Vitamin D is a steroid pro-hormone synthesised in the skin following UV exposure or through supplemental or dietary intake. In addition to maintaining bone and muscle health, vitamin D has recently been postulated to protect against immune dysfunction, cancer(Reference Lips1), cardiovascular conditions(Reference Kendrick, Targher and Smits2, Reference Nemerovski, Dorsch and Simpson3), hypertension(Reference Nemerovski, Dorsch and Simpson3, Reference Pilz, Tomaschitz and Ritz4), metabolic syndrome and diabetes(Reference Pittas, Lau and Hu5).
The concentration of 25-hydroxyvitamin D (25(OH)D), the major circulating form of vitamin D in the blood, rises and falls with the supply of vitamin D; on the other hand, the concentration of circulating 1,25-dihydroxyvitamin D (1,25(OH)2D) (the biologically active form) is thought to be kept under tight homeostatic control and is thus largely independent of vitamin D supply, except in severe deficiency(Reference Lips1).
As early as the 1980s, animal studies identified the expression of the vitamin D receptor in rat pancreatic cells and demonstrated that vitamin D deficiency inhibits the production of insulin(Reference Christakos, Friedlander and Frandsen6, Reference Norman, Frankel and Heldt7). To our knowledge, no large epidemiological analysis study has investigated simultaneously diabetes, hypertension and CHD risk together with vitamin D blood levels. Thus, the aim of the present study was to investigate the association between diabetes, hypertension and CHD and two serum bio-markers associated with vitamin D function, 25(OH)D and 1,25(OH)2D.
Materials and methods
In the present study, we analysed data from subjects selected as controls in five previous case–control studies of circulating vitamin D biomarkers (25(OH)D and 1,25(OH)2D) nested within the Prostate Lung Colon and Ovarian Trial (PLCO), which was a large, randomised, controlled multicentre trial in the USA with 155 000 men and women at sites in Birmingham, AL; Denver, CO; Detroit, MI; Honolulu, HI; Marshfield, WI; Minneapolis, MN; Pittsburgh, PA; Salt Lake City, UT; St Louis, MO; and Washington, DC, which was designed to evaluate selected methods for the early detection of these four cancers as well as non-Hodgkin's lymphoma, breast and pancreatic cancer. Enrolment began on 1 November 1993 and ended on 30 June 2001(Reference Hayes, Reding and Kopp8). Details of these studies of colorectal adenoma, non-Hodgkin's lymphoma and prostate, breast and pancreatic cancer are described elsewhere(Reference Brock, Huang and Fraser9). Briefly, we included 399 controls used for a study of colorectal adenoma (matched to cases by sex and race), 286 controls used for a study of non-Hodgkin's lymphoma, 713 controls used for a study of prostate cancer (matched to cases by age, time since screening and year of follow-up), 932 controls used for a study of breast cancer (matched to cases by age and year of blood draw) and 350 used for a study of pancreatic cancer (matched to cases by age, sex, race and date of blood draw). Of these controls, 59 were included in more than one study; thus, in total, 2621 control subjects from the Prostate Lung Colon and Ovarian Trial were included in this present data analysis. As there were only 157 non-Caucasians in these data, we only present results for these Caucasian subjects (25(OH)D, n 2465; 1,25(OH)2D, n 1369). At the initial screening, all participants were asked to complete a questionnaire on medical history, including diabetes, hypertension and CHD as well as demography, anthropometry, lifestyle factors (including smoking history, menopausal hormone therapy use and vigorous physical activity (PA) during the previous year) and usual dietary intake over the 12 months before enrolment (137-item FFQ and fourteen questions about intake of vitamin and mineral supplements)(Reference Subar, Midthune and Kulldorff10). Because of the self-reported nature of the questionnaire, the medical diagnosis questions were repeated on the same population 5 years later with 87 % repeatability for diabetes, 89 % for hypertension and only 55 % for CHD. Daily nutrient intake from foods was calculated by multiplying the reported frequency of consumption of each food item by the nutrient composition of the imputed sex-specific portion size, using the nutrient database from the US Department of Agriculture(Reference Tippett and Cypel11). Ca and vitamin D intakes were measured both from food and supplemental sources. Serum samples were collected during the baseline visit and stored at − 70°C. Levels of serum 25(OH)D and 1,25(OH)2D for subjects were determined at baseline using a radio-iodinated tracer assay in the laboratories of Horst and Hollis(Reference Wagner, Hanwell and Vieth12, Reference Hollis13). Replicate blinded quality-control samples from two to four different individuals were included in all 25(OH)D and 1,25(OH)2D batches. The overall CV for 25(OH)D were 16·3 % for the colorectal adenoma, 11·4 % for non-Hodgkin's lymphoma, 5·9 % for prostate, 8·2 % for the breast study and 4·7 % for the pancreas study and for 1,25(OH)2D were 16·3 and 12·8 % for adenoma and breast studies, respectively.
Dummy variables coded to represent each of the centres in the study and each of the control series (from the five case–control studies) were included as covariates in all models as well as age, smoking, educational status, BMI, total dietary energy and PA, as they are known risk factors for diabetes, hypertension and CHD in previous studies. All these variables were included as a priori confounders/covariates in all multivariable regression models. Unconditional logistic regression analyses were conducted with diabetes, hypertension and CHD as health outcomes, with 25(OH)D nmol/l (categorised as >80, 50–80, 37–50 and < 37) as either the independent variable for vitamin D status or 1,25(OH)2D pmol/l (in quartiles: ≥ 103, ≥ 86–103, ≥ 72–86 and < 72). Vitamin D and Ca intakes (diet plus supplements) were also calculated as potential independent variables and also included in all models.
Linear trends of ordered categorical variables (e.g. categories of 25(OH)D, 1,25(OH)2D, BMI and vitamin D and Ca intake) were assessed using continuous values for each variable and applying a likelihood ratio test(Reference Breslow and Day14). All statistical analyses were performed using the SPSS 15 statistical package (SPSS, Inc., Chicago, IL, USA). All P values are two-sided.
Results
The characteristics of the study participants are reported in Table 1. Serum 25(OH)D levels varied seasonally, with the highest levels during the summer and autumn months and the lowest levels during winter and spring (unlike 1,25(OH)2D levels). Unadjusted 25(OH)D and 1,25(OH)2D values were significantly lower for diabetics (Fig. 1(a) and (b)), those with hypertension and those with CHD (data not shown). Apart from sunshine exposure, other independent predictors of 25(OH)D levels in these data have been previously reported to be high dietary and supplement intake of vitamin D and Ca and menopausal hormone therapy use in women, as well as having a high BMI and low PA levels(Reference Brock, Huang and Fraser9). As 1,25(OH)2D levels are under tight metabolic control, 25(OH)D and 1,25(OH)2D levels were only minimally correlated (r 2 0·158, P < 0·001) and, also, do not vary by season. In contrast to 25(OH)D, there were no other lifestyle predictors for 1,25(OH)2D except menopausal hormone therapy use. Diabetes was significantly associated with lower levels of 25(OH)D and 1,25 (OH)2D in blood: after adjustment for confounding factors, including sex, geographical location, educational level, smoking history, BMI, PA, total dietary energy, vitamin D and Ca intake and blood vitamin D (25(OH)D or 1,25(OH)2D (as appropriate)). Caucasians who had 25(OH)D ≥ 80 nmol/l 25(OH)D were half as likely to have diabetes (OR 0·5 (95 % CI 0·3, 0·9)) compared with those who had 25(OH)D < 37 nmol/l (trend test: P = 0·05). Those in the highest quartile of 1,25(OH)2D ( ≥ 103 pmol/l) were a third as likely to have diabetes (OR 0·3 (95 % CI 0·1, 0·7)) than those in the lowest quartile ( < 72 pmol/l; trend test: P = 0·02). In contrast, there were no significant associations between 25(OH)D or 1,25 (OH)2D and CHD or hypertension risk once adjustment was made for confounding (BMI was the major confounder) (Table 2). There were no sex differences with any of these chronic conditions and 25(OH)D or 1,25(OH)2D (P interaction >0·05).
Table 1 Distribution of demographic, lifestyle habits and dietary intake by low serum 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D), diabetes, hypertension and CHD status in Caucasian healthy middle-aged men and women living across the USA

MHT, menopausal hormone therapy.
Values were significantly different: *P < 0·01, **P < 0·001.
† χ2 P value testing for difference between the category v. the converse.
‡ n 616 women.
§ n 813 women.
∥ P value for significance of Pearson correlation coefficient.
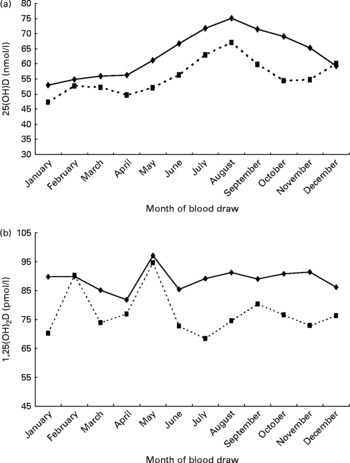
Fig. 1 (a) Distribution of 25-hydroxyvitamin D (25(OH)D) (nmol/l) levels by month of blood draw for diabetic (![]() ) and non-diabetic (
) and non-diabetic (![]() ) healthy middle-aged Caucasian US men and women. (b) Distribution of 1,25(OH)2D (pmol/l) levels by month of blood draw for diabetic (
) healthy middle-aged Caucasian US men and women. (b) Distribution of 1,25(OH)2D (pmol/l) levels by month of blood draw for diabetic (![]() ) and non-diabetic (
) and non-diabetic (![]() ) healthy middle-aged Caucasian US men and women.
) healthy middle-aged Caucasian US men and women.
Table 2 Association between dietary vitamin D and calcium intake and serum 25-hydroxyvitamin D (25(OH)D) (nmol/l) and 1,25-dihydroxyvitamin D (1,25(OH)2D) (pmol/l) and diabetes, hypertension and CHD prevalence in Caucasian men and women living across the USA

* Unadjusted for confounders and covariates.
† Adjusted for 1,25(OH)2D, study centre, different case–control study vitamin D analysis, date of blood draw, pack years of smoking, educational level, BMI, physical activity, sex, total dietary energy, dietary vitamin D and Ca plus supplements where appropriate.
‡ Missing data 25(OH)D (n 1).
§ Adjusted for 25(OH)D level, study centre, different case–control study vitamin D analysis, date of blood draw, pack years of smoking, educational level, BMI, physical activity, sex, total dietary energy, dietary vitamin D and Ca plus supplements where appropriate.
Discussion
The present findings for 25(OH)D and diabetes support the results of earlier clinical and animal studies(Reference Pittas, Lau and Hu5–Reference Norman, Frankel and Heldt7), and are consistent with other large epidemiological cross-sectional studies that have investigated the relationship between serum 25(OH)D levels and diabetes(Reference Pittas, Lau and Hu5, Reference Hyppönen and Power15). Two recent prospective investigations also support this hypothesis: in the first study, which was conducted among 524 women and men in the UK, baseline 25(OH)D levels were inversely associated with glycaemia and insulin resistance after a 10-year follow-up(Reference Forouhi, Luan and Cooper16). In the second study, which used data from two nested case–control studies in Finland, baseline 25(OH)D levels were significantly inversely associated with type 2 diabetes risk after a 22-year follow-up, but only in men(Reference Knekt, Laaksonen and Mattila17). None of these studies investigated associations with 1,25(OH)2D nor did they investigate CHD or the risk of hypertension.
Several small clinical intervention studies also support that vitamin D, or its active metabolite 1,25(OH)2D, improves insulin sensitivity, even in subjects with glucose metabolism parameters classified within normal ranges(Reference Pittas, Lau and Hu5). The mechanisms proposed to explain this effect include potential relationships with improvements in lean mass, regulation of insulin release, altered insulin receptor expression and specific effects on insulin action. These actions may be mediated by systemic or local production of 1,25(OH)2D or by suppression of the parathyroid hormone, which may function to negatively affect insulin sensitivity(Reference Pittas, Lau and Hu5–Reference Norman, Frankel and Heldt7, Reference Hyppönen and Power15–Reference Teegarden and Donkin19). However, since the present study is cross-sectional, we cannot rule out that being diabetic could modify the regulation of renal synthesis of 1,25(OH)2D, which would be reflected in the concentration of this hormone in the blood.
Vitamin D status is of interest with respect to diabetes because of its potential as a target for intervention. Maintaining adequate vitamin D status has proven to be challenging, as sunlight is the only substantial natural source of vitamin D for humans; given the low intensity of UV light in the winter and many people's indoor lifestyles, vitamin D supplied through sun exposure is often inadequate to avoid deficiency.
In contrast to our findings, others, but not all, have reported associations between low levels of vitamin D in the blood and hypertension(Reference Pilz, Tomaschitz and Ritz4); in summary, eighteen out of twenty-six cross-sectional studies (n 22–12 644) and of the two prospective studies, only one was positive(Reference Kendrick, Targher and Smits2, Reference Pittas, Lau and Hu5). As increased BMI is related to both hypertension and low vitamin D levels, it is important to consider it as a confounder in these studies. Only half of the positive observational studies adjusted for BMI; it should be noted that in the present data, a significant association with 25(OH)D or 1,25(OH)2D concentrations disappeared once BMI was added to the model. Similarly, the results from small, randomised, control trials have been variable with less than half positive(Reference Pittas, Lau and Hu5). Three of the large observational studies in the USA, UK and Germany reported significant associations between 25(OH)D and risk of hypertension after adjustment for diabetes prevalence(Reference Kendrick, Targher and Smits2, Reference Hyppönen and Power15, Reference Hintzpeter, Mensink and Thierfelder18). One postulated mechanism for vitamin D-mediated reduction of hypertension involves reno-protective effects, i.e. increased activation of the renin–angiotensin–aldosterone system, which is the main regulator of electrolyte and volume homeostasis that contributes to the development of arterial hypertension(Reference Nemerovski, Dorsch and Simpson3, Reference Pilz, Tomaschitz and Ritz4).
In contrast to our findings, some previous epidemiological studies have found a negative association between serum vitamin D and heart disease(Reference Nemerovski, Dorsch and Simpson3), with ten out of twelve observational studies (n 238–4839) of vascular disease (including two peripheral artery disease, five CVD) and five myocardial infarction) and six out of seven observational studies of heart failure (n 25–3299) showing a negative association between lower 25(OH)D levels and disease. Of these studies, only seven out of twelve adjusted for hypertension and diabetes, but none of these reported all three conditions separately in the same study(Reference Kendrick, Targher and Smits2, Reference Nemerovski, Dorsch and Simpson3). It is thought that vitamin D plays a role in maintaining cardiovascular homeostasis both through direct action of 1,25(OH)2D on cardiomyocytes and indirect actions on circulating parathyroid hormone and Ca levels. The lack of association with CHD seen in these data could have been because of the low repeatability of the self-reported nature of this outcome variable and its associated variability compared with that of hypertension or diabetes.
The strengths of the present investigation are its large sample size, relatively low deficiency levels and the fact that the vitamin D analyses were all performed by the same assay method. Moreover, the present analysis is unique in reporting, in a large cohort study, the association between diabetes and blood levels of 1,25(OH)2D; to our knowledge, only associations of 1,25(OH)2D with hypertension(Reference Pilz, Tomaschitz and Ritz4), CHD(Reference Nemerovski, Dorsch and Simpson3) and BMI(Reference Konradsen, Lindberg and Hexeberg20) have been reported in large epidemiological groups. Important limitations of the present study include its cross-sectional design and reliance upon self-report of diabetes, hypertension and CHD status; however, the measures of hypertension and diabetes had high reliability on follow-up. We had only one measure of adiposity (BMI), not total body fat, which is often thought to be more accurate; however, a recent validation study of BMI and dual-emission X-ray absorptiometry has reported very favourable correlations with adiposity(Reference Flegal, Shepherd and Looker21). We used only education as a marker for social class; however, this has been well accepted as the best marker for social class on an epidemiological level(Reference Kant and Graubard22–Reference Liberatos, Link and Kelsey24). In particular, we cannot rule out the possibility that changes in sun exposure and/or diet as a consequence of having diabetes may have led to our findings; however, the associations reported here warrant investigation in large prospective studies. In summary, these data outlining the positive association between vitamin D blood markers and diabetes indicated the need for more investigation both at the biochemical and epidemiological levels.
Acknowledgements
This research was supported in part by the Intramural Research Program of the National Cancer Institute (NCI), the National Institutes of Health (NIH). In addition, this research was supported by US Public Health Service contracts from NCI, NIH, the Department of Health and Human Services. All authors reviewed the final manuscript before submission, and none has a conflict of interest with regard to this work. K. E. B. was primary author in analysing the data and preparing the manuscript and coordinating the co-authors; R. Z. S.-S., D. M. F., J. A., U. P., C. M., R. G. Z. and M. P. P. were the primary investigators in the initial nested case–control studies used for this analysis and W.-Y. H. and M. P. P. are the overall co-ordinators of Prostate, Lung, Colorectal and Ovarian Screening Trial data, D. R. F. and R. S. M. and B. W. H. were involved in the vitamin D analysis aspects of the study; K. E. B., L. K. and B. I. G. were involved in the data analysis, and although K. E. B., M. T. and B. I. G. prepared the manuscript, all co-authors read and contributed to the final version, especially D. R. F. and R. S. M. read the vitamin D metabolism aspects.





