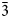Introduction
During the last twenty years, more than 40 new uranyl sulfates have been discovered as post-mining phases on the walls of mining tunnels in inactive uranium mines, mostly in mines in the White Canyon district of south-eastern Utah, USA and at Jáchymov, Czech Republic. We now realise that the diversity of secondary minerals formed by oxidation–hydration weathering of uraninite at ambient temperatures is much greater than previously thought. This diversity can be attributed to several factors including (1) relatively low pH caused by the release of sulfuric acid from altering sulfides (principally pyrite); (2) concentrations of ‘dissolved’ uranium in aqueous solutions; and (3) concentrations of various cations originating both from altered primary minerals or from the surrounding country rock (Plášil et al., Reference Plášil, Kampf, Ma and Desor2022). The new mineral shinarumpite, described herein, adds to the remarkable diversity of uranyl sulfate minerals.
Shinarumpite is named for the Shinarump member of the Upper Triassic Chinle formation. The uranium deposits in the White Canyon district occur within the Shinarump member. The Shinarump is the basal member of the Chinle formation. It consists primarily of sandstone, conglomerate and rare lenses of mudstone deposited by braided stream systems. The Shinarump is highly resistant to erosion and is prominently exposed over a large portion of the Colorado Plateau. It is the caprock for many of the mesas and buttes of Monument Valley. The word ‘shinarump’ is an old colloquial term used in the southwestern United States to refer to silicified wood; however, the Shinarump Conglomerate was first defined by G.K. Gilbert in 1875 and named for exposures in the Shinarump Cliffs, which straddle the Utah-Arizona border (Gilbert, Reference Gilbert1875).
The new mineral and name (symbol Sru) were approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2021-105, Kampf et al., Reference Kampf, Plášil, Olds, Ma and Marty2022a). The description is based on one holotype and three cotype specimens deposited in the collections of the Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, CA 90007, USA, catalogue numbers 76199 (holotype), 76200, 76201 and 76202.
Occurrence
Shinarumpite was first discovered on a specimen collected by one of the authors (JM) underground in the Scenic mine (37°38'43"N, 110°07'10"W) on Fry Mesa, White Canyon district, San Juan County, Utah, USA. The uranium deposits in the White Canyon district (Chenoweth, Reference Chenoweth1993) occur within the Shinarump member of the Upper Triassic Chinle Formation, in channels incised into the reddish-brown siltstones of the underlying Lower Triassic Moenkopi Formation. The Shinarump member consists of medium- to coarse-grained sandstone, conglomeratic sandstone beds and thick siltstone lenses. Ore minerals (uraninite, montroseite, coffinite, etc.) were deposited as replacements of wood and other organic material and as disseminations in the enclosing sandstone. Since the mine closed, oxidation of primary ores in the humid underground environment has produced a variety of secondary minerals, mainly carbonates and sulfates, as efflorescent crusts on the surfaces of mine walls.
Shinarumpite is a rare mineral in the secondary mineral assemblage. It occurs on matrix comprised mostly of subhedral to euhedral, equant quartz crystals that are recrystallised counterparts of the original grains of the sandstone. It is associated with gypsum, deliensite, Co-rich rietveldite, scenicite (Kampf et al., Reference Kampf, Plášil, Olds, Ma and Marty2022b), shumwayite and sulfur. Shinarumpite alters by apparent dehydration to Co-rich rietveldite.
Physical and optical properties
Shinarumpite crystals are flattened rectangular prisms, up to ~1 mm in length, occurring in subparallel and divergent intergrowths (Fig. 1). Blades are elongated parallel to [010], flattened on {100} and exhibit the forms {100}, {010} and {001}. Crystals are yellow and transparent with vitreous lustre and white streak. The mineral is nonfluorescent. The Mohs hardness is ~2½, based upon scratch tests. Crystals are brittle with irregular, curved fracture. There is perfect cleavage on {100}. Shinarumpite is readily soluble in room-temperature H2O. The density measured by flotation in a mixture of methylene iodide and toluene is 2.58(2) g⋅cm–3. The calculated density is 2.569 g⋅cm–3 for the empirical formula and 2.575 g⋅cm–3 for the ideal formula.

Fig. 1. Yellow shinarumpite prisms partially encrusted with Co-rich rietveldite on holotype specimen (#76199); field of view 0.84 mm across.
Optically, shinarumpite is biaxial (–), with α = 1.515(2), β = 1.526(2), γ = 1.529(2) (measured in white light). The measured 2V from extinction data analysed using EXCALIBRW (Gunter et al., Reference Gunter, Bandli, Bloss, Evans, Su and Weaver2004) is 55(1)°; the calculated 2V is 54.8°. Dispersion is r < v, strong. The optical orientation is Z = b, X ^ a = 30° in the obtuse angle β. The mineral is pleochroic with X = very pale yellow, Y = pale yellow, Z = light yellow; X < Y < Z. The Gladstone–Dale compatibility index 1 – (K P/K C) for the empirical formula is –0.024, in the excellent range (Mandarino, Reference Mandarino2007), using k(UO3) = 0.118, as provided by Mandarino (Reference Mandarino1976).
Raman spectroscopy
Raman spectroscopy was done using a Horiba XploRA PLUS using a 532 nm diode laser, a 100 μm slit, a 1800 gr/mm diffraction grating and a 100× (0.9 NA) objective. The Raman spectrum of shinarumpite from 4000 to 60 cm–1 is shown in Fig. 2.

Fig. 2. Raman spectrum of shinarumpite recorded with a 532 nm laser.
A broad band consisting of several overlapping vibrations in the 3600 to 3000 cm–1 range (the most prominent are those at 3519, 3490, 3408, 3399 and 3330 cm–1) are attributed to the ν O–H stretching vibrations of the H2O molecules. This set of bands is comparable to that observed, for instance, for shumwayite and the synthetic phase, UO2SO4⋅2.5H2O (Vlček et al., Reference Vlček, Čejka, Císařová, Goliáš and Plášil2009; Kampf et al., Reference Kampf, Plášil, Kasatkin, Marty, Čejka and Lapčák2017a). According to the correlation given by Libowitzky (Reference Libowitzky1999), the approximate O–H⋅⋅⋅O hydrogen bond lengths range between 3.0 and 2.7 Å, in excellent agreement with those observed in the crystal structure (2.95–2.69 Å). In the region of the ν2 (δ) H2O bending vibrations, no peaks were observed, which is not unusual in Raman spectroscopy of hydrated minerals. Instead, the higher background observed there is a spectral artifact.
The bands at 1188, 1123 and 1102 cm–1 are assigned to the split triply degenerate ν3 antisymmetric stretching vibrations of the SO4 tetrahedra. The band at 1013 cm–1 and shoulder at 1001 cm–1 are assigned to the ν1 symmetric stretching vibrations of structurally independent SO4 tetrahedra. Some overlaps of these bands with the librations of H2O are to be expected (see Colmenero et al., Reference Colmenero, Plášil and Němec2020).
The Raman band at 927 cm–1 is attributed to the ν3 antisymmetric stretching vibration of the uranyl ion, UO22+. The most prominent Raman band at 839 cm–1 is attributed to the ν1 symmetric stretching vibration of the uranyl ion. The inferred U–O bond-lengths (after Bartlett and Cooney, Reference Bartlett and Cooney1989) of the uranyl group, ~1.77 Å (from both ν1 and ν3), are within the range derived from the current X-ray study.
The bands at 658 and 602 cm–1 have been assigned to the ν4 (δ) triply degenerated antisymmetric stretching vibrations of SO4 tetrahedra. Raman bands centred at 448 and 438 cm–1 are related to the split ν2 (δ) doubly degenerate bending vibrations of the SO4 tetrahedra.
The ν2 (δ) doubly degenerate bending vibrations of UO22+ (see Kampf et al., Reference Kampf, Plášil, Kasatkin, Marty, Čejka and Lapčák2017a; Plášil et al., Reference Plášil, Buixaderas, Čejka, Sejkora, Jehlička and Novák2010; Colmenero et al., Reference Colmenero, Plášil and Němec2020 and others) probably contribute to the multiple bands centred at ~243 cm–1, whereas Colmenero et al. (Reference Colmenero, Plášil and Němec2020) showed that the contribution of the bending energies of the uranyl ions in the structure is distributed over a wider energy region and thus, probably the strong band at 195 cm–1 is also actually the result of energy overlap between ν2 (δ) UO22+ and, for instance, U–Oeq–(H2O) stretches and bends. Weak bands at the lowest energies can be assigned to unclassified lattice modes, most probably skeletal vibrations of the entire infinite sheets of polyhedra.
Chemical composition
Electron microprobe analysis (EPMA) of shinarumpite from the Scenic mine (7 points) were performed at Caltech on a JEOL 8200 electron microprobe in wavelength dispersive mode. Analytical conditions were 15 kV accelerating voltage, 10 nA beam current and 10 μm beam diameter. Insufficient material is available for CHN analysis; however, the fully ordered structure unambiguously established the quantitative content of H2O. Analytical data are given in Table 1. The empirical formula (calculated on the basis of 21 O atoms per formula unit) is Co0.51Ni0.28Fe0.21U1.00S2.00O21H22 or, arranged structurally, [(Co0.51Ni0.28Fe0.21)Σ1.00(H2O)6][(UO2)(SO4)2(H2O)]⋅4H2O. The ideal formula is [Co(H2O)6][(UO2)(SO4)2(H2O)]⋅4H2O, which requires CoO 10.42, UO3 39.77, SO3 22.26, H2O 27.55, total 100 wt.%.
Table 1. Chemical composition (in wt.%) for shinarumpite.

* Based on the structure; S.D. – standard deviation.
X-ray crystallography and structure refinement
Powder X–ray diffraction was done on an aggregate of unground crystal fragments using a Rigaku R-Axis Rapid II curved imaging plate microdiffractometer, with monochromatised MoKα radiation. A Gandolfi-like motion on the φ and ω axes was used to randomise the sample orientation and observed d values and intensities were derived by profile fitting using JADE Pro software (Materials Data, Inc.). The powder data are presented in Supplementary Table S1 (see below).
The single-crystal structure data were collected at room temperature using the same diffractometer and radiation noted above. Less than ideal crystal quality limited the data collection range to 50°2θ and contributed to somewhat low data completeness (93.1%). The Rigaku CrystalClear software package was used for processing structure data, including the application of an empirical multi-scan absorption correction using ABSCOR (Higashi, Reference Higashi2001). The structure was solved using the intrinsic-phasing algorithm of the SHELXT program (Sheldrick, Reference Sheldrick2015a). SHELXL-2016 (Sheldrick, Reference Sheldrick2015b) was used for the refinement of the structure. All non-hydrogen atom sites were refined successfully with anisotropic displacement parameters. Difference-Fourier synthesis located all H atom positions, which were then refined with soft restraints of 0.82(3) Å on the O–H distances and 1.30(3) Å on the H–H distances and with the U eq of each H atom set to 1.2 times that of the related O atom. The Co sites refined to very slightly less than full occupancy by Co; however, in the final refinement the sites were assigned full occupancy by Co, which had no effect on the R values. Because of the closeness in atomic numbers (and scattering powers) of Fe(26), Co(27) and Ni(28), we consider the EPMA to be the best indication of the amounts of these elements present in shinarumpite, including the crystal used in the structure determination; consequently, for calculation of the bond-valences associated with the Co sites, we used the proportions of Co, Ni and Fe provided by EPMA. Data collection and refinement details are given in Table 2, atom coordinates and displacement parameters in Table 3, selected bond distances in Table 4, and a bond valence analysis in Table 5. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available with the Supplementary material (see below).
Table 2. Data collection and structure refinement details for shinarumpite.*

*R int = Σ|F o2–F o2(mean)|/Σ[F o2]. GoF = S = {Σ[w(F o2–F c2)2]/(n–p)}½. R 1 = Σ||F o|–|F c||/Σ|F o|. wR 2 = {Σ[w(F o2–F c2)2]/Σ[w(F o2)2]}½; w = 1/[σ2(F o2)+(aP)2+bP] where a is 0.033, b is 7.44 and P is [2F c2+Max(F o2,0)]/3.
Table 3. Atom coordinates and displacement parameters (Å2) for shinarumpite.

Table 4. Selected bond distances (Å) and angles (°) for shinarumpite.

D = donor; A = acceptor
Table 5. Bond valence analysis for shinarumpite. Values are expressed in valence units.

Bond valence parameters from Gagné and Hawthorne (Reference Gagné and Hawthorne2015). The values for the Co sites are based on the proportions of Co, Ni and Fe provided by EPMA. Hydrogen-bond strengths are based on O–O bond lengths from Ferraris and Ivaldi (Reference Ferraris and Ivaldi1988).
Description of the structure
The single independent U site in the structure is surrounded by seven O atoms forming a UO7 pentagonal bipyramid. This is the most typical coordination for U6+, particularly in uranyl sulfates, where the two short apical bonds of the bipyramid constitute the uranyl group. Four of the five equatorial O sites of the UO7 bipyramid participate in two different SO4 tetrahedra (centred by S1 and S2); the other equatorial O site is an H2O group. The linkages of pentagonal bipyramids and tetrahedra form an infinite [(UO2)(SO4)2(H2O)]2– sheet in the {100} plane (Fig. 3).

Fig. 3. The uranyl sulfate sheets of formula [(UO2)(SO4)2(H2O)]2– parallel to {100} in shinarumpite and parallel to {001} in leydetite. The H atoms of the H2O groups are shown as small white balls. The hydrogen bonds are shown with thin black lines.
Two independent Co sites (Co1 and Co2) are located on special positions (0 ½ 0) and (½ ½ ½), respectively and each is octahedrally coordinated by H2O groups. These Co(H2O)6 octahedra, along with four isolated H2O groups (OW18, OW19, OW20 and OW21), constitute the [Co(H2O)6⋅4(H2O)]2+ interstitial complex, which links the [(UO2)(SO4)2(H2O)]2– sheets to one another via an extensive system of hydrogen bonds (Fig. 4).

Fig. 4. The structures of shinarumpite and leydetite viewed down [010]. The O atoms of the isolated H2O groups are shown as large white balls. The H atoms of the H2O groups are shown as small white balls. The hydrogen bonds are shown with thin black lines. The unit-cell outlines are shown with dashed lines.
The structure of shinarumpite is very similar to that of leydetite, [Fe2+(H2O)6][(UO2)(SO4)2(H2O)]⋅4H2O (Plášil et al., Reference Plášil, Kasatkin, Škoda, Novák, Kallistová, Dušek, Skála, Fejfarová, Čejka, Meisser, Goethals, Machovič and Lapčák.2013; Fig. 4). The most noteworthy structural difference is in the configurations of the [(UO2)(SO4)2(H2O)]2– structural units (Fig. 3). The sheet in shinarumpite is topologically identical to that in wetherillite, [Na(H2O)3]2[Mg(H2O)6][(UO2)(SO4)2(H2O)]2⋅4H2O (Kampf et al., Reference Kampf, Plášil, Kasatkin and Marty2015).
Discussion
Plášil et al. (Reference Plášil, Kampf, Ma and Desor2022) recently discussed the relationships between structure, chemical composition and occurrence for the uranyl sulfate minerals. Herein, we will expand upon that discussion by considering the structural typologies of known uranyl sulfate minerals as they relate to the structure hierarchy hypothesis (see Hawthorne, Reference Hawthorne2014). Figure 5 compares the numbers of uranyl sulfate minerals with structures containing cluster, chain, sheet and framework structural units constructed from linkages of uranyl bipyramids and sulfate tetrahedra. Sheet structural units are by far the most common and it is worth noting that this is also true for synthetic uranyl sulfates and even for U6+ phases not containing sulfate groups (see Lussier et al., Reference Lussier, Lopez and Burns2016). Interestingly, the average charge deficiency per anion (CDA; see Schindler and Hawthorne, Reference Schindler and Hawthorne2008 and references therein) is very similar for the chains and sheets at ~0.2 valence units (vu). However, the full range in CDA is slightly narrower for sheets, which might reflect the somewhat greater rigidity of the sheet-like structural units in adopting possible OH/H2O configurations. The average CDA value for cluster structural units is much higher, 0.3 vu, which is not very common among uranyl minerals (see Schindler and Hawthorne, Reference Schindler and Hawthorne2008). It is very likely that such a high CDA is due to more alkaline conditions under which these minerals form, despite a much higher proportion of SO4 within the structural units (see Plášil et al., Reference Plášil, Kampf, Ma and Desor2022). We should not forget that the activity of alkalis (manifested by a high proportion of Na and K in the structures of these cluster-based uranyl sulfates; see Plášil et al., Reference Plášil, Kampf, Ma and Desor2022) is due to, or is causing, relatively alkaline conditions. The relationship between the average Lewis basicity (or CDA) of the structural units and the pH of the corresponding parental solution has been provided by Schindler and Hawthorne (Reference Schindler and Hawthorne2001) for borate minerals. It is noteworthy that borate minerals also have relatively high CDA values, often exceeding 0.3 vu.

Fig. 5. The total numbers of known uranyl sulfate minerals with cluster, chain, sheet and framework structural units. Each class is further characterised by the value of the charge deficiency per anion (CDA) (N = number of uranyl sulfates in the class; aver. = average value of the CDA). The minerals metauranopilite (Nováček, Reference Nováček1935) and jáchymovite (Čejka et al., Reference Čejka, Sejkora, Mrázek, Urbanec and Jarchovský1996) are not included due to the lack of structure information.
The lack of framework structures based on linkages of uranyl bipyramids and sulfate tetrahedra is not surprising, as framework structures are rare for U6+ minerals in general (see Lussier et al., Reference Lussier, Lopez and Burns2016). Although there are no uranyl sulfate framework structures, frameworks involving SeO4 or MoO4 polyhedra are known (Lussier et al., Reference Lussier, Lopez and Burns2016) and provide a rich avenue for synthetic studies. It is also worth considering the possibility of a uranyl-sulfate-based nanocluster, either in a mineral or synthetic structure. The only known naturally occurring uranyl-nanocluster mineral ewingite, Mg8Ca8(UO2)24(CO3)30O4(OH)12(H2O)138 (Olds et al., Reference Olds, Plášil, Kampf, Simonetti, Sadergaski, Chen and Burns2017), contains a characteristic fundamental building unit (FBU), a pentagonal uranyl bipyramid trimer. This seems to be an important piece of the uranyl-nanocluster puzzle in natural minerals, particularly without utilising uranyl-peroxide bridges that provide effective curvature for their formation (see Burns and Nyman, Reference Burns and Nyman2018). Moreover, as yet, there is no evidence that peroxide-based uranyl nanoclusters are abundant in Nature. At least one known uranyl sulfate contains such an FBU. It is the mineral alwilkinsite-(Y), Y(H2O)7[(UO2)3(SO4)2O(OH)3]⋅7H2O (Kampf et al., Reference Kampf, Plášil, Čejka, Marty, Škoda and Lapčák2017b), which contains a [(UO2)3O5(OH)5]9– trimer. The CDA of alwilkinsite-(Y), 0.18 vu, is close to the average CDA value for both chain and sheet uranyl sulfate structures. This suggests that some potential exists for uranyl sulfates to form exotic or novel structures based on trimers of uranyl pentagonal bipyramids. In particular, mineral associations that include zippeite-group minerals, mathesiusite, seaborgite and others having similar CDA values (see Plášil et al., Reference Plášil, Kampf, Ma and Desor2022) may provide opportunistic hunting grounds for new species.
Acknowledgements
Reviewer Fabrice Dal Bo and an anonymous reviewer are thanked for their helpful comments on the manuscript. A portion of this study was funded by the John Jago Trelawney Endowment to the Mineral Sciences Department of the Natural History Museum of Los Angeles County. This research was also financially supported by the Czech Science Foundation (project 20-11949S to JP).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.128
Competing interests
The authors declare none.