurements, statistical presentations and analyses, and environmental and ecological research experimentation.
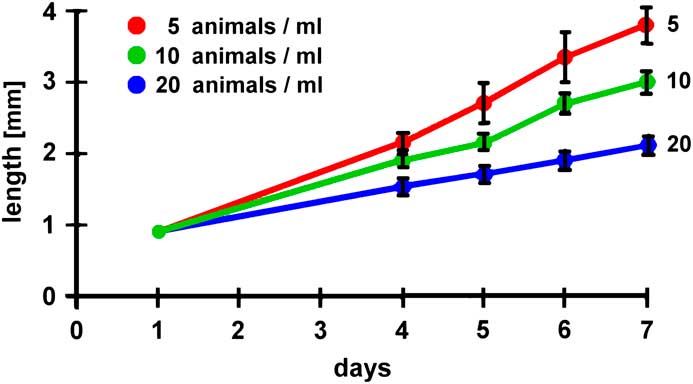
Figure 11 Influence of population density on the growth kinetics of Artemia (modified from [Reference Evjemo and Olsen7]).
Discussion
Young people (children and school students) of today are the scientists of tomorrow. Their interest in exploring nature should be stimulated as early and successfully as possible. In this respect, Artemia can be regarded as an attractive “model organism” of high pedagogical value. These aquatic “household pets” can be purchased at low cost, and they can be cultivated without difficulty. Moreover, these organisms tolerate wide ranges of temperature, pH-values, and salt concentrations. On the other hand, one must feed Artemia carefully so that over-eating is avoided and the quality of water can be maintained. When the water appears somewhat cloudy or foul, about one-third of the volume should be replaced by a new portion of fresh salt water.
Since individual sizes range from 0.5 to 15 mm over the course of development, these animals can be observed from day one, even with naked eyes. On the other hand, various magnifying equipment can be used by young (and advanced) explorers, from simple loupes and student microscopes to stereo microscopes and high-end laboratory microscopes. Examinations can be carried out with low or medium magnifying lenses that operate at long working distances from the specimen and can be handled well by young users. While brightfield is always available and is the most widely used illumination mode, standard dark-field mode is especially recommended as a tool for impressive microscopy. In the latter technique, Artemia appears in natural colors, as long the specimen’s surface is hit by the illuminating light beams, and fine details such as hairs and eye pigments can be seen at higher resolution and clarity. In bright-field images, however, elderly Artemia may appear as dark silhouettes because of their thickness and density.
Through daily observations young explorers can comprehend the phenomena of growth, differentiation, and maturation. The actual length of growing Artemia can be measured on the screen or on photographic prints. For this task photographs should be projected or printed as large as possible in order to obtain appropriate precision in measurements so that estimations can be made to 0.1 of a millimeter. Body lengths should be measured from the head to the caudal furca (see Figure 2). For each magnification used, students can learn how to evaluate the respective horizontal field width. The millimeter scale of a small transparent ruler imaged with the microscope is sufficient for this task at low magnifications. For higher magnifications (10× objective lenses, for instance), the ruler should be replaced by a calibration slide (slide micrometer).
Young students also can learn that higher levels of differentiation correspond with greater variation in locomotion patterns. Motion patterns can be documented by video clips. Even slow-motion videos can be produced by most consumer digital cameras and modern smartphones (for example, see web sources [g] and [h]). Pedagogically, it can be regarded as an advantage that the whole “metamorphosis” from young larvae to mature animals can be observed within a rather short period of 4 or 5 weeks. The animals show morphological changes every few days. Thus, young “scientists” will not be overstrained or bored. Young investigators might be motivated to pursue further experiments involving digital image processing, especially creating digital “dark-field” or digital “phase contrast” images. For advanced students a variety of further experiments can be suggested so that Artemia can be used for introductions to statistical analyses and several types of physiological and ecological investigations. Thus, by studying Artemia students can comprehend some general principles of scientific workflows.
Conclusion
Artemia sp. is well suited for educational projects leading to insights about important phenomena of life, such as growth, differentiation, maturation, and locomotion. Because of their size these organisms can be examined with simple low-cost microscopes equipped with a simple digital camera or a smartphone adapted to the microscope. For high-quality photomicrographs a flash is needed. Video clips can be made, using the video modes implemented in many cameras, to provide documentation of various locomotion characteristics. Further experiments for advanced students can be designed that lead to insights about the principles of scientific investigations in general.
Acknowledgements
The Author thanks the Immunocons Company, Germany, for Figure 3e, and Timm Piper for all photomicrographs presented which were made by Timm from May to June 2007.