Osteoporosis is a skeletal disease associated with low bone mass, microarchitectural deterioration of bone tissue and, as a consequence, increased bone fragility and susceptibility to fracture( Reference Harvey, Dennison and Cooper 1 ). The incidence of osteoporosis increases with age and is most frequently observed in postmenopausal women, as bone tissue loss and remodelling are accelerated by decreased ovarian oestrogen levels associated with menopause( 2 ). Osteoporotic bone fractures may be particularly devastating in the case of elderly women. Fracture prevention is one of the key goals of osteoporosis therapy in postmenopausal women, with targets to inhibit bone loss processes, maintain proper bone strength, and minimise or eliminate the factors contributing to slips or falls( 3 ).
The relationship between Ca intake and bone mineral density (BMD) is a widely analysed issue; higher Ca intake is associated with increased bone density in children( Reference Wosje and Specker 4 ), as well as higher bone mass( Reference Nieves, Golden and Siris 5 ), reduced risk of osteoporosis( Reference Nieves, Barrett-Connor and Siris 6 ) and lower fracture risks( Reference Heaney 7 ) in adults and the elderly. The above-mentioned association is very complex and some authors emphasise the role of other factors, such as environmental factors for example, while doubting any strong correlation with Ca( Reference Badurski, Dobreńko and Nowak 8 , Reference Badurski, Jeziernicka and Dobreńko 9 ). Simultaneously, besides gender and age, body mass is the third important element which influences the risk of osteoporosis( Reference Varenna, Binelli and Casari 10 ), while being also associated with Ca intake( Reference Bueno, Cesar and Martini 11 , Reference Dicker, Belnic and Goldsmith 12 ).
The most important sources of Ca are dairy products, providing over 60 % of daily Ca intake in women's diets( Reference Gonnelli, Rossi and Montomoli 13 , Reference Poliquin, Joseph and Gray-Donald 14 ) and even 74 % in some Polish studies( Reference Wądołowska, Pabjan and Słowińska 15 ). Simultaneously, they are at least as efficacious as Ca supplements in osteoporosis prevention( Reference Heaney 16 ).
It was hypothesised that Ca intake from dairy sources will be associated with BMD in women over 55 years of age. The objective of the present population-based cohort study was to assess the influence of Ca intake from dairy sources on hip BMD and hip fracture incidence in a group of Polish women aged >55 years.
Methods
The RAC-OST-POL study was carried out in a group of Polish women aged >55 years, randomly recruited from the general population of women over 55 years of age in the District of Raciborz in the south of Poland. The total number of eligible women inhabiting the region at the time of enrolment was 17 500, from whom 1750 were randomly selected and invited by regular mail to participate in the study. A blind list of women, selected for the study, was provided by the local government and each woman was assigned a number without showing her name. A group of 625 women responded positively to the invitation and declared their intention to take part in the study, which was performed in May 2010. Among these women, six were still menstruating. All 625 participants were submitted to the study protocol, including BMD measurements and an FFQ to assess Ca intake. Body weight and height were measured with a standard medical balance and used to calculate BMI (kg/m2). The study complied with the guidelines of the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee of the Medical University of Silesia, Katowice, Poland. A written informed consent was obtained from all participants. The study group was described in a previous paper( Reference Pluskiewicz, Adamczyk and Czekajło 17 ).
Osteoporosis has been operationally defined on the basis of BMD assessment. According to the WHO criteria, osteoporosis is defined as a BMD level that lies 2·5 sd or more below the average value for young healthy women (T-score of ≤–2·5)( 18 ). The skeletal status was assessed by a Lunar DPX bone densitometer (GE Healthcare, Waukesha, WI, USA), assessing femoral neck and total hip bone density. Densitometric variables are presented as BMD (g/cm2) and T-score. All measurements were performed by one operator. The CV for measurements (calculated on the basis of fifty measurements – two for each participant with reposition) was 1·6 % for femoral neck and 0·82 % for total hip. Participants were queried about previous fractures of non-traumatic origin, e.g. caused by a fall from standing height or less; since no radiograms were obtained, some spine fractures might have been missed.
Participants were asked questions from the applied FFQ to assess their Ca intake from dairy products; they were asked by a dietitian about the frequency of consumption of forty-nine dairy products most common in Poland (e.g. milk, yoghurt, other milk beverages, rennet cheese, cottage cheese) and of meals containing dairy products, as well as typical portion sizes. Ca intake from a particular product was estimated by the following formula: Ca intake (mg) = typical portion size (g) × Ca content (mg/g). The FFQ results were analysed using the Dietetyk 2 software package (the Polish dietetic software) and the Polish database of nutritional values of food products( Reference Kunachowicz, Nadolna and Przygoda 19 ). The use of an FFQ to assess Ca intake is a commonly accepted and widely applied method, taking into account specified sources of Ca in the diet, mainly dairy products( Reference Gonnelli, Rossi and Montomoli 13 , Reference Khan, Mai le and Hien 20 – Reference Pereira, Genaro and Santos 23 ). The standard error of the Ca estimate for the FFQ administered (calculated on the basis of two measurements for fifty participants with repetition during the period of 6 weeks) was 180 mg, while the median difference for the two estimations of daily Ca intake was 12 %. A similar questionnaire, taking into account similar groups of products and applied to assess Ca intake in the Polish population, was also characterised by high sensitivity( Reference Szymelfejnik, Wądołowska and Cichon 24 ).
Data are presented as means and standard deviations, along with minimum, maximum and median values. The distribution of the analysed factors was verified using the Shapiro–Wilk test, while the Spearman correlation coefficient was used to characterise relationships among the analysed factors and the Mann–Whitney U test was used to analyse differences between groups.
An additional statistical analysis was performed in order to confirm that the analysed population might be treated as a representative sub-sample. It was verified whether the mean age in the analysed sub-population matched the mean age in the general population. The two-sided level of significance P ≤ 0·05 was accepted to define the significance of correlations. Statistical analysis was carried out using the Statistica software version 8·0.
Results
Table 1 presents the characteristic features of the participants: age, body weight, body height and BMI. According to the 2010 Statistical Yearbook, the general population of women over 55 years of age in Poland comprises the following age subgroups: 29·8 % aged 55–59 years, 14·3 % aged 60–64 years and 55·9 % aged 65 years and older( 25 ). These age subgroups accounted for respectively 27·0 %, 19·8 % and 53·2 % of the women in the present study. The χ 2 test performed for the subgroup aged 60–64 years revealed a significant difference between typical and observed numerical strength in the analysed group. A significant correlation was found between age and BMI values (P = 0·0017; r = 0·12).
Table 1 Characteristic features of the participants: Polish women (n 625) aged >55 years, RAC-OST-POL Study, May 2010

*Variable was not normally distributed (verified by the Shapiro–Wilk test; P ≤ 0·05).
Dairy Ca intakes of the women, grouped according to femoral neck and total hip T-scores >–2·5 or ≤–2·5, are presented in Table 2. Ca intake from dairy products was lower in the group of women with femoral neck T-scores ≤–2·5 than in the group with T-scores >–2·5 (P = 0·0019). For the total hip scores, the difference was borderline significant (P = 0·0698).
Table 2 Dairy calcium intake (mg/d) according to femoral neck and total hip bone mineral density T-scores: Polish women (n 625) aged >55 years, RAC-OST-POL Study, May 2010
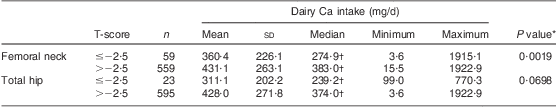
*Differences assessed by the Mann–Whitney U test.
†Variable was not normally distributed (verified by the Shapiro–Wilk test; P ≤ 0·05).
Table 3 presents the associations between dairy Ca intake and fractures. Ca intake from dairy products was lower in the group of women with previous fractures than in those without fracture history (P = 0·0254).
Table 3 Dairy calcium intake (mg/d) according to fracture status: Polish women (n 625) aged >55 years, RAC-OST-POL Study, May 2010
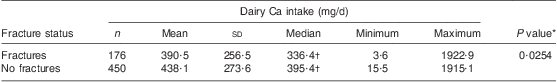
*Differences assessed by the Mann–Whitney U test.
†Variable was not normally distributed (verified by the Shapiro–Wilk test; P ≤ 0·05).
Ca intake from various dairy sources is presented in Table 4. The median Ca intake from total dairy products was 373·2 mg/d in the analysed group; however with a considerable variability among particular individuals, ranging from 3·6 mg/d to 1922·9 mg/d. Other milk drinks (i.e. drinks other than milk, such as yoghurt, kefir, buttermilk) were the main dairy source of Ca, providing a median of 99·1 mg Ca/d. Important sources of Ca included rennet cheese (median 72·6 mg/d) and milk (median 70·8 mg/d). The other analysed sources of Ca included cottage cheese, dairy dishes and other products.
Table 4 Calcium intake (mg/d) from dairy sources: Polish women (n 625) aged >55 years, RAC-OST-POL Study, May 2010
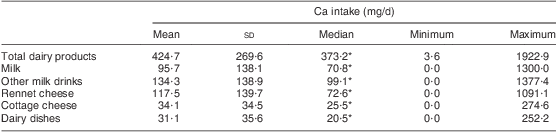
*Variable was not normally distributed (verified by the Shapiro–Wilk test; P ≤ 0·05).
The correlations of age and BMI with Ca intake from various dairy sources are presented in Table 5. In the case of older individuals, a lower intake of Ca was observed, both from total dairy products and particular dairy products groups (including milk, other milk drinks, cottage and rennet cheese), while a higher intake of Ca from dairy dishes was confirmed. BMI values were inversely correlated with Ca intake levels from cottage cheese only.
Table 5 Correlations of age and BMI with calcium intake from dairy sources: Polish women (n 625) aged >55 years, RAC-OST-POL Study, May 2010
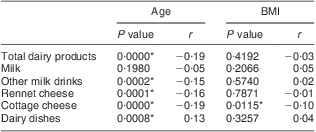
*Significant correlations (assessed by the Spearman correlation coefficient; P ≤ 0·05).
Table 6 presents correlations between Ca intake from various dairy sources and measured BMD values. Positive correlations between femoral neck and total hip BMD were observed for total dairy Ca intake as well as Ca intake from milk and other milk drinks, while a negative correlation was found between total hip BMD values and Ca intake from dairy dishes.
Table 6 Correlations between calcium intake from dairy sources and femoral neck and total hip bone mineral density (BMD): Polish women (n 625) aged >55 years, RAC-OST-POL Study, May 2010
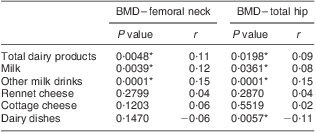
*Significant correlations (assessed by the Spearman correlation coefficient; P ≤ 0·05).
Discussion
The present data were obtained from research carried out in a group of Polish women aged >55 years, analysing their dietary Ca intake from dairy products and its association with osteoporosis. The correlation between dietary Ca intake and osteoporosis development arouses a great deal of controversy, as Ca intake is not the only factor affecting BMD( Reference Badurski, Jeziernicka and Dobreńko 9 ), but remains a vital issue that is evaluated intensively in various countries( Reference Fardellone, Cotté and Roux 26 – Reference Park, Heo and Park 30 ).
The group of women analysed in the present study was characterised by excessive body mass, with a median BMI of 30·84 kg/m2. The high BMI values in these women are congruent with BMI levels observed in osteoporosis studies conducted in Europe( Reference Fardellone, Cotté and Roux 26 , Reference Manios, Moschonis and Panagiotakos 27 ). Increased BMI may be associated with bone mineral loss( Reference Tseng, Huang and Liu 31 ) and during weight reduction overweight postmenopausal women are also more susceptible to bone loss, even if they are characterised by daily Ca intake of 1000 mg( Reference Riedt, Cifuentes and Stahl 32 ).
Femoral neck T-score is the main indicator of osteoporosis in BMD assessment( 18 ), with fractures being the most adverse effect of the disease( Reference Burge, Dawson-Hughes and Solomon 33 ). In the present study, women with either lower femoral neck T-scores or previous fractures were characterised by lower dairy Ca intake. Other reports indicate that postmenopausal women with higher intakes of dairy products are also characterised by higher lumbar BMD values( Reference Varenna, Binelli and Casari 10 ). Some researchers have emphasised a strong negative correlation between Ca intake and hip fracture incidence among women( Reference Yaegashi, Onoda and Tanno 34 ), as well as a correlation between Ca intake and broadband ultrasound attenuation Z-score in elderly women( Reference Lin, Chiu and Lin 35 ). However, other studies of Ca intake and osteoporosis indicated associations between Ca intake and BMD to be rather contradictory. Some researchers claim that, even if a certain influence of Ca intake on bone mass improvement is observed, neither Ca nor vitamin D supplementation demonstrates any short-term effect on fracture risk( Reference Nieves, Barrett-Connor and Siris 6 ); thus coming to the conclusion that no correlation exists between Ca intake and fracture incidence( Reference Zhong, Okoro and Balluz 28 ).
Dairy Ca intake may be perceived as a strong predictor of total Ca intake, as Ca intake from products other than dairy products is fairly constant. The results of other authors indicate that dairy products provide over 60 % of daily Ca intake in women's diets; in a group characterised by Ca intake of about 800 mg/d the contribution from dairy products was about 62 %, while in group characterised by Ca intake of about 900 mg/d the contribution amounted to about 84 %( Reference Gonnelli, Rossi and Montomoli 13 ). As the daily Ca intake from non-dairy products remains at a fairly stable level, a higher total daily Ca intake suggests a higher contribution of Ca from dairy products in the diet. The total Ca intake of the women in the present study may be estimated as the sum of Ca intakes from all products in the FFQ and about 250 mg Ca from products other than dairy products. Thus, in the present group of Polish women, Ca intake can be estimated at the level of about 675 mg/d. In other research conducted in postmenopausal women in Poland, the total Ca intake was similar – over 600 mg daily( Reference Bruyere, De Cock and Mottet 36 ). The latter study, performed in nine European countries, indicated that Polish women are characterised by low Ca intake compared with women in the other countries evaluated( Reference Bruyere, De Cock and Mottet 36 ). Ca intake in non-European populations was lower than in Poland – below 600 mg/d( Reference Pongchaiyakul, Kosulwat and Charoenkiatkul 37 – Reference Kumar, Mittal and Orito 39 ). The recommended daily Ca intake for postmenopausal women is 1000–1500 mg, depending on age and recommending institution( 40 ). As a result, a number of women in the present study were unable to satisfy their Ca requirement exclusively from their diet.
The present women had lower intakes of Ca from milk and milk drinks, as well as from cheeses and other products, as compared with the results of other studies( Reference Gonnelli, Rossi and Montomoli 13 ). The proportion of dairy products as sources of Ca was also different. In the study by Poliquin et al.( Reference Poliquin, Joseph and Gray-Donald 14 ) the proportion of Ca from milk to Ca from other dairy products was about 3·2:4 and in the study by Fardellone et al.( Reference Fardellone, Cotté and Roux 26 ) it was about 1·8:4, whereas the proportion was 0·7:4 in the present study. Simultaneously, Gonelli et al.( Reference Gonnelli, Rossi and Montomoli 13 ) found the proportion of Ca from milk and milk drinks to Ca from cheeses and other dairy products to be about 3:4, while this proportion was 5·5:4 in our study. Therefore it may be concluded that while milk consumption was low in the present study group, the consumption of other milk drinks was fairly high in comparison with the results from other research.
The strong effect of age on Ca intake we observed is also reported by other researchers( Reference Fardellone, Cotté and Roux 26 , Reference Pongchaiyakul, Kosulwat and Charoenkiatkul 37 , Reference Kumar, Mittal and Orito 39 ). According to other authors, the daily Ca intake of postmenopausal women is significantly lower than that of premenopausal women( Reference Kumar, Mittal and Orito 39 ) and the proportion of women with low Ca intake rises with age( Reference Fardellone, Cotté and Roux 26 , Reference Pongchaiyakul, Kosulwat and Charoenkiatkul 37 ). However, the character of the relationship is not obvious, as other authors report lower Ca inadequacy in women above 75 years of age( Reference Bruyere, De Cock and Mottet 36 ), probably associated with higher intake of Ca supplements( Reference Poliquin, Joseph and Gray-Donald 14 ).
In the present study it can be concluded that the lower Ca intake observed in the case of older women can be attributed to lower consumption of dairy products, probably as a result of changing nutritional habits. Simultaneously, milk consumption remains stable, independently of age, while the consumption of dairy dishes is higher, without any major impact on the total dairy Ca intake. With age, everyday activities such as preparing meals become more difficult( Reference Seidel, Brayne and Jagger 41 ) and chewing ability also decreases( Reference Locker 42 ). So, the above-mentioned higher consumption of dairy dishes may probably result from the fact that dairy dishes are easy to prepare and eat.
In the research of Zhong et al.( Reference Zhong, Okoro and Balluz 28 ), a close to significant association was observed between Ca intake and BMI: postmenopausal women with higher BMI values reported lower Ca intake. No such correlation was observed in our study group. The only correlation we observed, that between cottage cheese consumption and BMI, could have been attributed to the general observation that individuals with lower BMI consume more cottage cheese, also confirmed by other researchers( Reference Mullie, Godderis and Clarys 43 ).
In the present group of females, correlations were observed between Ca intake from various dairy sources and BMD that corresponded to the results of other authors, claiming similar correlations between Ca intake and BMD( Reference Kumar, Mittal and Orito 39 , Reference Napoli, Thompson and Civitelli 44 , Reference Farrell, Harris and Lohman 45 ).
In contrast, the observed negative correlation between Ca intake from dairy dishes and total hip BMD score is rather difficult to explain. Only the fact indicated previously, that dairy dishes are generally easier to prepare and to eat and may be more often chosen by elderly people, may provide an explanation. The positive correlation between dairy Ca intake and BMD in the case of Ca from milk and other milk drinks, but not for Ca from rennet cheese and cottage cheese, may be associated with lactose. Lactose is contained in milk and other milk drinks, being a factor promoting Ca absorption( Reference Uenishi and Yamaura 46 ). The content of lactose in milk and other milk drinks is significantly higher than in either rennet cheese or cottage cheese; according to the Polish food composition database, the lactose content is 4·6–4·9 g/100 g and 4·1–5·0 g/100 g for cow's milk and other milk drinks, as opposed to 0·1–1·0 g/100 g and 1·0–3·3 g/100 g for rennet cheese and cottage cheese, respectively( Reference Kunachowicz, Nadolna and Przygoda 19 ). So, it may be concluded that BMD is correlated not only with Ca from dairy products but with Ca intake from lactose-containing dairy products, where lactose improves Ca absorption.
One of the factors that influences Ca metabolism in the human body, and as a consequence reduces the risk of osteoporosis, is vitamin D3. In the present study, neither vitamin D intake nor its serum level was assessed. This may be a limitation of the study, but nevertheless the proper intake of Ca is the main dietary factor influencing bone density, and in the research of other authors a similar attitude is chosen( Reference Varenna, Binelli and Casari 10 , Reference Nguyen, Center and Eisman 47 ). Other potential limitations of our study may be associated with the fact that only 36 % of invited women participated and that BMD was measured at the hip only and spine radiograms were not available, thus some spine fractures might not have been taken into account.
Conclusions
In the present group of Polish women above 55 years of age, it may be concluded that lower BMD (femoral neck T-score ≤–2·5) and previous fractures were associated with lower reported dairy Ca intake. Due to the insufficient consumption of dairy products, a number of women were unable to satisfy their Ca requirement exclusively from their diet. The main dairy sources of Ca in the analysed group included milk, other milk drinks and rennet cheese. It is therefore important to recommend the consumption of dairy products with lactose, a Ca-absorption improving factor, such as milk and milk drinks, to improve BMD and reduce fracture risks in the population of postmenopausal women with osteoporosis.
Acknowledgements
Sources of funding: This research received no specific grant from any funding agency in the public, commercial or non-for-profit sectors. Conflicts of interest: The authors have no conflicts of interest to declare. Authors’ contributions: D.W. and W.P. designed study; D.W., D.G., A.K., P.A., A.C., W.G., B.D. and W.P. conducted the research; D.W., D.G. and A.K. analysed the data and performed the statistical analysis; D.W. and D.G. wrote the paper; D.W. had primary responsibility for final content.








