Type 2 diabetes mellitus (T2DM) is considered one of the major epidemics of the twenty-first century. In 2014, WHO estimated that, worldwide, 422 million suffer from diabetes, almost doubling the prevalence in 1980( 1 ). This trend is expected to continue over the coming years, and the International Diabetes Federation estimates that in 2040 there will be 642 million people living with diabetes( 2 ). Moreover, T2DM is a leading cause of many severe complications such as CVD, blindness, kidney failure and lower limb amputation( 3 ) with the consequent costs to the healthcare system( Reference Zimmet, Magliano and Herman 4 ). Therefore, it is essential to assess lifestyle interventions effects on risk factors related to T2DM.
Obesity is a major preventable risk factor for T2DM( 1 , 2 ). A new approach in the dietary control of overweight and obesity for the prevention of T2DM should include well known, healthy (cardio-protective), high-quality and palatable dietary patterns. One dietary paradigm that may be beneficial in this context is a traditional Mediterranean diet (MedDiet), relatively rich in fat from vegetable sources (extra-virgin olive oil, tree nuts) and including an abundance of minimally processed plant-foods (vegetables, fruits, whole grains, legumes), moderate fish consumption, low consumption of meat and meat products, and wine in moderation, usually consumed with meals.
Recent studies support that a better adherence to MedDiet could mitigate the adverse consequences of obesity on CVD even in obese persons at high cardiovascular risk( Reference Eguaras, Toledo and Hernández-Hernández 5 , Reference Eguaras, Toledo and Buil-Cosiales 6 ). There is strong evidence that modifications in the overall dietary pattern and the adoption of high-quality diets, such as the traditional MedDiet, together with an intervention aimed to promote weight loss may play an important role in decreasing the incidence of T2DM( Reference Kastorini and Panagiotakos 7 – Reference Martínez-González, Salas-Salvadó and Estruch 10 ). Nevertheless, it is not known whether any dietary change different from weight loss could attenuate the acknowledged adverse effects of obesity on the risk of T2DM.
In this study we aimed to assess if only changes in the composition of the food pattern, without any additional weight loss, physical activity or energy restriction can reduce the obesity-associated risk of T2DM. We tested the hypothesis that a higher adherence to a non-energy-restricted MedDiet may mitigate the adverse effect of obesity on the incidence of T2DM.
Methods
Study population
The Seguimiento Universidad de Navarra (SUN) Project is a dynamic multipurpose prospective Spanish cohort of university graduates. This cohort started in 1999 with biennial collection of updated information through self-administered questionnaires and it is permanently open to recruitment of new participants. The aim of this cohort was to assess associations between diet or lifestyles and the incidence of several chronic diseases and mortality. Details of the design, methods and objectives of the SUN Project have been described previously( Reference Martínez-González, Sanchez-Villegas and de Irala 11 , Reference Segui-Gomez, de la Fuente and Vazquez 12 ).
For the present analyses we assessed 22 476 participants who had answered the baseline questionnaire before December 2015 (Fig. 1). We excluded 406 participants who had prevalent diabetes at baseline and also participants who had not remained in the cohort enough time for being followed-up for at least 2 years (2376). In addition, 1469 participants were excluded because they reported a total daily energy intake out of pre-defined limits (>2092 or <23 012 kJ/d (>500 or <5500 kcal/d) for female, >3347 or <25 104 kJ/d (>800 or <6000 kcal/d) for male). After exclusions, the final population sample included a total of 18 225 participants.

Fig. 1 Flow chart of participants in the Seguimiento Universidad de Navarra Project, 1999–2016.
Ethical approvals
The study protocol was approved by the Institutional Review Board of the University of Navarra. Voluntary completion of the first questionnaire was considered to imply informed consent.
All clinical investigation were conducted according to the guidelines laid down in the Declaration of Helsinki and it was approved by the Human Research Ethical Committee of the University of Navarra.
Anthropometric variables
Information about weight was recorded at baseline and at each follow-up questionnaire. BMI, defined as weight in kilograms divided by the square of height in metres, was calculated in the baseline questionnaire. Reliability of self-reported weight and height to compute BMI was assessed in a subsample of the cohort( Reference Bes-Rastrollo, Perez Valdivieso and Sanchez-Villegas 13 ). A high correlation was found with directly measured weight (r 0·99; 95 % CI 0·99, 0·99) and BMI (r 0·94; 95 % CI 0·91, 0·97), with mean relative errors of 1·45 and 2·64 %, respectively.
Dietary assessment
A validated semi-quantitative 136-item FFQ( Reference Martin-Moreno, Boyle and Gorgojo 14 ) was used to assess dietary intakes over the previous year. The validity( Reference Martin-Moreno, Boyle and Gorgojo 14 , Reference Fernández-Ballart, Piñol and Zazpe 15 ) and reproducibility( Reference De la Fuente-Arrillaga, Vazquez Ruiz and Bes-Rastrollo 16 ) of this FFQ have been repeatedly reported. In order to calculate each nutrient score, nutrient composition of specified portion sizes (using data from food composition tables valid for Spain( Reference Mataix 17 , Reference Moreiras 18 )) was multiplied by the frequency of consumption of each participant. Consumption frequencies were grouped in nine categories (ranging from never/almost never, to >6 times/d) for each food item. A nine-item scale proposed by Trichopoulou et al.( Reference Trichopoulou, Costacou and Bamia 19 ) was used to classify participants according to their baseline adherence to the MedDiet( Reference Trichopoulou, Kouris-Blazos and Wahlqvist 20 ). One point was assigned to persons whose consumption was above the sex-specific median of components most in line with the traditional MedDiet (vegetables, fruits/nuts, legumes, fish/seafood, cereals and MUFA:SFA lipid ratio). One point was assigned to persons whose consumption was below the sex-specific median of components against the traditional MedDiet (meat/meats products, dairy products). For ethanol, 1 point was assigned to men consuming 10–50 g/d and to women consuming 5–25 g/d, otherwise, no point was assigned.
Outcome assessment
Ascertainment of T2DM in the SUN Project has been reported before( Reference Martínez-González, de la Fuente-Arrillaga and Nunez-Córdoba 21 ). Participants who reported at baseline having been treated with either oral antidiabetic agents or insulin or reported a medical diagnosis of T2DM were considered prevalent cases of diabetes at baseline and were excluded. We considered probable cases of new-onset diabetes to those participants who reported a T2DM clinical diagnosis during any follow-up questionnaire but did not have diabetes at baseline( Reference Martínez-González, de la Fuente-Arrillaga and Nunez-Córdoba 21 ). These participants were asked to confirm their diagnosis with additional-specific confirmation questionnaires where they specified further details (i.e. type of diabetes, date of diagnosis, whether the diagnosis was gestational diabetes, highest fasting glucose value, eventual oral glucose tolerance testing, glycosylated Hb (HbA1c), current use of oral antidiabetic agents or insulin and occurrence of complications) and to provide a copy of their medical reports to ensure a sufficiently high specificity in the classification of incident cases. An endocrinologist, blinded to the dietary variables, revised the information collected with the diabetes-specific questionnaires and the medical records of participants to adjudicate new-onset (incident) cases of T2DM. The American Diabetes Association’s criteria were used to classify incident cases of T2DM( 22 ).
Other covariates
At baseline questionnaire, information was gathered about socio-demographic variables (age, marital status, years of university education), health-related habits (smoking status, energy intake, physical activity, sedentary lifestyles, hours of television watching) and clinical variables (medications, personal history of hypertension, diabetes, hypercholesterolaemia, cancer, depression, CVD).
Physical activity was assessed at baseline using a previously validated questionnaire that contained time spent in seventeen different activities( Reference Martínez-González, López-Fontana and Varo 23 ). Physical activity was expressed in metabolic equivalent tasks-h/week as calculated from the time spent at each activity in h/week multiplied by its typical energy expenditure( Reference Ainsworth, Haskell and Whitt 24 ). The validity of this questionnaire of physical activity was formally tested in a specific study within a subset of this cohort( Reference Martínez-González, López-Fontana and Varo 23 ).
Statistical analysis
We estimated statistical power assuming an absolute total cumulative incidence of T2DM=0·8 %, sample sizes of 12 000 and 700 in extreme categories of BMI (<25 and >30 kg/m2, respectively), with expected relative risks between 6 and 8 (a realistic assumption based on previous literature). Under these assumptions and with a two-tailed α error of 5 %, the expected statistical power will range between 0·78 and 0·84. Specifically for interactions, the proposed minimum sample size in each group to obtain a sufficiently high statistical power for assessing interactions was 900/group in the article by Demidenko( Reference Demidenko 25 ) and we had a similar sample size in our groups.
We examined baseline characteristics of participants stratified by their baseline BMI and according to their baseline adherence to MedDiet. Adherence to MedDiet was categorised into two groups (≤4 and >4 points in the Trichopoulou’s score).
We used Cox regression models to assess the hazard ratios (HR) and their 95 % CI for incident T2DM across categories of BMI (cut off points: 25 and 30 kg/m2). Age was used as the underlying time variable and we stratified all Cox models by broad categories of age (decades). The fully adjusted model included the following potential confounders: sex, year of recruitment (four categories), adherence to the MedDiet (continuous within each strata of poor and good adherence); smoking status (three categories: former smokers, current smokers and never smokers), physical activity during leisure time (continuous), hours of television watching, hypertension status, hypercholesterolaemia status, depression, cancer, CVD, years of university education, energy intake, marital status, following special diets and between-meal snacking.
In subgroup analyses we stratified the results by baseline adherence to the MedDiet (categorised into two groups: poor adherence (≤4 points) and good adherence (>4 points). The P value for multiplicative interaction was calculated by comparing a full model including a multiplicative interaction term to a reduced model without an interaction term, using a likelihood ratio test. We used both a 2 df product-term (dichotomous MedDiet and three categories for BMI) and a 1 df product-term (both variables as continuous).
To address the possibility that the beneficial effect of the MedDiet on T2DM might be explained only by changes in weight during follow-up we conducted an ancillary analysis where we assessed whether the inverse association between better adherence to the MedDiet and T2DM was attenuated after adjusting for weight changes during follow-up.
To assess non-linear associations we fitted fully adjusted restricted cubic spline models for the association between BMI and incident diabetes stratified by adherence to the MedDiet.
A P value <0·05 was considered statistically significant. Analyses were performed using STATA SE version 12.1 (StataCorp LP).
Results
Baseline characteristics of participants stratified by their baseline BMI and according to their baseline adherence to MedDiet are shown in Table 1. Those participants who reported higher levels of adherence to MedDiet were on average older, more likely to be married and more physically active. In the baseline cross-sectional analyses, they also were more likely to have a previous diagnosis of hypercholesterolaemia, hypertension, CVD, cancer or depression, probably because these conditions may have led them to improving their dietary habits. In addition, these participants with better adherence to the MedDiet at baseline were also less likely to be current smokers but more prone to being former smokers. They were also more likely to follow special diets and to consume more alcohol, but were less likely to consume snacks between meals.
Table 1 Baseline characteristics of participants according to their baseline BMI and their adherence to the Mediterranean diet (MedDiet) (Mean values and standard deviations; percentages)

* P values for the comparison of percentages or means across the three BMI categories, separately within each group of adherence to the MedDiet (≤4 or >4).
After a median of 9·5 years of follow-up we observed 136 incident T2DM cases.
The relationship between categories of BMI and the risk of T2DM according to their baseline adherence to MedDiet (≤4 and >4 points in the Trichopoulou’s score) is shown in Table 2. We observed that the HR increased across categories of BMI in both groups built according to conformity with the MedDiet. However, after multivariable adjustment, we observed that in the stratum of low adherence to MedDiet, the obesity-associated HR for T2DM were significantly higher than in the stratum of high adherence to MedDiet. The P value for multiplicative interaction between MedDiet and BMI was statistically significant (P=0·002). In fully adjusted models, the association between BMI and the risk of diabetes was stronger when the adherence to the MedDiet was poorer (HR=2·50; 95 % CI 1·93, 3·24 for each additional standard deviation in BMI) than when the MedDiet score was higher than 4 points (HR 2·01; 95 % CI 1·72, 2·36). The P value for interaction between MedDiet and BMI (both as continuous variables) was marginally significant (P=0·051). When the non-confirmed cases were included (in total 169 cases) the results were similar (and the P for interaction became significant, P=0·025).
Table 2 Relative risks of type 2 diabetes in the Seguimiento Universidad de Navarra project according to baseline BMI and adherence to Mediterranean diet (MedDiet) (Hazard ratios (HR) and 95 % confidence intervals)
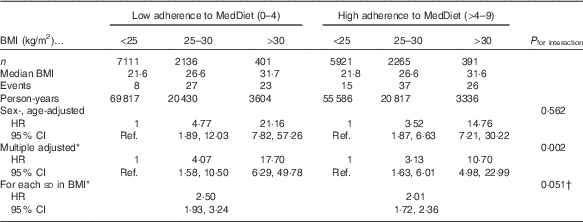
Ref., referent values.
* Adjusted for age, sex, recruitment year, smoking (three categories: former smokers, current smokers and never smokers), adherence to the Mediterranean diet (continuous), hypercholesterolaemia, hypertension, physical activity, marital status, prevalent CVD, prevalent cancer, prevalent depression, years of university studies, television watching time, snacks intake and special diets. The multiplicative interaction was assessed with a 2 df product-term (dichotomous MedDiet and three categories for BMI).
† Both the nine-item Mediterranean score and BMI were introduced as continuous variables in the product-term used to assess effect modification. 1 df product-term was used to test the P value of interaction.
We conducted an ancillary analysis after adjusting for weight changes during follow-up. The average yearly weight changes during follow-up were –0·467 and +0·225 kg/year among participants who eventually developed and did not develop T2DM during follow-up, respectively. Therefore, there was no indication that weight gain during follow-up may explain the development of T2DM because weight loss (and not weight gain) during follow-up occurred more likely in cases than in non-cases. After additionally adjusting for weight changes, the HR for each additional standard deviation in BMI were 2·31 (95 % CI 1·77, 3·01) kg/m2 when adherence to the MedDiet was poor (0–4 points) and 1·95 (95 % CI 1·66, 2·30) when it was good (>4 points), and the interaction remained statistically significant (P=0·025).
We fitted spline models to represent graphically the relationship between baseline BMI and the risk of developing T2DM during the follow-up period according to baseline adherence to the MedDiet (≤4 points and >4 points). We observed that in the group with poor adherence to MedDiet the BMI-associated relative risk of T2DM was visibly higher than in the group with better adherence (Fig. 2).

Fig. 2 Dose–response association between baseline BMI and the incidence of type 2 diabetes according to baseline adherence to the Mediterranean diet (MedDiet). The Seguimiento Universidad de Navarra cohort (1999–2015). Values are hazard ratios and 95 % CI.
Discussion
In this multipurpose cohort of university graduates we observed an attenuation in the association between high BMI and the risk of developing T2DM in participants with better adherence to MedDiet, after adjusting for other common risk factors in T2DM. This study supports our hypothesis that a higher adherence to MedDiet could mitigate the pernicious effect of obesity on the incidence of T2DM even without inducing loss of weight. In fact, we found a statistically significant interaction (assuming a multiplicative scale for interaction), and our finding is consistent with the inverse association between the MedDiet and the risk of T2DM observed in this cohort( Reference Martínez-González, de la Fuente-Arrillaga and Nunez-Córdoba 21 ) and in other previous studies such as the observational Nurses’s Health Study( Reference Fung, Rexrode and Mantzoros 26 ), the EPIC Study( 27 ) or the Prevención con dieta Mediterránea (PREDIMED) randomised trial( Reference Salas-Salvadó, Martínez-González and Bulló 8 , Reference Salas-Salvadó, Bulló and Babio 9 ). In addition, a recent meta-analysis( Reference Koloverou, Esposito and Giugliano 28 ) reported a strong association between better adherence to MedDiet and a reduction in the risk of T2DM.
Although at baseline we observed that participants with better adherence to the MedDiet were more likely to have a previous diagnosis of hypercholesterolaemia, hypertension, CVD, cancer or depression, these results could be explained because those differences were based on simple cross-sectional analyses where reverse causality could not be excluded.
It is well known that there is a strong relationship between overweight/obesity and the risk of developing T2DM. In fact, the main measure proposed to prevent T2DM is a weight reduction through an intervention with diet and lifestyle( Reference Colditz, Willett and Rotnitzy 29 , Reference Scherer and Hill 30 – Reference Balk, Early and Raman 32 ). Interventions addressing lifestyles, including physical activity, weight reductions and energy-restricted diets, have been successful in achieving a reduction in the incidence of diabetes mellitus in the long term( Reference Balk, Early and Raman 32 , 33 ). However, the role of the overall nutritional quality in the prevention of T2DM independent of weight changes, has not been fully addressed by these trials. The paradigm used in most of these trials, including a recent trial for cardiovascular prevention in participants who were already diabetics at baseline (the Look Action for Health in Diabetes (Look AHEAD) trial( Reference Wing and Bolin 34 )), was a low-energy, low-fat diet. In contrast with the low-energy, low-fat diet, the MedDiet represents an updated paradigm of overall dietary quality, with demonstrated effectiveness and sustainability and with the potential to be globally applied( Reference Martínez-González, Salas-Salvadó and Estruch 10 , Reference Anand, Hawkes and de Souza 35 , Reference Trichopoulou, Martínez-González and Tong 36 ).
The novelty of our research is the suggestion of a reduction in the risk of T2DM by the MedDiet that may attenuate the detrimental effects of increased body weight. We did extra analyses in the multivariable adjustment to take into account the observed yearly average changes in the weight of our participants, and we did not observe any substantial attenuation of our results after adjusting for weight changes during follow-up. This result points to an inherent beneficial effect of a high-quality overall dietary pattern on diabetes risk independent of weight loss. Interestingly, our results are in line with recent studies that assessed a reduction in major CVD events associated with closer conformity with the MedDiet in obese patients with high cardiovascular risk, thereby mitigating the adverse effect of abdominal adiposity( Reference Eguaras, Toledo and Hernández-Hernández 5 , Reference Eguaras, Toledo and Buil-Cosiales 6 ). In this line, the PREDIMED trial assessed a significant reduction in the risk of T2DM( Reference Salas-Salvadó, Bulló and Babio 9 , Reference Salas-Salvadó, Bulló and Estruch 37 ). Our results are of particular interest in the context of the current concerns to assess whether interventions with a rationale different from the Look AHEAD trial( Reference Alberti, Zimmet and Straw 31 , Reference Balk, Early and Raman 32 ) can provide a better answer to the current epidemics of obesity and T2DM. Specifically, the quality of the diet could be a more important factor for the prevention of T2DM and its cardiovascular complications than the weight loss( Reference Després and Poirier 38 ). A new large trial focused on MedDiet, weight loss and physical activity (PREDIMED-PLUS) is ongoing (http://medpreventiva.es/cD5Mp1). Almost 7000 participants have been already randomised to two equally sized arms in the new PREDIMED-PLUS trial, these two arms are an energy-restricted MedDiet plus physical activity and weight loss in the intensive intervention group, but only MedDiet (without energy restriction or physical activity) in the control group. They will be in the trial for the next 5 years. The primary end-point is a composite of hard cardiovascular events (myocardial infraction, stroke or CVD death). Results are expected in 2021. An intervention based in an energy-restricted MedDiet together with physical activity in order to obtain weight loss could achieve even greater benefits in obese subjects, than the benefit assessed by the initial PREDIMED study.
Obesity is a well-known risk factor for CVD independent of BMI( Reference Alberti, Zimmet and Straw 31 , Reference Casanueva, Moreno and Rodriguez-Azeredo 39 ) and the MedDiet could play an important role by reducing the inflammatory mediators involved in the adverse consequences of abdominal adiposity( Reference Kastorini and Panagiotakos 7 , Reference Martínez-González, de la Fuente-Arrillaga and Nunez-Córdoba 21 , Reference Esser, Legrand-Poels and Piette 40 , Reference Estruch, Martínez-González and Corella 41 ). It is known that all food intake is accompanied by a mild inflammatory oxidative condition that increases plasma levels of inflammatory biomarkers reducing the sensitivity of tissues to insulin that leads to a state of insulin resistance( Reference Vogel 42 , Reference Hu 43 ). Plant foods typical of the MedDiet are rich in antioxidants and anti-inflammatory elements. Their joint and synergistic effects are likely to be important because the effect of the overall dietary pattern captures interactions between nutrients and results in a stronger effect( Reference De la Rosa and Luluaga 44 ). The MedDiet pattern may reduce the risk of T2DM by increasing adiponectin levels( Reference Razquin, Martínez and Martínez-González 45 ), reducing oxidative stress( Reference Razquin, Martínez and Martínez-González 45 , Reference Dai, Jones and Goldberg 46 ) as well as reducing low-grade inflammation( Reference Esposito, Marfella and Ciotola 47 , Reference Urpi-Sarda, Casas and Chiva-Blanch 48 ).
There are several strengths in our research. We used a cohort with a prospective design, including a large number of participants and with a high retention rate. Besides, we used multiple-adjusted models to control for a wide array of potential confounders. Our study shows a strong internal validity due to a high retention rate and sufficiently reliable self-reported measures reported by highly educated participants.
On the other hand, some limitations of our study deserve to be acknowledged. The information on several variables was assessed through self-reporting. However, parameters such as self-reported weight and height or usual diet have been previously validated in sub-samples of this cohort( Reference Bes-Rastrollo, Perez Valdivieso and Sanchez-Villegas 13 , Reference Martin-Moreno, Boyle and Gorgojo 14 ). Another possible caveat might be the fact that the cohort is composed of middle-aged, highly educated persons, with a high level of physical activity which could limit the generalisability of our findings to other populations. We acknowledge that the SUN cohort is a relatively young cohort for diabetes research. Previous cohorts have usually included older participants. The advantage of a younger cohort is that it may offer unique characteristics to ascertain the earliest steps in the pathophysiological mechanisms relating dietary exposures to the risk of T2D. Therefore, our findings provide interesting clues with relevance for diabetes prevention research. The disadvantage of assessing these associations in a young cohort is that absolute risks are low, and the statistical power might be limited due to the low number of new cases of T2D. Given that the participants in our study live in a Mediterranean country, they are relatively young (mean baseline age was 38 years) and are, in general, health-conscious subjects, their consumption of products typical of the traditional MedDiet was high, even in participants with lower scores of conformity to the MedDiet. Therefore, under the assumption that the MedDiet plays a protective role against the development of diabetes, in a cohort with these characteristics it is not surprising to find a low incidence of T2DM. However, our findings need to be confirmed in future cohort studies and trials, given the low absolute risks of T2DM in our cohort of young, slim and highly educated adults.
Conclusions
Our prospective study suggests that a high adherence to MedDiet could mitigate the adverse effects of obesity on incidence of T2DM, without specifically requiring a loss of weight. However, due to the low incidence of T2DM further research is needed to confirm our findings.
Acknowledgements
The authors thank the SUN project participants for their enthusiastic collaboration and participation. The authors also thank the other members of the SUN study group: A. Alonso, I. Álvarez, A. Balaguer, I. Barrientos, M. T. Barrio-López, F. J. Basterra-Gortari, P. Bazal, S. Benito, J. J. Beunza, P. Buil-Cosiales, M. Canales, L. Carmona, S. Cervantes, C. Cristobo, J. de Irala, C. de la Fuente-Arrillaga, M. Delgado-Rodríguez, J. Díaz-Gutiérrez, J. Díez Espino, L. Domínguez, C. Donat-Vargas, M. Donazar, A. Fernández-Montero, U. Fresán, C. Galbete, A. García-Arellano, M. García López, I. Gardeazábal, A. Gea, E. Gómez-Gracia, E. Goñi, F. Guillén, M. Gutiérrez-Bedmar, P. Henríquez, A. Hernández, E. Hu, F. Lahortiga, A. Leone, J. Llorca, C. López del Burgo, A. Marí, I. Marques, A. Martí, N. Martín Calvo, J. A. Martínez, R. Mendonça, P. Molero, J. M. Núñez-Córdoba, P. Pérez de Ciriza, A. Pérez Cornago, A. M. Pimenta, J. Pons, R. Ramallal, C. Razquin, A. Rico, C. Ruano, A. Ruiz Zambrana, E. Salgado, B. San Julián, D. Sánchez, A. Sánchez-Tainta, A. Sánchez-Villegas, S. Santiago, C. Sayón-Orea, E. Toledo, J. Toledo, Z. Vázquez, D. Zarnowiecki and I. Zazpe
We have received funding from the European Research Council (Advanced Grant (AdG), LS7, ERC-2013-ADG, PREDIMEDPLUS, PI: M. A. M.-G.), the Spanish Government-Instituto de Salud Carlos III, and the European Regional Development Fund (FEDER) (RD 06/0045, CIBER-OBN, grants PI10/02658, PI10/02293, PI13/00615, PI14/01668, PI14/01798, PI14/01764 and G03/140), the Navarra Regional Government (45/2011, 122/2014) and the University of Navarra.
M. A. M.-G. conceived and designed the study; S. E. performed the research; S. E. and M. A. M.-G. analysed data and wrote the paper; all authors critically reviewed the manuscript and approved the final version.
The authors declare that there are no conflicts of interest.







