Twinning rates vary considerably across the world, ranging from 6–9 per 1,000 maternities in South Asia, South-East Asia, and Latin America, 11–20 per 1,000 maternities in Europe and North America, to above 18 per 1,000 maternities in Central Africa (Hoekstra et al., Reference Hoekstra, Zhao, Lambalk, Willemsen, Martin, Boomsma and Montgomery2008; Smits & Monden, Reference Smits and Monden2011). In addition to regional differences, there are secular differences as well. Rates of twinning started to decline from around the year 1900 to the mid-20th century, but began to increase again in the late 1970s in most developed countries, including the United States, Japan, South Korea, and Western European countries (Hur & Song, Reference Hur and Song2009; Imaizumi, Reference Imaizumi, Blickstein, Keith and Keith2005; Macfarlane & Blondel, Reference Macfarlane, Blondel, Blickstein, Keith and Keith2005; Martin et al., Reference Martin, Hamilton, Osterman, Curtin and Matthews2015). In developing countries, however, changes in twinning rates over time are small and not in a specific direction (Smits & Monden, Reference Smits and Monden2011).
Since monozygotic (MZ) twinning generally occurs at a constant rate of about 4 per 1,000 maternities worldwide, the variation in twinning rates is mostly due to differences in dizygotic (DZ) twinning (Blickstein et al., Reference Blickstein, Keith and Keith2005; Bulmer, Reference Bulmer1970). Spontaneous DZ twinning is influenced by genetic, maternal, and environmental factors (Campbell, Reference Campbell, Blickstein, Keith and Keith2005; Hoekstra et al., Reference Hoekstra, Zhao, Lambalk, Willemsen, Martin, Boomsma and Montgomery2008). Maternal age has played a major role in twinning rate fluctuations during the past 100 years, following demographic trends (Bulmer, Reference Bulmer1970; Hoekstra et al., Reference Hoekstra, Zhao, Lambalk, Willemsen, Martin, Boomsma and Montgomery2008), but the rise in DZ twins seen in developed countries during the past two or three decades has been related to the increase in the use of fertility treatments (Fauser et al., Reference Fauser, Devroey and Macklon2005; Martin et al., Reference Martin, Hamilton, Osterman, Curtin and Matthews2015; Tandberg et al., Reference Tandberg, Bjorge, Bordahl and Skjaerven2007). Moreover, some studies have found that mothers of DZ twins are significantly taller and heavier and smoke more often before the twin pregnancy than mothers of MZ twins (Corney et al., Reference Corney, Seedburgh, Thompson, Campbell, MacGillivray and Timlin1979; Hoekstra et al., Reference Hoekstra, Willemsen, van Beijsterveldt, Lambalk, Montgomery and Boomsma2010; Nylander, Reference Nylander1981; Reddy et al., Reference Reddy, Branum and Klebanoff2005). Although MZ twinning has been considered an essentially random event, it has also been observed that the odds of producing MZ twins associated with fertility treatments are higher than in natural conception (Vitthala et al., Reference Vitthala, Gelbaya, Brison, Fitzgerald and Nardo2009).
Approximately two-thirds of MZ twins are monochorionic and share the same placenta and nutritive source, and thus may have higher risk of experiencing intrauterine growth restriction as indicated by lower birth weight in MZ than in DZ twins (Boomsma et al., Reference Boomsma, Willemsen, Geus, Kupper, Posthuma, Ijzerman, Dolan, Kordon, Gaillard and Christen2005; Corney et al., Reference Corney, Seedburgh, Thompson, Campbell, MacGillivray and Timlin1979; Johansson & Rasmussen, Reference Johansson and Rasmussen2001; Loos et al., Reference Loos, Beunen, Fagard, Derom and Vlietinck2001; Ramos-Arroyo et al., Reference Ramos-Arroyo, Ulbright, Yu and Christian1988). Twin studies from infancy to adulthood have reported non-significant or very small mean differences in height and relative weight by zygosity; however, a closer look at these results indicates a trend toward greater body size in DZ compared with MZ twins (Antoniades et al., Reference Antoniades, MacGregor, Andrew and Spector2003; Boomsma et al., Reference Boomsma, Willemsen, Geus, Kupper, Posthuma, Ijzerman, Dolan, Kordon, Gaillard and Christen2005; Estourgie-van Burk et al., Reference Estourgie-van Burk, Bartels, van Beijsterveldt, Delemarre-van de Waal and Boomsma2006; Hur et al., Reference Hur, Kaprio, Iacono, Boomsma, McGue, Silventoinen and Mitchell2008; Jelenkovic et al., Reference Jelenkovic, Ortega-Alonso, Rose, Kaprio, Rebato and Silventoinen2011; Lajunen et al., Reference Lajunen, Kaprio, Keski-Rahkonen, Rose, Pulkkinen, Rissanen and Silventoinen2009; Schousboe et al., Reference Schousboe, Willemsen, Kyvik, Mortensen, Boomsma, Cornes and Harris2003; Silventoinen et al., Reference Silventoinen, Sammalisto, Perola, Boomsma, Cornes, Davis and Kaprio2003, Reference Silventoinen, Bartels, Posthuma, Estourgie-van Burk, Willemsen, van Beijsterveldt and Boomsma2007a, Reference Silventoinen, Pietiläinen, Tynelius, Sørensen, Kaprio and Rasmussen2007b, Reference Silventoinen, Pietiläinen, Tynelius, Sørensen, Kaprio and Rasmussen2008b). It is largely unknown how these differences vary by age. Studies on age-dependent zygosity differences in height and body mass index (BMI) are scarce, and insufficient sample sizes make comparisons of the existing results problematic. Further, whether the variance of height and BMI differs between MZ and DZ twins has not been systematically studied previously.
Using international data obtained from twin cohorts in 22 countries, the present study aims to analyze zygosity differences in mean values and variances of height and BMI among males and females from infancy to old age, and to determine how these zygosity differences vary with age.
Materials and Methods
Sample
This study is based on the data from the COllaborative project of Development of Anthropometrical measures in Twins (CODATwins; Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Honda, Aaltonen, Yokoyama and Kaprio2015). Briefly, the CODATwins project was launched in 2013 and was intended to recruit all twin projects in the world with information on zygosity and height and weight measurements. The present study included a total of 54 twin cohorts from 22 countries: one cohort from Africa (Guinea-Bissau Twin Study), three cohorts from Australia (Australian Twin Registry, Peri/Postnatal Epigenetic Twins Study, and Queensland Twin Register), nine cohorts from East-Asia (Guangzhou Twin Eye Study, Japanese Twin Cohort, Korean Twin-Family Register, Mongolian Twin Registry, Osaka University Aged Twin Registry, South Korea Twin Registry, Qingdao Twin Registry of Adults, Qingdao Twin Registry of Children, and West Japan Twins and Higher Order Multiple Births Registry), 22 cohorts from Europe (Adult Netherlands Twin Registry, Berlin Twin Register, Bielefeld Longitudinal Study of Adult Twins, Danish Twin Cohort, East Flanders Prospective Twin Survey, Finnish Older Twin Cohort, FinnTwin12, FinnTwin16, Gemini Study, Genesis 12–19 Study, Hungarian Twin Registry, Italian Twin Registry, Murcia Twin Registry, Norwegian Twin Registry, Portugal Twin Cohort, Swedish Twin Cohorts, Swedish Young Male Twins Study of Adults, Swedish Young Male Twins Study of Children, TCHAD-study, Twins Early Developmental Study, TwinsUK, and Young Netherlands Twin Registry), three cohorts from South-Asia and Middle-East (Longitudinal Israeli Study of Twins, Sri Lanka Twin Registry, and Turkish Twin Study) and 16 cohorts from North-America (Boston University Twin Project, California Twin Program, Carolina African-American Twin Study of Aging (CAATSA), Colorado Twin Registry, Michigan Twins Study, Mid-Atlantic Twin Registry, Minnesota Twin Family Study, Minnesota Twin Registry, NAS-NRC Twin Registry, Quebec Newborn Twin Study, SRI-International, Texas Twin Project, University of British Columbia Twin Project, University of Southern California Twin Study, University of Washington Twin Registry, and Vietnam Era Twin Study of Aging). From these cohorts, 35 are longitudinal and included from two to more than 10 measurements. A more detailed description of the participating twin cohorts was presented previously (Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Honda, Aaltonen, Yokoyama and Kaprio2015).
In the original database, there were 960,859 height and weight measures from MZ and DZ (the same sex and opposite sex) twins, at ages ranging from 1 to 103 years. Most of the height and weight measures were self-reported (67%) or parentally reported (19%), and only a minority was based on measured values (14%). Age was classified to single-year age groups from age 1 to 19 years (e.g., age 1 refers to 0.5–1.5 years range) and decade age groups from age 20 to 103 years (e.g., 20–29, . . ., 70–79, and age ≥ 80 years). BMI was calculated as follows: weight (kg)/height (m2). Impossible values and outliers were checked by visual inspection of histograms for each age and sex group. Outliers were removed to obtain an approximately normal distribution of height, whereas the distribution of BMI was allowed to be positively skewed. The number of observations removed represented less than 0.2% of the whole database. For the purpose of this article, we restricted the analyses to one observation per individual in each year/decade age group. In the final database, we had 842,951 observations for both height and BMI, and the maximum age at measurement was 102 years.
Statistical Analyses
Equality of mean values between MZ and DZ twins by age group and sex was tested using linear regression adjusted for birth year and cohort, and corrected for clustering of twin pairs. Equality of variances was tested using the Levene's clustered test based on the 10% trimmed mean as proposed by Iachine et al. (Reference Iachine, Petersen and Kyvik2010). This clustered version of the Levene's test is robust under the non-normality of outcomes. Percentage difference (%) between DZ and MZ twins in mean values [(DZ mean/MZ mean) × 100 - 100] and standard deviations (SD) [(DZ SD/MZ SD) × 100 - 100] of height and BMI were calculated. Statistical analyses were conducted using the Stata statistical software package (version 12.0; StataCorp, College Station, Texas, USA).
Results
Descriptive statistics by zygosity, age, and sex are listed in Tables 1 and 2 for height and BMI, respectively. Sample size for each zygosity, age, and sex group ranged between 1,154 and 11,426 individuals from age 1 through 19 years, and between 970 and 32,777 individuals in adulthood (≥20 years). The 6 and ≥80-year age groups had the smallest sample sizes. Briefly, mean height increased with age in childhood and adolescence and slightly decreased over adulthood (Table 1). Males were expectedly taller than females; only at the age of 11 and 12 years were girls slightly taller than boys. The SD of height was highest at 13 years in boys and 12 years in girls. Mean values for BMI declined slightly from the age of 1 to 5 years and then started to increase; these mean values were higher in males than in females from age 1 to 6 years and from the age of 16 years onwards (Table 2). The SD of BMI increased with age but slightly decreased for the oldest age groups.
TABLE 1 Number of Twin Individuals, Mean, and SD of Height (cm) by Zygosity, Age, and Sex

a p-value for equality of mean values.
b p-value for equality of variances; SD: standard deviation.
TABLE 2 Number of Twin Individuals, Mean, and SD of BMI (kg/m2) by Zygosity, Age, and Sex

a p-value for equality of mean values.
b p-value for equality of variances; SD: standard deviation.
Dizygotic twins were consistently taller than MZ twins, demonstrating zygosity differences in mean height. Statistical significance was attained particularly in adulthood because of the larger sample size, but also at many ages during childhood and adolescence (Table 1). Figure 1 illustrates the percentage difference (%) in the mean value and SD of height between DZ and MZ twins. DZ twins presented up to 1.7% greater height than MZ twins; the greatest differences were observed in middle and late childhood and decreased with age to <0.6% in adulthood. The SD of height was not significantly different between MZ and DZ twins at most ages, and the greatest zygosity differences were observed at the age of 1 and 2 years (higher SD in MZ twins) and at the age of 6 (higher SD in DZ twins) for both sexes.
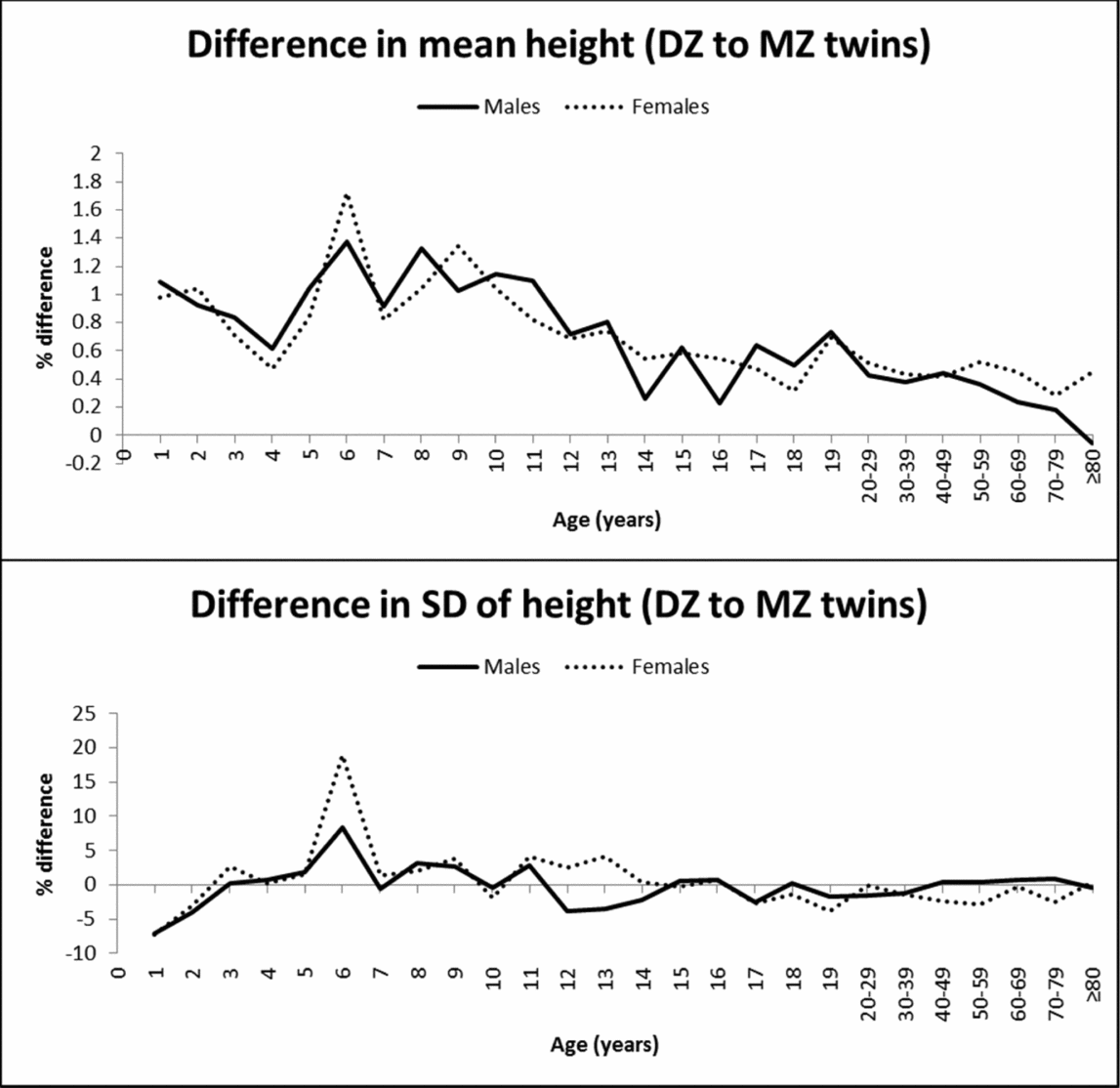
FIGURE 1 Mean and SD differences (%) in height between DZ and MZ twins across ages.
In contrast to the observations for height, mean BMI was not significantly different between MZ and DZ twins at young ages (Table 2). Significantly higher mean values in DZ than in MZ twins were observed at some ages from 11 to 30–39 years in males and from 10 to 50–59 years in females. The greatest mean differences between DZ and MZ twins ranged from 1.3–1.7% in males (at the age of 11, 14, and 17 years) and reached 1.9% in females (at the age of 6, 8, 9, and 11 years), and then decreased with age (Figure 2). The SD of BMI was significantly higher in DZ than in MZ twins, particularly in middle and late childhood; the highest difference was observed at the age of 6 years for females (24%) and was below 20% for the rest of the age groups. MZ twins presented a slightly greater SD at the age of 4 and 18 years in females and 1 and 50–59 years in both sexes. Finally, because of the positively skewed distribution of BMI, we tested the equality of mean values and variances for the log-transformed data, which produced very similar results (results not shown).
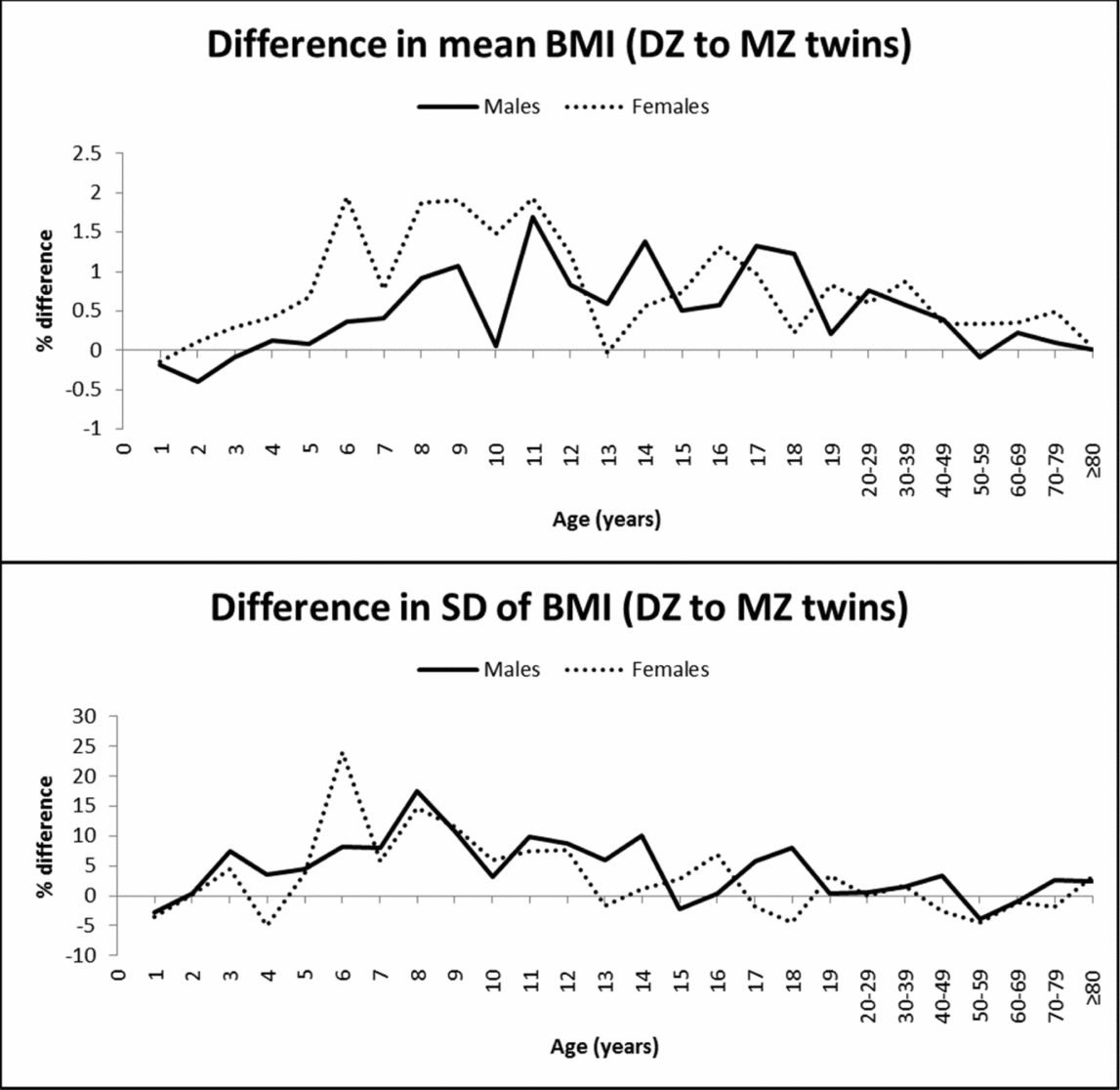
FIGURE 2 Mean and SD differences (%) in BMI between DZ and MZ twins across ages.
Discussion
The present study, based on an international database of twin cohorts with 842,951 measurements from infancy to old age, revealed zygosity differences in mean height and BMI in both male and female twins. Although zygosity was not associated with variance differences in height in most age groups, the variance of BMI was significantly different in MZ and DZ twins, particularly in childhood. However, these zygosity differences in mean values and variances of height and BMI were generally modest and age-dependent.
Zygosity differences have been analyzed previously for several health-related outcomes. For example, Oberg et al. (Reference Oberg, Cnattingius, Sandin, Lichtenstein, Morley and Iliadou2012) reported no substantial differences in cumulative morbidity in cardiovascular disease (CVD) and overall cancer in adult Swedish MZ and DZ twins. Some studies have reported higher risks of breast and testicular cancers in DZ than in MZ twins (Swerdlow et al., Reference Swerdlow, De Stavola, Swanwick and Maconochie1997; Verkasalo et al., Reference Verkasalo, Kaprio, Pukkala and Koskenvuo1999), but this has not been corroborated with data from the Nordic Twin Cancer project (Hjelmborg et al., Reference Hjelmborg, Scheike, Holst, Skytthe, Penney, Graff and Mucci2014). Large-scale register studies found no zygosity differences in the risk of diabetes (Johansson et al., Reference Johansson, Iliadou, Bergvall, de Faire, Kramer, Pawitan and Cnattingius2008; Kaprio et al., Reference Kaprio, Tuomilehto, Koskenvuo, Romanov, Reunanen, Eriksson and Kesäniemi1992; Lehtovirta et al., Reference Lehtovirta, Pietiläinen, Levalahti, Heikkila, Groop, Silventoinen and Kaprio2010; Petersen et al., Reference Petersen, Nielsen, Beck-Nielsen and Christensen2011), and although some studies have suggested that MZ twins have more adverse levels of glucose metabolism-related traits (Poulsen & Vaag, Reference Poulsen and Vaag2006; Poulsen et al., Reference Poulsen, Levin, Beck-Nielsen and Vaag2002), the findings are inconsistent (Benyamin et al., Reference Benyamin, Sørensen, Schousboe, Fenger, Visscher and Kyvik2007; Lehtovirta et al., Reference Lehtovirta, Kaprio, Forsblom, Eriksson, Tuomilehto and Groop2000; Rahman et al., Reference Rahman, Bennet, Pedersen, de Faire, Svensson and Magnusson2009; Souren et al., Reference Souren, Paulussen, Loos, Gielen, Beunen, Fagard and Zeegers2007). Regarding height and BMI, a trend toward greater mean values in DZ than in MZ twins has been observed in several studies. In Swedish males from birth to 18 years, although MZ twins tend to be taller at the age of 2 and 4 years, DZ twins showed slightly greater height at later ages (Silventoinen et al., Reference Silventoinen, Pietiläinen, Tynelius, Sørensen, Kaprio and Rasmussen2007b) and BMI in most age groups (Silventoinen et al., Reference Silventoinen, Pietiläinen, Tynelius, Sørensen, Kaprio and Rasmussen2008b). A study of 5-year-old children from the Netherlands found that MZ twins were significantly shorter than DZ twins, but inconsistent differences were found for weight and BMI (Estourgie-van Burk et al., Reference Estourgie-van Burk, Bartels, van Beijsterveldt, Delemarre-van de Waal and Boomsma2006). Finnish DZ twins at the age of 12, 14, and 17 years showed slightly higher values for height and BMI in both sexes (Jelenkovic et al., Reference Jelenkovic, Ortega-Alonso, Rose, Kaprio, Rebato and Silventoinen2011; Lajunen et al., Reference Lajunen, Kaprio, Keski-Rahkonen, Rose, Pulkkinen, Rissanen and Silventoinen2009). In a comparative study between Caucasian and East Asian adolescent twins of 13–15 years of age, a trend toward greater height in DZ twins was observed in Caucasian populations, but not in East Asians (Hur et al., Reference Hur, Kaprio, Iacono, Boomsma, McGue, Silventoinen and Mitchell2008). Hur et al. (Reference Hur, Kaprio, Iacono, Boomsma, McGue, Silventoinen and Mitchell2008) found no differences for BMI in either ancestry group. In adulthood, Dutch DZ twins were significantly taller (Boomsma et al., Reference Boomsma, Willemsen, Geus, Kupper, Posthuma, Ijzerman, Dolan, Kordon, Gaillard and Christen2005), and DZ women from the United Kingdom showed greater height, weight, and BMI than MZ twins (Antoniades et al., Reference Antoniades, MacGregor, Andrew and Spector2003). Accordingly, twin studies in seven European populations and Australia found that DZ men and women had slightly greater height and BMI in the majority of populations (Schousboe et al., Reference Schousboe, Willemsen, Kyvik, Mortensen, Boomsma, Cornes and Harris2003; Silventoinen et al., Reference Silventoinen, Sammalisto, Perola, Boomsma, Cornes, Davis and Kaprio2003).
Our results from this very large international database confirmed previous findings of a greater mean height and BMI in DZ than in MZ twins, and in addition showed that these differences (lower than 2% in all age groups) decrease with age. The small but significant zygosity differences observed in this study demonstrate the importance of large sample sizes to detect such differences; for example, to detect a difference of 1 cm in mean adult height (equal variances by zygosity) at a significance level of 0.05 and a power of 90%, we would need about 1,000 twins in each zygosity, age, and sex group. Thus, the non-significant findings reported in many earlier studies, based on smaller samples, would be primarily due to the lack of statistical power to detect such small differences.
The reasons for zygosity differences in height and BMI are not clear. It is possible that vascular and placental circumstances characterizing monochorionic pregnancies might be important; an indicator of the more adverse intrauterine environment of monochorionic MZ twins is their significantly lower birth weight compared with dichorionic MZ and DZ twins (Dube et al., Reference Dube, Dodds and Armson2002; Loos et al., Reference Loos, Beunen, Fagard, Derom and Vlietinck2001). Low birth weight predicts lower adult height and BMI in twins (Johansson & Rasmussen, Reference Johansson and Rasmussen2001; Pietiläinen et al., Reference Pietiläinen, Kaprio, Rasanen, Winter, Rissanen and Rose2001); however, the difference in body size between monochorionic and dichorionic twins has been observed to diminish during childhood (Falkner & Matheny, Reference Falkner, Matheny, Keith, Papiernik, Keith and Luke1995). The decreasing mean differences between MZ and DZ twins observed with age in our study, which were more evident for height, could be explained by the rapid catch-up growth that occurs in MZ twins, especially during the first years of life. Accordingly, a study on zygosity and chorion type showed that the prenatal disparities between monochorionic and dichorionic MZ twins did not result in larger intra-pair differences in adult height and BMI in monochorionic twins, as would be predicted from the prenatal programming hypothesis (Loos et al., Reference Loos, Beunen, Fagard, Derom and Vlietinck2001).
According to the ‘natural selection’ hypothesis, women who are predisposed to having twins are more likely to produce them in a healthy reproductive environment (Helle et al., Reference Helle, Lummaa and Jokela2004; Lummaa et al., Reference Lummaa, Haukioja, Lemmetyinen and Pikkola1998). Since variation in twinning is mostly due to differences in DZ twinning rates, and favorable reproductive conditions would be expected to result in more robust phenotypes in offspring, our findings of a greater height and BMI in DZ twins are in line with this hypothesis. Since height and BMI are highly heritable traits, the evidence that mothers of DZ twins are taller and heavier than mothers of MZ twins (Corney et al., Reference Corney, Seedburgh, Thompson, Campbell, MacGillivray and Timlin1979; Hoekstra et al., Reference Hoekstra, Willemsen, van Beijsterveldt, Lambalk, Montgomery and Boomsma2010; Nylander, Reference Nylander1981; Reddy et al., Reference Reddy, Branum and Klebanoff2005) offers a further possible explanation. Basso et al. (Reference Basso, Nohr, Christensen and Olsen2004) observed that the association of maternal height and BMI with the odds of twinning was slightly stronger when singleton mothers were compared with opposite-sex twin mothers (i.e., DZ twin mothers) than with all twin mothers. Although information on the zygosity of the same-sex twin pairs was not available in that study, it may reflect that DZ twin mothers not only differ from MZ twin mothers but also from non-twin mothers. Therefore, DZ twin parents might represent a group from the population with enrichment for a particular set of genes, and the greater height and BMI in DZ twins would be a reflection of this inheritance. However, our finding of decreasing zygosity differences with age suggests that genetics is not the only reason for the observed differences.
Another explanation for the observed zygosity differences might be fertility treatments, which generally produce DZ twins. It has been reported that parents of twins conceived via fertility treatments are better educated and are better off financially than those of naturally conceived twins (Burt & Klump, Reference Burt and Klump2012; Davies et al., Reference Davies, Moore, Willson, Van Essen, Priest, Scott and Chan2012). Due to the expenses of fertility treatments in many countries, these treatments would be more accessible to parents of a better socio-economic status (SES), which is in turn associated with taller height (Bogin, Reference Bogin2001). The association of SES with BMI is more complex and depends on the country's social and economic prosperity, and is generally inverse in developed countries (McLaren, Reference McLaren2007). However, because obesity has been associated with a higher risk of infertility (Lash & Armstrong, Reference Lash and Armstrong2009; Ramlau-Hansen et al., Reference Ramlau-Hansen, Thulstrup, Nohr, Bonde, Sørensen and Olsen2007), an increased use of fertility treatments among overweight and obese women could also account for higher BMI in DZ compared with MZ twins. Since the larger increase in DZ twinning rates started in the late 1980s (Blickstein et al., Reference Blickstein, Keith and Keith2005), it can be assumed that virtually no twins born before 1980 are the result of fertility treatments. Additional analyses of the data reported herein revealed that zygosity differences were also present in cohorts born before 1980 (results not shown), thus suggesting that differences between MZ and DZ twins are not related to fertility treatments.
The variance of height was overall similar in MZ and DZ twins, except at the age of 1 and 2 years. Similarly, other studies have reported no zygosity difference in height variance, and small differences between MZ and DZ twins did not show any consistent pattern (Antoniades et al., Reference Antoniades, MacGregor, Andrew and Spector2003; Boomsma et al., Reference Boomsma, Willemsen, Geus, Kupper, Posthuma, Ijzerman, Dolan, Kordon, Gaillard and Christen2005; Hur et al., Reference Hur, Kaprio, Iacono, Boomsma, McGue, Silventoinen and Mitchell2008; Jelenkovic et al., Reference Jelenkovic, Ortega-Alonso, Rose, Kaprio, Rebato and Silventoinen2011; Silventoinen et al., Reference Silventoinen, Sammalisto, Perola, Boomsma, Cornes, Davis and Kaprio2003, Reference Silventoinen, Bartels, Posthuma, Estourgie-van Burk, Willemsen, van Beijsterveldt and Boomsma2007a, Reference Silventoinen, Pietiläinen, Tynelius, Sørensen, Kaprio and Rasmussen2008b). It should be noted that the zygosity difference in the variance of both height and BMI observed in females at age 6 was considerably greater than for the rest of age and sex groups, and thus its significance should be interpreted with caution.
In contrast to the observations for height, we found significant differences in the variance of BMI between MZ and DZ twins in middle and late childhood. Our findings are in agreement with the slightly greater variance in MZ twins until the age of 4 years but greater in DZ twins from the age of 5 years in Swedish males (Silventoinen et al., Reference Silventoinen, Pietiläinen, Tynelius, Sørensen, Kaprio and Rasmussen2007b). Other studies have also shown a trend toward a slightly greater variance of BMI for DZ twins in adolescence and adulthood (Antoniades et al., Reference Antoniades, MacGregor, Andrew and Spector2003; Lajunen et al., Reference Lajunen, Kaprio, Keski-Rahkonen, Rose, Pulkkinen, Rissanen and Silventoinen2009; Schousboe et al., Reference Schousboe, Willemsen, Kyvik, Mortensen, Boomsma, Cornes and Harris2003). A possible explanation is social interaction, which causes variance of a phenotype to depend on the degree of relationship of social actors (Rietveld et al., Reference Rietveld, Posthuma, Dolan and Boomsma2003). Social interactions can have important implications for quantitative genetic models because they produce systematic differences in twin variances; cooperation results in greater total phenotypic variance in MZ than in DZ twins, whereas competition results in greater total phenotypic variance in DZ twins. Competition or contrast effects, in which a high trait value in one sibling tends to act in the opposite direction in the other, might be expected to be especially marked in environments in which there is competition for limited resources (Rietveld et al., Reference Rietveld, Posthuma, Dolan and Boomsma2003). The greatest zygosity differences in the variance of BMI observed during childhood in our study might be indicating competition for nutritional resources in a period highly sensitive to environmental influences, when the individualized parental care provided during the first years of life becomes less important.
The main strength of the present study is the large sample size of our international database of twin cohorts, with height and weight measures covering the whole lifespan. In contrast to earlier meta-analyses of twin data on height and BMI, our analysis is based on individual (although anonymized) data. However, a limitation is that countries or regions are not equally represented, and the database is heavily weighted toward Caucasian populations following westernized lifestyles. Another limitation of the data is that overall unadjusted descriptive statistics reflect not only within population differences but also differences in the distribution within each age group of different cohorts. Multiple testing may have resulted in false-positive differences between MZ and DZ twins; however, mean values and variances showed a quite consistent pattern across age and sex groups, which provides considerable robustness to the results. Moreover, information on chorionicity is crucial to determine whether the observed zygosity differences in height and BMI are explained, at least in part, by differences in monochorionic and dichorionic MZ twins. Finally, another important issue is whether twins differ from singletons in their height and BMI. Some studies reported that the differences in body size between twins and singletons disappear in childhood, while others showed these differences to remain until adulthood (Buckler & Green, Reference Buckler and Green2004; Eriksen et al., Reference Eriksen, Sundet and Tambs2013; Estourgie-van Burk et al., Reference Estourgie-van Burk, Bartels, van Beijsterveldt, Delemarre-van de Waal and Boomsma2006, Reference Estourgie-van Burk, Bartels, Boomsma and Delemarre-van de Waal2010; Pietiläinen et al., Reference Pietiläinen, Kaprio, Rissanen, Winter, Rimpelä, Viken and Rose1999; Silventoinen et al., Reference Silventoinen, Magnusson, Tynelius, Kaprio and Rasmussen2008a). In the present study, we do not have comparable sampling schemes for singletons; however, differences between twins and singletons would not invalidate the twin method, but depending on the cause of these differences offer an interesting opportunity for further research. Further research in twins and their sibling first needs to determine whether early life differences in body size between twins and the general population disappear in childhood or remain until adulthood. Mechanistic searches for possible causes for complete or incomplete catch-up growth in twins may focus on whether these causes differ for DZ and MZ twins, and maybe even shed light on the genes that are associated with twinning itself.
We observed that DZ twins were generally taller and had greater BMI than MZ twins. However, these zygosity differences were modest and decreased with age in both sexes, but still may be associated with genes that also influence DZ twinning itself. Alternatively, social explanations may be of importance, where, for example, the greater variance observed in DZ twins for BMI in childhood might indicate competition for nutritional resources. These findings have theoretical significance and might help to shed light on the underlying mechanisms linking zygosity status and body size in future research.
Acknowledgments
This study was conducted within the CODATwins project (Academy of Finland #266592). Support for participating twin projects: The University of Southern California Twin Study is funded by a grant from the National Institute of Mental Health (R01 MH58354). The Carolina African-American Twin Study of Aging (CAATSA) was funded by a grant from the National Institute on Aging (grant 1RO1-AG13662-01A2) to K. E. Whitfield. The NAS-NRC Twin Registry acknowledges financial support from the National Institutes of Health (grant No. R21 AG039572). Waves 1–3 of Genesis 12–19 were funded by the W T Grant Foundation, the University of London Central Research fund, and a Medical Research Council Training Fellowship (G81/343) and Career Development Award (G120/635) to Thalia C. Eley. Wave 4 was supported by grants from the Economic and Social Research Council (RES-000-22-2206) and the Institute of Social Psychiatry (06/07-11) to Alice M. Gregory, who was also supported at that time by a Leverhulme Research Fellowship (RF/2/RFG/2008/0145). Wave 5 was supported by funding to Alice M. Gregory from Goldsmiths, University of London. Anthropometric measurements of the Hungarian twins were supported by Medexpert Ltd., Budapest, Hungary. South Korea Twin Registry is supported by National Research Foundation of Korea (NRF-371-2011-1 B00047). Danish Twin Registry is supported by the National Program for Research Infrastructure 2007 from the Danish Agency for Science, Technology and Innovation, The Research Council for Health and Disease, the Velux Foundation, and the US National Institute of Health (P01 AG08761). Since its origin, the East Flanders Prospective Survey has been partly supported by grants from the Fund of Scientific Research, Flanders and Twins, a non-profit Association for Scientific Research in Multiple Births (Belgium). Korean Twin-Family Register was supported by the Global Research Network Program of the National Research Foundation (NRF 2011-220-E00006). Colorado Twin Registry is funded by NIDA center grant DA011015, and Longitudinal Twin Study HD10333. Author Brooke M. Huibregtse is supported by 5T32DA017637-10. Vietnam Era Twin Study of Aging was supported by National Institute of Health grants NIA R01 AG018384, R01 AG018386, R01 AG022381, and R01 AG022982, and, in part, with resources of the VA San Diego Center of Excellence for Stress and Mental Health. The Cooperative Studies Program of the Office of Research & Development of the US Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of NIA/NIH, or VA. The Australian Twin Registry is supported by the Centre of Research Excellence (grant ID 1079102) from the National Health and Medical Research Council administered by the University of Melbourne. The Michigan State University Twin Registry has been supported by Michigan State University as well as grants R01-MH081813, R01-MH0820-54, R01-MH092377-02, R21-MH070542-01, and R03-MH63851-01 from the National Institute of Mental Health (NIMH), R01-HD066040 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD), and 11-SPG-2518 from the MSU Foundation. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of NIMH, NICHD, or the National Institutes of Health. California Twin Program was supported by The California Tobacco-Related Disease Research Program (7RT-0134H, 8RT-0107H, and 6RT-0354H) and the National Institutes of Health (1R01ESO15150-01). Guangzhou Twin Eye Study is supported by National Natural Science Foundation of China (grant #81125007). PETS was supported by grants from the Australian National Health and Medical Research Council (grant Nos. 437015 and 607358 to Jeffrey M Craig, and Richard Saffery), the Bonnie Babes Foundation (grant No. BBF20704 to Jeffrey M. Craig), the Financial Markets Foundation for Children (grant No. 032-2007 to Jeffrey M. Craig), and by the Victorian Government's Operational Infrastructure Support Program. Data collection and analyses in Finnish twin cohorts have been supported by ENGAGE — European Network for Genetic and Genomic Epidemiology, FP7-HEALTH-F4-2007, grant agreement No. 201413, National Institute of Alcohol Abuse and Alcoholism (grants AA-12502, AA-00145, and AA-09203 to R J Rose, the Academy of Finland Center of Excellence in Complex Disease Genetics (grant Nos. 213506 and 129680), and the Academy of Finland (grants 100499, 205585, 118555, 141054, 265240, 263278, and 264146 to J Kaprio). K Silventoinen is supported by Osaka University's International Joint Research Promotion Program. S.Y. Öncel and F. Aliev are supported by Kırıkkale University Research Grant: KKU, 2009/43 and TUBITAK grant 114C117. Longitudinal Israeli Study of Twins was funded by the Starting grant No. 240994 from the European Research Council (ERC) to Ariel Knafo. Data collection and research stemming from the Norwegian Twin Registry is supported, in part, by the European Union's Seventh Framework Programmes ENGAGE Consortium (grant agreement HEALTH-F4-2007-201413, and BioSHaRE EU (grant agreement HEALTH-F4-2010-261433). The Murcia Twin Registry is supported by the Seneca Foundation, Regional Agency for Science and Technology, Murcia, Spain (08633/PHCS/08 and 15302/PHCS/10) and Ministry of Science and Innovation, Spain (PSI11560-2009). The Twins Early Development Study (TEDS) is supported by a program grant (G0901245) from the UK Medical Research Council, and the work on obesity in TEDS is supported, in part, by a grant from the UK Biotechnology and Biological Sciences Research Council (31/D19086). Madeira data comes from the following project: Genetic and environmental influences on physical activity, fitness and health: the Madeira family study project reference: POCI/DES/56834/2004 founded by the Portuguese agency for research (The Foundation for Science and Technology [FCT]). The Boston University Twin Project is funded by grants (Nos. R01 HD068435 and R01 MH062375) from the National Institutes of Health to K. Saudino. TwinsUK was funded by the Wellcome Trust; European Community's Seventh Framework Programme (FP7/2007–2013). The study also receives support from the National Institute for Health Research (NIHR) BioResource Clinical Research Facility and Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King's College London. University of Washington Twin Registry was supported by the grant NIH RC2 HL103416 (D. Buchwald, PI). Netherlands Twin Register acknowledges the Netherlands Organization for Scientific Research (NWO) and MagW/ZonMW grants 904-61-090, 985-10-002, 912-10-020, 904-61-193, 480-04-004, 463-06-001, 451-04-034, 400-05-717, Addiction-31160008, Middelgroot-911-09-032, Spinozapremie 56-464-14192, VU University's Institute for Health and Care Research (EMGO+), the ERC (ERC-230374), and the Avera Institute, Sioux Falls, South Dakota (USA). Gemini was supported by a grant from Cancer Research UK (C1418/A7974). The West Japan Twins and Higher Order Multiple Births Registry was supported by Grant-in-Aid for Scientific Research (B) (grant No. 15H05105) from the Japan Society for the Promotion of Science. The Quebec Newborn Twin Study acknowledges financial support from the Fonds Québécois de la Recherche sur la Société et la Culture, the Fonds de la Recherche en Santé du Québec, the Social Science and Humanities Research Council of Canada, the National Health Research Development Program, the Canadian Institutes for Health Research, Sainte-Justine Hospital's Research Center, and the Canada Research Chair Program (Michel Boivin).






