Atherosclerosis is the major source of morbidity and mortality in the developed world, representing the result of several underlying mechanisms and closely related to endothelial function and oxidative stress(Reference Libby and Theroux1, Reference Tousoulis, Antoniades and Tentolouris2). It is well known that the vascular endothelium regulates the balance between vasodilatation and vasoconstriction, including a number of different processes. Whenever this balance is upset, endothelial dysfunction occurs(Reference Libby and Theroux1, Reference Davignon and Ganz3). In addition, endothelial dysfunction is considered to be an early marker for atherosclerosis. Studies have shown that endothelial dysfunction is present even in the preclinical stage of atherosclerosis(Reference Celermajer, Sorensen and Bull4–Reference Reddy, Nair and Sheehan6).
Evidence suggests that increased production of reactive oxygen species and vascular inflammation play an important role in endothelial dysfunction. Several vascular sources of superoxide have been implicated including NADPH, xanthine oxidase, lipoxygenases and myeloperoxidase(Reference Droge7, Reference Hsich, Segal and Pagano8). Superoxide reacts with NO, resulting in the formation of peroxynitrite(Reference Ogita and Liao9). Moreover, vascular inflammation plays an important role in the process of endothelial dysfunction mainly though the pro-inflammatory cytokines, selectins and adhesion molecules(Reference Tousoulis, Antoniades and Stefanadis10).
Epidemiological and clinical studies showed that the Mediterranean diet is associated with significantly lower cardiovascular risk. Although it is difficult to distinguish specific dietary factors, data suggest that olive oil, used as primary component of the Mediterranean diet, may play a key role in cardiovascular protection(Reference Carluccio, Massaro and Scoditti11). It is still not clear whether other types of oil could be beneficial. Therefore, in the present study, we examined the effects of maize oil, cod liver oil, soya oil and extra virgin olive oil on endothelial function, vascular cell adhesion molecule 1 (VCAM-1) and oxidative stress status in healthy subjects.
Experimental methods
Study population and protocol
A total number of thirty-seven healthy young subjects were recruited for the present parallel study. Exclusion criteria were the presence of clinical evidence for atherosclerotic peripheral vascular disease or coronary atherosclerosis, the presence of any acute or chronic inflammatory diseases (e.g. liver diseases) or any other cardiac disease (including coronary artery disease documented by medical history). Subjects with any classic risk factor for atherosclerosis such as smoking, as well as those receiving any systemic medication, were also excluded from the study.
All the participants were randomly allocated into five groups to receive four different types of oil or water, in order to receive an equivalent amount of 50 ml as reported previously(Reference Vogel, Coretti and Plotnick12). Each type of oil had a known macronutrients composition commercially available. Thus, the composition per 100 ml of each type of oil was: extra virgin olive oil (824 kcal or 3449·9 kJ, 91·6 g fatty acids, 12·8 g saturated, 70·5 g monounsaturated and 8·3 g polyunsaturated); maize oil (825 kcal or 3454·1 kJ, 92 g fatty acids, 12 g saturated, 25 g monounsaturated and 55 g polyunsaturated); soya oil (825 kcal or 3454·1 kJ, 92 g fatty acids, 14 g saturated, 23 g monounsaturated and 55 g polyunsaturated); cod liver oil (830 kcal or 3475·0 kJ, 92 g cod oil, 0·00 828 g EPA, 0·00 736 g DHA, 0·0112 g vitamin A, 0·0001 g vitamin D and 0·068 g vitamin E). It is worth to mention that extra virgin olive oil was administered, as minor components of virgin olive oil are thought to be responsible for much of its beneficial effects(Reference Covas13, Reference Fitó, Guxens and Corella14). Oils were administered directly from their commercial form (liquid) and not as part of a meal. Endothelial function was evaluated by gauge-strain plethysmography at baseline, 1, 2 and 3 h after oil consumption, and blood samples were obtained at baseline and at 3 h. The group of subjects, who received water, allowed us to correct for any circadian variation of serum sVCAM-1, total lipid peroxides (PEROX) and endothelial function. The protocol was approved by the ethics committee of our institution and a written informed consent was given by each subject.
Forearm blood flow measurements
In the present study, we evaluated post-ischaemic hyperaemia as an index of endothelial function in the right forearm(Reference Tousoulis, Antoniades and Stefanadis15). Briefly, all measurements were performed in the morning in a dark quiet room under controlled temperature (22–24°C). All subjects abstained from food, tobacco, alcohol and caffeine-containing drinks for at least 12 h before vascular study. Before measurements were started, subjects were rested in a supine position for 30 min. Forearm blood flow (FBF) was measured using venous occlusion, gauge-strain plethysmography (EC-400, D.E. Hokanson, Inc., Bellevue, WA, USA) as previously described(Reference Tousoulis, Antoniades and Stefanadis15). Briefly, a mercury-in-silastic strain gauge, which had been electrically calibrated, was positioned on the widest part of the forearm. The pressure of the collecting cuff was set at 40 mmHg. After obtaining the baseline FBF value, hyperaemic FBF was recorded after occluding the wrist with a pressure set at 250 mmHg for 5 min. FBF output signal was transmitted to a computer with Hokanson arterial inflow NIVP3 software, and the arterial blood flow signal was analysed at a later time by an independent reader blinded to the subject's history. By the method, FBF in the forearm is calculated as the volume of blood flow (in ml) per 100 ml of forearm tissue per minute. Forearm vasodilatory response to reactive hyperaemia (RH) was defined as the % change in FBF from baseline to the maximum FBF during the period of RH as we have previously described in details(Reference Tousoulis, Antoniades and Stefanadis15). It was previously shown that RH is endothelium dependent, and this method is widely used as an index of endothelial function in human forearm resistance vessels. It is considered to be a marker of NO bioavailability (since infusion of endothelial NO synthase inhibitor NG-monomethyl-l-arginine inhibits RH by approximately 50 %)(15).
Subjects rested in a supine position and were asked to remain in the same position 15 min before obtaining measurements for the examination of endothelium-independent dilation. Fifteen minutes after RH and while blood flow was confirmed to have returned to the baseline level, baseline measurements were obtained and subsequently 0·4 mg of nitroglycerine (NTG) was sublingually administrated by a spray device. It is worth to mention that before measurements, all participants had a blood pressure test in order to avoid hypotensive response or other problems (subjects with systolic blood pressure lower than 105 mmHg were excluded from NTG measurements). After baseline measurements, NTG was administered. A 2–3 min period of post-administration is adequate for NTG to act. Following this 2 min time, continuous measurements (curves) were obtained for the examination of endothelium-independent dilation. Forearm vasodilatory response to NTG was defined as the percentage change in FBF from baseline to the maximum FBF achieved after sublingual administration of NTG 0·4 mg, respectively(Reference Tousoulis, Antoniades and Stefanadis15). NTG was expressed as the percentage change in FBF from baseline to the maximum FBF observed during the period post-NTG administration. Mean intra- and inter-observer variabilities of RH measurements in our laboratory in thirty-seven healthy volunteers were 3·1 (sem 1·7) and 4·1 (sem 1·9) %, respectively.
Biochemical determinations
Venous blood samples were taken at the beginning of each protocol and at 3 h post-intervention, before the final plethysmographic measurement, to avoid a possible effect of NTG administration on the measured parameters. After centrifugation at 1150 g at 4°C for 10 min, serum was collected and stored at − 80°C until assayed. Total lipid PEROX were measured by using a commercially available photometric technique (kit by ImmunDiagnostik, Bensheim, Germany). The intra-assay CV was 3·1 %, while the inter-assay CV was 5·1 % (a detection limit of 7 μmol/l). Quantitative sandwich enzyme immunoassay technique was used to measure sVCAM-1 serum levels (ELISA kit by R&D Systems, Wiesbaden, Germany). The intra-assay CV was 3·5 %, while the inter-assay CV 7·7 % (a detection limit of 0·17–1·26 ng/ml).
Statistical analysis
All variables were normally distributed, as confirmed by using the Kolmogorov–Smirnov test, and are expressed as means with their standard errors. Comparisons of baseline characteristics were performed by χ2 test for categorical variables or one-way ANOVA, followed by Bonferoni post hoc correction for multiple comparisons. The effect of treatment on each variable was examined by using ANOVA for repeated measures. Correlations between variables were performed by univariate analysis, and the Pearson's r coefficient was estimated. A pre-study sample size calculation indicated that a sample of six patients was able to detect a 20 % change in RH after an intervention, with a power of 90 % and α = 0·05. All analyses were carried out by using the SPSS v12.0 statistical package (SPSS Inc., Chicago, IL, USA).
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Institutional Ethics Committee. Written informed consent was obtained from all subjects/patients.
Results
The demographic characteristics and lipid profile of the participants are presented in Table 1. There were no significant differences between the five groups at baseline.
Table 1 Demographic characteristics and lipid profile of the participants*
(Mean values with their standard errors)
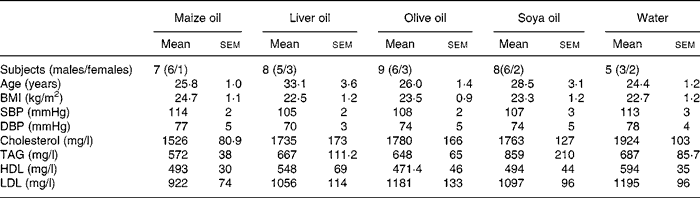
SBP, systolic blood pressure; DBP, diastolic blood pressure.
* There was no significant difference in any of the examined baseline characteristics between the five groups.
Effects on forearm blood flow measurements
Acute consumption of maize oil, extra virgin olive oil, soya oil, cod liver oil or water had no significant effect on absolute FBF at rest or during post-ischaemic hyperaemia (Fig. 1). However, maize oil induced a significant decrease in endothelial function as that was evaluated by RH, Fig. 2) 1 h after consumption. An opposite effect was observed for cod liver oil and soya oil, which improved RH 1 h post-consumption (Fig. 2). Surprisingly, extra virgin olive oil had no effect on RH during the 3 h post-consumption (Fig. 2). The change in RH (from baseline to 1 h post-oil consumption) was significantly lower in maize oil-treated subjects compared to water-treated (control) subjects (P < 0·05). The improvement of RH in the cod liver oil- and soya oil-treated subjects 1 h after consumption was significantly greater compared with controls (P < 0·05). There was no significant difference in NTG between the all study groups (P = NS).

Fig. 1 There was no significant effect of (a) water (![]() , rest;
, rest; ![]() , hyperaemia), (b) maize oil (
, hyperaemia), (b) maize oil (![]() , rest;
, rest; ![]() , hyperaemia), (c) extra virgin olive oil (
, hyperaemia), (c) extra virgin olive oil (![]() , rest;
, rest; ![]() , hyperaemia), (d) cod liver oil (
, hyperaemia), (d) cod liver oil (![]() , rest;
, rest; ![]() , hyperaemia) or (e) soya oil (
, hyperaemia) or (e) soya oil (![]() , rest;
, rest; ![]() , hyperaemia) on resting forearm blood flow (FBF) or maximum hyperaemic FBF during the first 3 h post-oil consumption.
, hyperaemia) on resting forearm blood flow (FBF) or maximum hyperaemic FBF during the first 3 h post-oil consumption.

Fig. 2 (a) Forearm vasidilatory response to reactive hyperaemia (RH) remained unchanged in the control group after water consumption (![]() ), while (b) it was significantly reduced after maize oil consumption (
), while (b) it was significantly reduced after maize oil consumption (![]() ). (c) Extra virgin olive oil had no effect on RH (
). (c) Extra virgin olive oil had no effect on RH (![]() ), while (d) cod liver oil induced a borderline improvement of RH at 1 h (
), while (d) cod liver oil induced a borderline improvement of RH at 1 h (![]() ). Importantly, (e) soya oil induced a significant improvement of RH 1 h post-consumption (
). Importantly, (e) soya oil induced a significant improvement of RH 1 h post-consumption (![]() ). * P < 0·05 and † P < 0·1 v. baseline.
). * P < 0·05 and † P < 0·1 v. baseline.
Effects on oxidative stress status and vascular inflammation
There were no significant differences between PEROX values at baseline and 3 h after consumption in all study groups. PEROX remained unchanged in all groups during the 3 h period (Fig. 3(a)). Similarly, acute oil consumption had no effect on serum levels of sVCAM-1 (Fig. 3(b)), suggesting that the acute consumption of oils has no effect on vascular inflammation in healthy individuals.

Fig. 3 (a) Total lipid peroxides (PEROX) as well as (b) soluble vascular cells adhesion molecule 1 (sVCAM-1) remained unchanged after oil consumptions in all study groups.
Discussion
In the present study, we examined the effects of acute consumption of equivalent amount of extra virgin olive oil, soya oil, cod liver oil and maize oil on endothelial function adhesion molecules and oxidative stress status in a population of healthy adults. We found that acute consumption of maize oil leads to impaired endothelial function, while cod liver oil and soya oil may improve endothelial function in human subjects.
Oil consumption and endothelial function
Previous studies have shown that oil consumption may affect endothelial function. The results have been controversial and still not clear as the implicated mechanisms affecting endothelial function are still under investigation. Cortés et al. (Reference Cortés, Núñez and Cofán16) found that consumption of olive oil decreased flow-mediated dilatation in healthy subjects and hypercholesterolaemic patients after 4 h. In controversy, flow-independent dilatation remained unchanged. Recent observations have shown that olive oils with different phenolic compounds content have different effects on endothelial function. Specifically, hypercholesterolaemic volunteers faced an improvement in ischaemic RH after high phenolic acid virgin olive oil(Reference Ruano, Lopez-Miranda and Fuentes17). Similar to Cortés et al., Rueda-Clausen et al. (Reference Rueda-Clausen, Silva and Lindarte18) supported that both olive oil and soyabean oil reduced flow-mediated dilatation after 3 h. Moreover, fish oil was superior to maize oil, according to its effects on endothelial function. Healthy volunteers fed with fish oil for a period of 14 d had a significant increase both in endothelial-dependent vasodilatation and endothelial-independent vasodilatation, while a similar effect did not exist with maize oil(Reference Shah, Ichiuji and Han19). Despite the beneficial effects on healthy subjects, fish oil supplementation significantly changed endothelium-dependent coronary vasodilatation in heart transplant recipients, but not endothelium-independent vasodilatation(Reference Fleischhauer, Yan and Fischell20). Finally, cod liver oil (fish oil) appeared to delay the impairment of endothelium-dependent relaxations in hypercholesterolaemia and atherosclerosis models, partly due to improved release of endothelium-derived relaxing factor and improved relaxation of coronary smooth muscle to the factor(Reference Shimokawa and Vanhoutte21).
In the present study, we have shown that vascular endothelium responds in a different way to different types of oil, despite the equivalent amount of oil received. We observed that endothelium-dependent dilatation was significantly reduced 1 h after consumption of maize oil, while soya oil induced a significant improvement of endothelial function 1 h after consumption. On the other hand, cod liver oil induced a borderline improvement of RH 1 h post-consumption. Interestingly, the present study showed that both the negative and positive effects of different oils consumption on endothelial function were diminished after 3 h time. This could be attributed to the fact that the healthy vascular endothelium, which was examined in the present study, has the potential to develop unknown defensive mechanisms after short time alterations (in 1–3 h). Additionally, the fact that both inflammatory process and oxidative stress status were unaffected after 3 h could imply that the association between these processes and endothelial function was also unaffected. Finally, like other studies, the present study was designed so that baseline testing was performed in the early morning and post-prandial testing was done about 3 h later. Probably, the physiologic improvement of endothelial function during the morning counteracted any changes due to effect of the different oil consumption.
Oil consumption, oxidative stress and inflammatory mechanisms
Several studies have investigated the association between oil consumption and oxidative stress status. Cortés et al. (16) were able to show such an association in hypercholesterolaemic patients and healthy individuals. Both the two groups faced a decrease in oxidative stress markers after olive oil consumption (as well as walnuts). It is well known that olive oil reduces oxidative stress due to polyphenols(Reference Droge7, Reference Covas, Nyyssönen and Poulsen22). In addition, a single dose of olive oil resulted in an increase in plasma lipid peroxidation products reaching a peak at 4–6 h and returning to baseline values at 24 h after oil ingestion(Reference Fitó, Gimeno and Covas23). More recently, consumption of olive oil at real-life doses has improved the fatty acid profile in LDL, the changes being associated with a reduction in the oxidative damage to lipids(Reference Cicero, Nascetti and López-Sabater24). In addition, diet modification with free virgin olive oil showed a significant reduction in oxidative stress markers after 3 months(Reference Fitó, Guxens and Corella14).
Furthermore, Suzukawa et al. (Reference Suzukawa, Abbey and Howe25) compared the effects of fish and maize oil on hypertensive patients. The results of the present study showed that from the standpoint of atherosclerosis, fish oil fatty acids adversely raise the susceptibility of LDL to Cu-induced and macrophage-mediated oxidation. Maize oil had no effect on the points measured in the study. Other studies comparing the effects of different kind of oils reported that fish oil is much more beneficial than olive oil regarding its antioxidant properties. Interestingly, another study evaluated that soyabean oil had a higher antioxidant capacity than olive oil too(Reference Pellegrini, Serafini and Colombi26). Regarding to soya, a recent study has found that consumption of soya protein in conjunction with a high-fat meal does not modify acutely postprandial oxidative stress in young healthy men(Reference Campbell, Brown and Dufner27).
Evidence suggests that dietary habits or modifications may affect inflammatory processes(Reference Estruch, Martínez-González and Corella28). It has been shown that Mediterranean diet reduced the levels of inflammatory markers in patients with metabolic syndrome after a long-term period(Reference Esposito, Marfella and Ciotola29). Similarly, n-3 fatty acids supplementation reduced sVCAM-1 levels in patients with coronary artery disease(Reference Johansen, Seljeflot and Høstmark30), which is a key adhesion molecule contributing to atherogenesis(Reference Tousoulis, Antoniades and Stefanadis10) and a marker of endothelial injury and activation(Reference O'Brien, McDonald and Chait31).
In addition, Fuentes et al. (Reference Fuentes, López-Miranda and Pérez-Martínez32) showed that different types of fat composition meals affected differentially (in 4 h) VCAM-1 levels, as MUFA appeared to be more beneficial than α-linoleic acid by reducing VCAM-1 levels. However, data seem to be more confusing when referring to healthy adults, as fish oil had no impact on VCAM-1 levels after a 12-week period(Reference Miles, Thies and Wallace33). Furthermore, the effect of different types of diet (different fat composition) on inflammation has also been examined. NF-κB, which plays a key role in the inflammatory response, was diminished after Mediterranean diet. On the contrary, Western diet increased 2·7-fold NF-κB compared with the Mediterranean diet(Reference Perez-Martinez, Lopez-Miranda and Blanco-Colio34). In addition, fish oil appeared to act beneficially in experimental model regarding to inflammation. Suppression of tumor necrosis factor-α and IL-6 expression by fish oil was attributed to its inhibitory effect on NF-κB activation, implicating that fish oil may ameliorate atherosclerosis by its immunomodulating effects(Reference Ma, Wang and Wang35).
In the present study, we have found that different types of oil have no effect on oxidative stress status in healthy adults. Specifically, total lipid PEROX remained unchanged after consumption of maize oil, extra virgin olive oil, soya oil and liver oil. Similarly, none of the used types of oils affected serum levels of sVCAM-1, a finding that implies that despite the effect of maize oil, soya and cod liver oil on vascular endothelium, these types of oil have no impact on vascular inflammatory process. The decision for the measurement of VCAM-1 levels needs further justification. It is well documented that the expression of adhesion molecules such as sVCAM-1 is increased in the presence of impaired endothelial function, and the soluble form of this molecule (sVCAM-1) cleaved and released to the circulation is a marker of endothelial injury and activation(Reference O'Brien, McDonald and Chait31). Therefore, by examining the impact of oils consumption on endothelial function (assessed by plethysmography), we evaluated NO release by vascular endothelium; while by measuring the changes in sVCAM-1, we searched for a possible effect on endothelial cell pro-atherogenic activation.
On the other hand, serum levels of sVCAM-1 are rapidly affected by various interventions, mainly due to the rapid cleavage of the soluble form of this molecule from endothelial surface and its release to the circulation, after endothelial activation. A rapid response of circulating sVCAM-1 after endothelial activation was documented by various studies in the past, and it seems that a period of 3–4 h is enough to observe this effect after an acute intervention(Reference Fuentes, López-Miranda and Pérez-Martínez32).
In conclusion, we have demonstrated that acute maize oil consumption blunts endothelial function in healthy individuals. On the contrary, acute consumption of codfish oil or soya oil slightly improves endothelial function 1 h after consumption, while olive oil has a rather neutral effect. The neutral effects of olive oil should be considered with caution as major components such as oleic acid act beneficially on endothelial function, while preventing from further alterations in inflammatory process and oxidative stress. In addition, other minor components of extra virgin olive oil such as oleuropein, hydroxytyrosol and tyrosol are strong antioxidants and radical scavengers, thus appearing to be protective for the vascular endothelium. However, such an effect was not observed in the present study. Most importantly, we observed that none of the four types of oil had an impact on PEROX and vascular adhesion molecule levels, suggesting that the observed effect on endothelial function is largely independent of oxidative stress alterations and vascular inflammatory process in healthy, young and low-risk individuals.
Study limitations
The present study showed that consumption of maize oil leads to impaired endothelial function, while soya oil and cod liver oil slightly improve endothelial function. However, no type of oils affected inflammatory process and systemic oxidative stress, suggesting that their effect on endothelial function may not be mediated by free radicals' bioavailability. Despite the fact that the results of the present study are indicative of the effects, that different types of oil exert on endothelial function, our findings cannot be widely generalised as there are some points that need further investigation and evaluation. Thus, the present study is characterised by a short follow-up period, as it investigates the acute effects of different types of oil. A longer follow-up period may have given different results. In addition, the study population was relatively small. However, the present study was adequately powered to identify significant differences. A pre-study sample size calculation indicated that a sample of six patients was able to detect a 20 % change in RH after an intervention, with a power of 90 % and α = 0·05. Finally, another limitation of the present study could be the absence of data regarding the lipid profile changes in the participants after oil consumption. Although this is an important issue to address, previous studies showed controversial results and this was the reason for not measuring post-prandial lipid levels.
Acknowledgements
The present research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
D. T. designed the study and wrote the manuscript. N. P. wrote the manuscript and collated all statistical information. C. A. designed the study and wrote the manuscript. A. G., G. B., P. G., E. S., A. M., G. S., T. P. and C. T. collated all statistical information. C. S. designed the study. All authors read and approved the findings of the study. Disclosures. There is no disclosure or any potential conflict of interest.






