Monozygotic dichorionic diamniotic (DCDA) twin pregnancies are believed to occur if the cleavage-stage embryo divides within 3 days of fertilization, before differentiation of the inner cell mass. Monochorionic diamniotic (MCDA) twin pregnancies occur if the blastocyst-stage embryo divides between days 4 and 8 postfertilization, at the time of differentiation of the inner cell mass. Monochorionic monoamniotic (MCMA) twin pregnancies occur if the embryo divides between days 9 and 12, when the amnion has become established (Knopman et al., Reference Knopman, Krey, Lee, Fino, Novetsky and Noyes2010). A blastocyst ovum is generally believed to divide into monochorionic twin pregnancy.
However, recently, DCDA twin pregnancies after single blastocyst embryo transfer have been reported (Kyono, Reference Kyono2013; Shibuya & Kyono, Reference Shibuya and Kyono2012; Sundaram et al., Reference Sundaram, Ribeiro and Noel2018; Van Langendonckt et al., Reference Van Langendonckt, Wyns, Godin, Toussaint-Demylle and Donnez2000). Single blastocyst transfer is usually believed to result in MCDA or MCMA twin pregnancies, although it could result in DCDA twin pregnancies.
Among previous studies, there is only one known case wherein the zygosity in DCDA twin pregnancies after single blastocyst embryo transfer was analyzed accurately using genetic testing; the case was of a monozygotic DCDA twin pregnancy (Kyono, Reference Kyono2013). The mechanism of how a monozygotic DCDA twin pregnancy occurs by splitting a single blastocyst embryo is yet unknown. Processes such as micromanipulation of the zona pellucida during intracytoplasmic sperm injection (ICSI), preimplantation genetic screening (PGS) or preimplantation genetic diagnosis (PGD), or assisted hatching (AH) might cause herniation and subsequent splitting of the inner cell mass. Furthermore, DCDA twin pregnancies after single blastocyst embryo transfer could occur through a combination of natural conception and embryo transfer, which is dizygotic.
The aim of this study was to estimate the incidence of DCDA twin pregnancies after single blastocyst embryo transfer and to analyze their zygosity of using genetic testing.
Methods
All consecutive twin pregnancies managed in our institution from 2006 to 2014 were included in this study. We studied how the patients became pregnant, and the chorionicity and amnionicity. Chorionicity and amnionicity were determined using first-trimester ultrasonography and/or postnatal placental pathology. A pregnancy was diagnosed as a DCDA twin pregnancy if a thick septum membrane between the two fetuses and the λ sign was observed in ultrasonography, or two chorionic membranes and two amniotic membranes were observed in postnatal placental pathology. MCDA twin pregnancy was diagnosed if the following were observed: a thin septum membrane between two fetuses, consisting of two amniotic cavities, and the T sign on ultrasonography, or one chorionic membrane and two amniotic membranes were detected in postnatal placental pathology. MCMA twin pregnancy was diagnosed if the following were detected: no septum membrane between two fetuses on ultrasonography, or one chorionic membrane and one amniotic membrane on postnatal placental pathology.
Zygosity was analyzed if the cases were DCDA twins after a single blastocyst embryo transfer. Umbilical cord blood samples were obtained from the twin babies (baby 1 and baby 2) and whole blood samples were from their respective parents. Genomic DNA was extracted from the blood cells. Each sample was genotyped at 16 microsatellite markers using the AmpFISTR® Identifiler® PCR Amplification Kit (Applied Biosystems, Foster City, CA, USA). DNA was amplified by PCR, and PCR products were analyzed using a 3100 Genetic Analyzer™ (Applied Biosystems, Foster City, CA, USA). At informative loci showing a discordant genotype between parents, all twin babies inherited one copy each of alleles from their respective parents. When there were discordant genotypes at one or more informative loci, twins were diagnosed as dizygotic (Case 2, Figure 1). When genotypes of baby 1 and baby 2 were identical at all informative loci, twins were diagnosed as a monozygotic (Case 4, Figure 1).
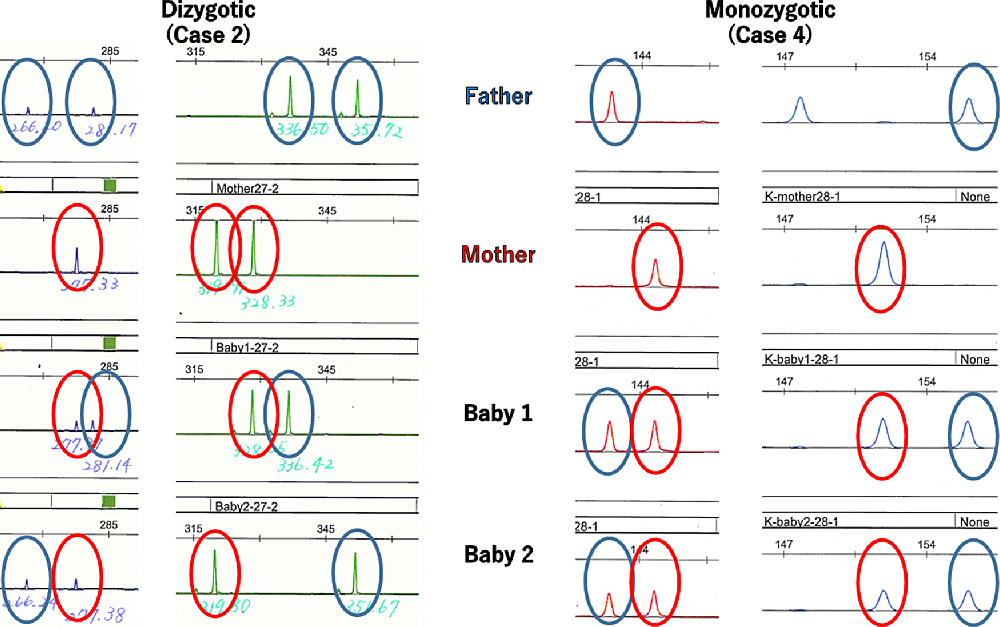
Fig. 1. Determination of twin zygosity by DNA genotyping at microsatellite marker loci. When there were discordant genotypes at one or more informative loci, twins were diagnosed as dizygotic (Case 2). When genotypes of baby 1 and baby 2 were identical at all informative loci showing a discordant genotype between parents, twins were diagnosed as a monozygotic (Case 4).
This study was approved by the ethics committee and the institutional research review board. Informed consent was sought and received from participating mothers. The data analysis was carried out according to the principles of the Declaration of Helsinki.
Results
Among the 655 twin pregnancies, there were 348 DCDA cases, 295 MCDA cases and 12 MCMA cases. The characteristics of all cases are shown in Table 1. Assisted reproduction was performed in 130 cases. Blastocyst embryo transfer was performed in 108 cases, among which 43 were single blastocyst embryo transfer. The characteristics of single blastocyst transfer are shown in Table 2. Six out of these 43 (14%) were DCDA twin pregnancies and the other 37 cases were MCDA twin pregnancies (Figure 2).
Table 1. Maternal characteristics in all cases
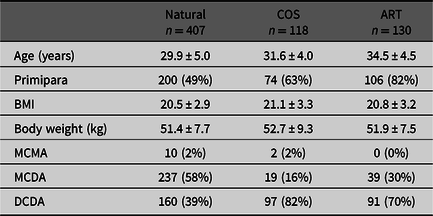
Note: Data are presented as mean ± SD or number (%).
COS, controlled ovarian stimulation; ART, assisted reproductive technology; BMI, body mass index; MCMA, monochorionic monoamniotic twin; MCDA, monochorionic diamniotic twin; DCDA, dichorionic diamniotic twin.
Table 2. Maternal characteristics of single blastocyst transfer

Note: Data are presented as mean ± SD, median (range) or number (%).
DCDA, dichorionic diamniotic twin; MCDA, monochorionic diamniotic twin; BMI, body mass index.
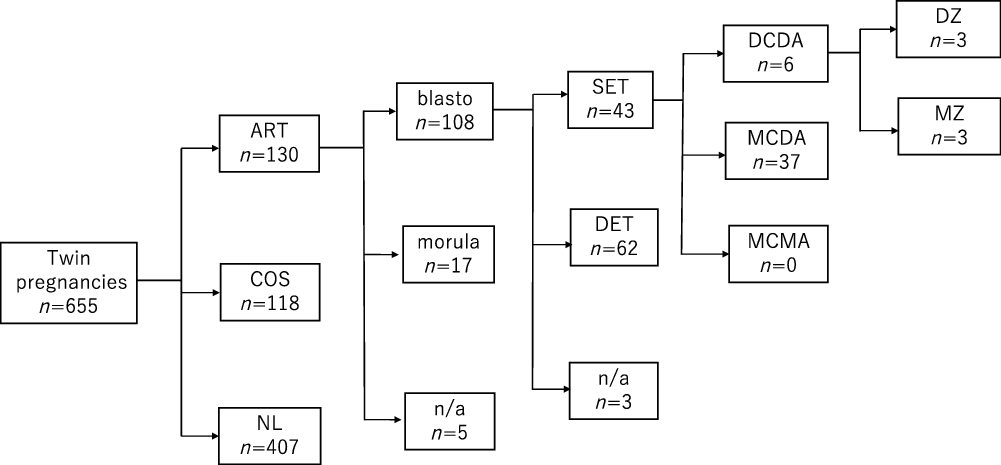
Fig. 2. Overview of all cases.
The zygosities of all six DCDA twins after single blastocyst embryo transfer were analyzed by means of the combined DNA index system. The characteristics of these six cases are shown in Table 3. Three cases were dizygotic and the other three cases were monozygotic DCDA twin pregnancies (Figure 1).
Table 3. Maternal characteristics of DCDA twins by single blastocyst transfer
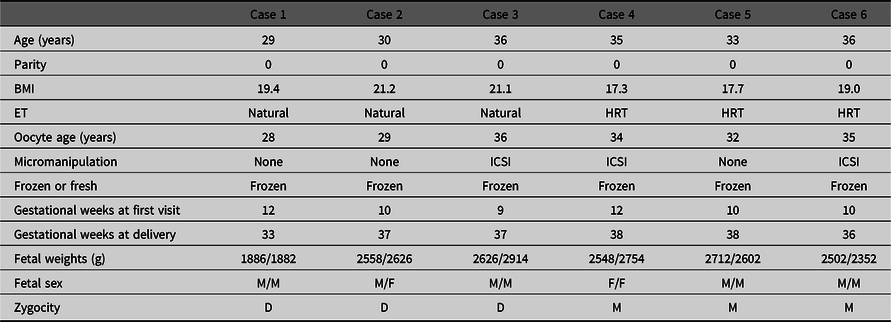
Note: DCDA, dichorionic diamniotic twin; BMI, body mass index; ET, embryo transfer; HRT, hormone replacement therapy; ICSI, intracytoplasmic sperm injection; M, male; F, female; D, dizygotic; M, monozygotic.
Discussion
DCDA twin pregnancies are either of monozygotic or dizygotic origin. DCDA twin pregnancies after single-embryo transfer are believed to result from separate cleavage-stage embryos until day 3 after fertilization (Knopman et al., Reference Knopman, Krey, Lee, Fino, Novetsky and Noyes2010). However, DCDA twin pregnancies after single blastocyst embryo transfer have been reported (Kyono, Reference Kyono2013; Shibuya & Kyono, Reference Shibuya and Kyono2012; Sundaram et al., Reference Sundaram, Ribeiro and Noel2018; Van Langendonckt et al., Reference Van Langendonckt, Wyns, Godin, Toussaint-Demylle and Donnez2000). Single blastocyst transfer is believed to result in MCDA or MCMA twin pregnancies, although it could result in DCDA twin pregnancies as well.
In previous studies, 12 multichorionic cases occurring after single blastocyst transfer have been reported (Sundaram et al., Reference Sundaram, Ribeiro and Noel2018). There has been only one case where zygosity was confirmed using genetic testing in DCDA twin pregnancies after a single blastocyst embryo transfer, and this case was a monozygotic pregnancy (Kyono, Reference Kyono2013). To the best of our knowledge, there are no studies that have accurately analyzed the incidence of zygosity of multichorionic cases after single blastocyst transfer using genetic testing.
In this study, 43 cases underwent single blastocyst embryo transfer. Furthermore, the zygosities of 6 DCDA twin pregnancies among these 43 twins were analyzed using the combined DNA index system. Three cases were confirmed to be dizygotic and the other three cases were confirmed to be monozygotic. This indicates that the occurrence of monozygotic DCDA twin pregnancies by single blastocyst transfer was the result of a separate blastocyst, and the occurrence of dizygotic DCDA twin pregnancies by single blastocyst transfer was pregnancies that occurred due to natural ovulation and concurrent blastocyst transfer. In three dizygotic cases, frozen embryo transfer (FET) was performed in the natural cycle without hormone supplements, which meant that spontaneous ovulation could have occurred. Dizygotic cases could be explained by a single embryo with a concurrent natural conception. On the other hand, in three monozygotic cases, FET was performed with hormone replacement therapy, which means that spontaneous ovulation could not have occurred. Monozygotic cases can be explained only by splitting of a single blastocyst embryo.
The mechanism of monozygotic DCDA twins by splitting a single blastocyst embryo has been inferred in several studies. A previous study reported visualization of an atypical hatching pattern of a vitrified or warmed blastocyst that resulted in two separate and complete blastocysts in vitro using time-lapse cinematography (Nishimura et al., Reference Nishimura, Tsuchiya, Kaneko, Matsui, Iizumi, Sato and Araki2014). A frozen-thawed blastocyst could split into two separate blastocysts and escape the zona pellucida. Some studies have reported that micromanipulation of the zona pellucida during ICSI, PGS/PGD or AH might cause herniation and subsequent splitting of the inner cell mass (Alikani et al., Reference Alikani, Noyes, Cohen and Rosenwaks1994, Elizur et al., Reference Elizur, Levron, Shrim, Sivan, Dor and Shulman2004; Hershlag et al., Reference Hershlag, Paine, Cooper, Scholl, Rawlinson and Kvapil1999; Nakasuji et al., Reference Nakasuji, Saito, Araki, Nakaza, Nakashima, Kuwahara and Sakumoto2014). In this study, micromanipulation was done in two cases of three monozygotic DCDA twin pregnancies. Although not all cases can be explained by micromanipulation, the relation cannot be denied.
Three cases (7%) of 43 single blastocyst transfer were monozygotic DCDA cases. In our study, the incidence of monozygotic DCDA twin pregnancies after single blastocyst transfer was not rarer than expected. Some studies have reported that the risk factors of monozygotic twin pregnancy after single-embryo transfer are young oocyte age, extended embryo culture and micromanipulation, such as ICSI or AH (Busnelli et al., Reference Busnelli, Dallagiovanna, Reschini, Paffoni, Fedele and Somigliana2019). In this study, blastocyst transfer was found to likely increase the incidence of monozygotic twin pregnancy after in vitro fertilization, which is comparable to the results of prior studies.
This study had several limitations, namely that we reported a small number of DCDA twin pregnancies by single blastocyst transfer, and not all the cases we studied underwent assisted reproductive technology in our institution. We need to expand the study to multiple centers in Japan and possibly in different countries. However, the primary strength of this study was that all DCDA twin pregnancies by single blastocyst transfer underwent genetical analysis of their zygosity using the combined DNA index system.
In conclusion, DCDA twin pregnancies were found to occur in six (14%) cases of twin pregnancies after single blastocyst embryo transfer, three (7%) cases of dizygotic pregnancies and three (7%) cases of monozygotic pregnancies. Dizygotic DCDA twin pregnancies after single blastocyst embryo transfer can result from a combination of natural conception and embryo transfer. A monozygotic DCDA twin pregnancy can also exist commonly after a single blastocyst embryo transfer, although the splitting mechanism is unknown.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.







