INTRODUCTION
Myocardial hypertrophy commonly involves the ventricular septumReference Chikamori, Doi and Akizawa 1 but may occasionally predominantly involve the cardiac apex. Since the initial characterization by Japanese researchers SakamotoReference Sakamoto, Tei and Murayama 2 and Yamaguchi,Reference Yamaguchi, Ishimura and Nishiyama 3 apical hypertrophic cardiomyopathy (ApHCM) has been recognized as a clinical entity with distinct electrocardiographic (ECG) findings including deep precordial T-wave inversion and echocardiographic findings involving a left ventricular (LV) ace-of-spades morphology.Reference Yamaguchi, Ishimura and Nishiyama 3 Patients are largely asymptomatic and have had a favourable prognosis in both a Japanese populationReference Sakamoto, Amano and Hada 4 , Reference Minami, Haruki and Hagiwara 5 (in which it is more common) and a non-Japanese North American population.Reference Eriksson, Sonnenberg and Woo 6 However, if these characteristic ECG findings are present with chest pain, patients may be inadvertently treated for an acute coronary syndrome (ACS).Reference Lin, Chen and Ding 7 - Reference Diaconu, Dumitru, Fruntelata, Lacau and Bartos 11 Given the time-sensitive nature and possible iatrogenic risks of reperfusion therapy, it is important to recognize ApHCM as a potential imitator of an acute myocardial infarction.
CASE REPORT
A 67-year-old man of Sudanese origin with no standard cardiac risk factors presented to our emergency department (ED) 30 minutes after awakening with new-onset continuously severe retrosternal chest pain and diaphoresis. There was no antecedent angina at baseline. Initial vital signs were as follows: oral temperature of 36.7°C (98.1°F), non-invasive blood pressure of 169/77, heart rate of 78, respiratory rate of 16, and oxygen saturation of 98% by pulse oximetry. A 12-lead ECG was performed within 15 minutes of arrival, showing a sinus rhythm with 2 mm of concave ST-segment elevation in leads V1-V2; ST-segment depression in leads V4-V6; and T-wave inversion in leads I, II, III, aVF, and V3-V6. In lead V4, the T wave was symmetrically inverted to a depth of 10 mm and the R wave was prominent, measuring at least 30 mm. Chest radiographs were unremarkable.
The patient was treated for an acute ST-elevation myocardial infarction (STEMI) in the ED and was later admitted to the coronary care unit (CCU) after the initial treatment. He was initiated on aspirin, clopidogrel, intravenous heparin infusion, and atorvastatin. As the patient presented to a non–percutaneous coronary intervention (PCI) capable hospital, the anticipated first medical contact to device time was >120 minutes; tenecteplase 50 mg was administered intravenously 60 minutes into his presentation. The patient was free of chest discomfort 90 minutes into admission. During admission, serial ECGs showed no resolution of the ST-segment elevation and persistent T-wave inversion (Figure 1). High-sensitivity cardiac troponin T was within normal limits, and both troponin and creatine kinase remained stable over several days. A coronary angiogram performed 13 hours into his presentation revealed normal coronary arteries without evidence of obstructive coronary disease with a left ventriculography demonstrating obliteration of the apical cavity at end-systole (Figure 2). Transthoracic echocardiography revealed a progressive increase in LV wall thickness toward the apex without evidence of LV systolic dysfunction or outflow tract obstruction (Figure 3). However, apical views were suboptimal, and no spade-like appearance of the apex was observed. On admission day three, the patient was discharged home on metoprolol.
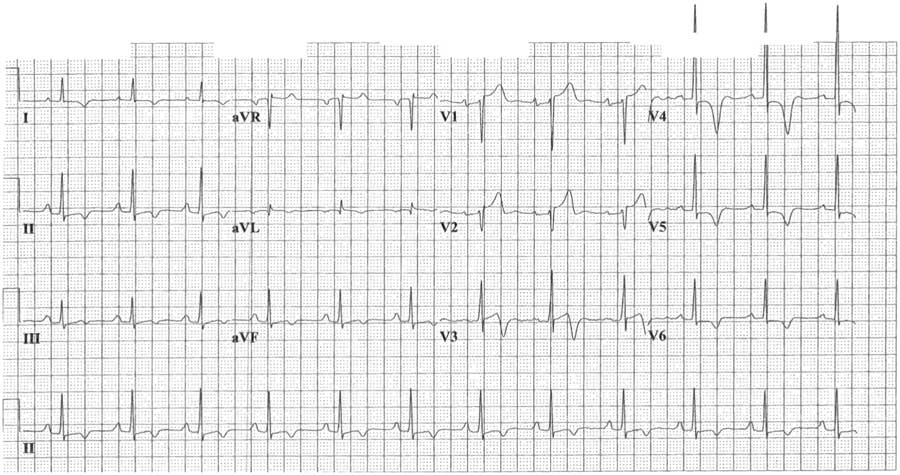
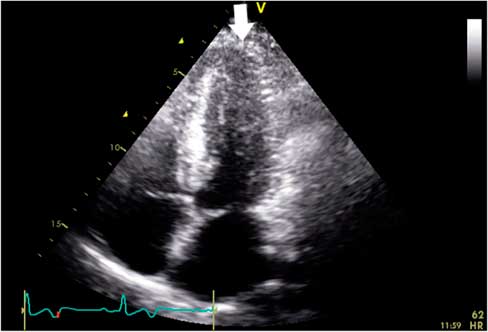
Figure 1 Index 12-lead electrocardiogram showing concave ST-segment elevation in leads V1-V2, ST-segment depression in leads V4-V6, and diffuse T wave inversion. In lead V4, the T wave was symmetrically inverted to a depth of 10 mm and the R wave was prominent, measuring at least 30 mm.
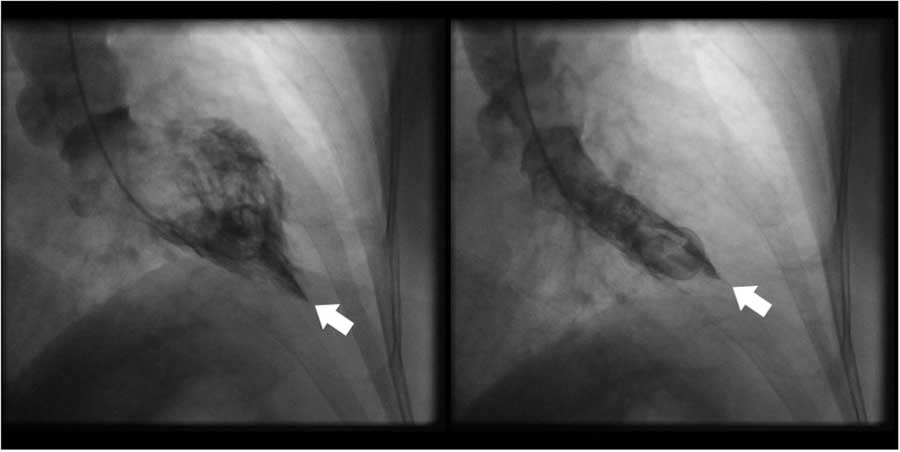
Figure 2 Left ventriculogram in right anterior oblique orientation showing (a) end-diastolic ace-of-spades configuration and (b) end-systolic obliteration of the apical cavity. White arrow indicates cardiac apex.
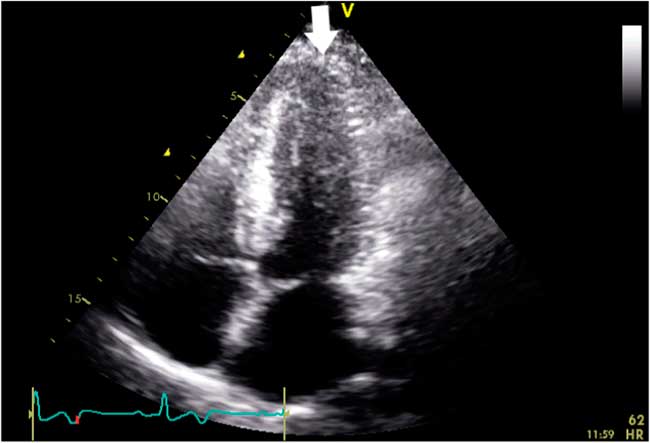
Figure 3 Transthoracic echocardiography showing progressive increase in wall thickness toward the left ventricular apex (white arrow) in the apical four-chamber view
Four months later, the patient was seen at an outpatient follow-up by his cardiologist and was well. Oral metoprolol was continued. Cardiac magnetic resonance imaging (MRI) was suboptimal but appeared to show an “ace-of-spades” configuration (Figure 4). A repeat transthoracic echocardiogram confirmed ApHCM with a progressive increase in wall thickness toward the apex with a wall thickness greater than 15 mm. A 24-hour Holter monitor showed predominantly a sinus rhythm with infrequent asymptomatic premature atrial contractions and no ventricular ectopy.
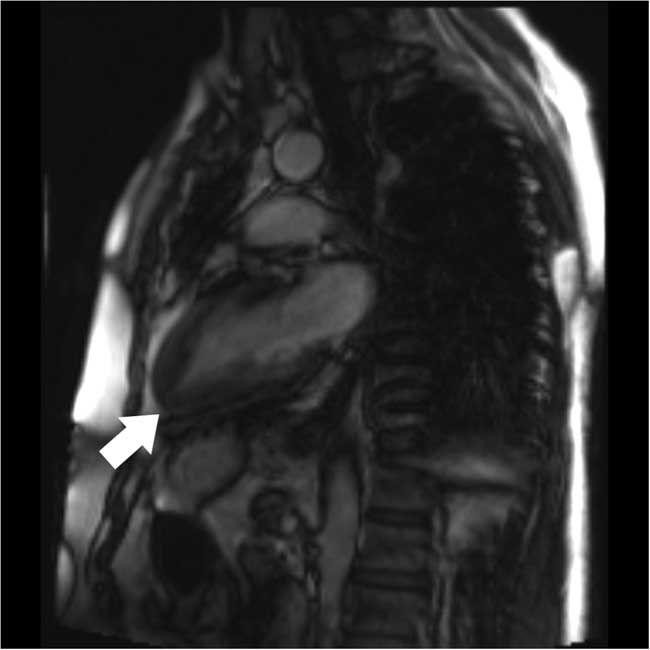
Figure 4 Cardiac magnetic resonance imaging demonstrating progressive thickening of myocardium toward the left ventricular apex (white arrow)
DISCUSSION
ApHCM is a mostly non-obstructive asymptomatic cardiomyopathy.Reference Sakamoto, Amano and Hada 4 - Reference Eriksson, Sonnenberg and Woo 6 In rare situations in which patients are symptomatic, they may present with adverse sequelae including heart failure (related to LV diastolic dysfunction and elevated filling pressures),Reference Webb, Sasson, Rakowski, Liu and Wigle 12 angina, syncope, arrhythmia including atrial fibrillation, stroke from apical thromboembolism, and sudden cardiac death.Reference Jan, Todaro, Oreto and Tajik 13 , Reference Moon, Shim and Ha 14 ApHCM was historically regarded as a phenotype largely unique to Japanese patients, in whom it was first described. It occurs more frequently in the Japanese population, with one comparative study revealing apical variants accounting for 15% and 3% of all hypertrophic cardiomyopathy within Japanese and Minnesotan cohorts, respectively.Reference Kitaoka, Doi and Casey 15 Smaller studies have suggested a prevalence between 3% and 11% in North American cohorts.Reference Chikamori, Doi and Akizawa 1 , Reference Eriksson, Sonnenberg and Woo 6 , Reference Wigle, Sasson and Henderson 16 , Reference Cannan, Reeder, Bailey, Melton and Gersh 17 The etiology of ApHCM is multifactorial, with studies suggesting variants with a genetic predispositionReference Towe, Bos, Ommen, Gersh and Ackerman 18 and also demonstrating exclusive development during adulthood.Reference Sakamoto, Amano and Hada 4 A family history is uncommon,Reference Sakamoto, Amano and Hada 4 , Reference Klarich, Attenhofer Jost and Binder 19 and genomic data regarding inheritance patterns, penetrance, and expressivity of known genetic mutations are presently unknown.Reference Towe, Bos, Ommen, Gersh and Ackerman 18
Classically described ECG findings comprise “giant negative T waves (greater than 10 mm) associated with high QRS voltage (R wave greater than 26 mm in lead V5 or the sum of the S wave in lead V1 and the R wave in lead V5 35 mm or more).”Reference Yamaguchi, Ishimura and Nishiyama 3 These voltage criteria may be related to both LV hypertrophy or to differences in localized wall thickness leading to disparities in the duration of repolarization.Reference Sakamoto, Tei and Murayama 2 Later studies emphasized any precordial giant negative T waves as the hallmark of ApHCM, although giant negative T waves may be present in as little as 11% of American Caucasian patients with ApHCM.Reference Klarich, Attenhofer Jost and Binder 19 Giant negative T waves diminish with ageReference Eriksson, Sonnenberg and Woo 6 and are significantly more common in Japanese patients.Reference Kitaoka, Doi and Casey 15 Notably, giant negative T waves may be seen in myocardial ischemia, right ventricular hypertrophy, electrolyte abnormalities, hypertensive heart disease, and cerebrovascular disease.Reference Sakamoto, Tei and Murayama 2 , Reference Yamanari, Saito and Mikio 20 , Reference Jacobson and Schrire 21
Echocardiographic criteria described in research protocols include two-dimensional echocardiography showing asymmetric LV hypertrophy with apical predominance, an apical wall thickness of >15 mm, and a ratio of maximal apical to LV posterior wall thickness of >1.5 at end-diastole.Reference Eriksson, Sonnenberg and Woo 6 An end-diastolic “ace-of-spades” configuration on left ventriculography is considered pathognomonic for ApHCM. Moreover, this may reveal a midventricular obstruction and an apical aneurysm, two potentially overlapping morphologies with significant clinical implications.Reference Minami, Haruki and Hagiwara 5 Cardiac MRI may facilitate diagnosis even if echocardiography or ventriculography fail to help in the diagnosis of ApHCM initiallyReference Eriksson, Sonnenberg and Woo 6 and may allow for early detection in the absence of the “ace-of-spades” configuration.Reference Suzuki, Shimamoto and Nishikawa 22
Chest pain mimicking ACS in the setting of ApHCM has been previously reported,Reference Cubukçu, Scott and Williams 8 , Reference Olearczyk, Gollol-Raju and Menzies 10 , Reference Diaconu, Dumitru, Fruntelata, Lacau and Bartos 11 including ST-segment elevation in contiguous leads resulting in the administration of thrombolytics,Reference Lin, Chen and Ding 7 , Reference Sayin, Koçum and Kervancioglu 9 as in this case. If patients with ApHCM develop angina, several mechanisms have been proposed. Apical infarction may occur because of systolic midventricular obstruction from cavitary obliteration, with subsequent diminished coronary perfusion or high wall tension from an apical aneurysm.Reference Lin, Chen and Ding 7 Impaired diastolic relaxation or systolic compression of vessels by a hypertrophied myocardial mass may result in inadequate coronary flow.Reference Cubukçu, Scott and Williams 8 , Reference Jan, Todaro, Oreto and Tajik 13 Lastly, there may be a component of a supply-demand mismatch with an increased apical myocardial mass without sufficient coronary perfusion.
There is no specific treatment for ApHCM.Reference Olearczyk, Gollol-Raju and Menzies 10 Patients should be referred to a cardiologist for risk stratification, monitoring, and management. Given the heterogenous clinical spectrum, medical therapy is directed toward specific symptoms, including the judicious use of beta-blockers and diuretics for angina, arrhythmia, and heart failure. Patients with a concomitant apical aneurysm have a particularly poor prognosis.Reference Chen, Lei, Hsu, Chung and Sung 23 Overall survival has ranged greatly from 47% at 20 yearsReference Klarich, Attenhofer Jost and Binder 19 to 95% at 15 years,Reference Eriksson, Sonnenberg and Woo 6 with heterogeneity in age at the first presentation playing a role in the discrepancies.
The diagnosis of ApHCM can prove challenging if patients present with acute chest discomfort and an abnormal ECG. Integral components of the initial diagnostic process in this instance include a careful evaluation of coronary risk factors, quality of chest discomfort, ECG pattern and progression, and cardiac biomarker evolution. Despite these factors, if patients present without a known history of apical hypertrophy, activation of ACS protocols with reperfusion may be warranted based on the best evidence available to clinicians at the time. While ApHCM may interfere with the clinical diagnosis because of ECG findings indicating infarction, it is equally as important to consider alternative explanations for chest pain in this setting.
In a retrospective evaluation of this case, given the equivocal ECG and lack of cardiac biomarker elevation, point-of-care echocardiography (POCE) may have been useful in distinguishing ApHCM from ACS, leading one to consider causes of pain such as a supply-demand mismatch as opposed to plaque rupture resulting in STEMI. The use of POCE in the setting of suspected ACS to recognize regional wall-motion abnormalities has been studied,Reference Kontos, Arrowood, Paulsen and Nixon 24 including by emergency physicians using a standardized protocol.Reference Frenkel, Riguzzi and Nagdev 25 Kerwin and others have demonstrated that with brief standardized training, emergency physicians can improve their ability to recognize regional wall-motion abnormalities.Reference Kerwin, Tommaso and Kulstad 26 In the ED, the absence of regional wall-motion abnormalities on POCE has been used to rule out ACS in the setting of ST-segment elevation resulting from pulmonary embolismReference Shy, Gutierrez and Strayer 27 and hypercalcemia.Reference Strand, Aung and Agarwal 28 Herein, we suggest that POCE performed in the ED with the support of a cardiologist may have allowed for recognition of the apical hypertrophy as the cause of the patient’s ECG findings.
Our case illustrates the complex manner in which ApHCM may present in the era of rapid early reperfusion therapy. It should reinforce the idea that not all chest pain with simultaneous ST-segment elevation is a result of myocardial infarction. For patients with ECG findings suggestive of alternate diagnoses, POCE may be useful in guiding further management.
Acknowledgements
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests: None declared.