Introduction
Measles virus is highly contagious. Measles disease is characterised by fever, cough, coryza, conjunctivitis and a maculopapular rash lasting 3–5 days [Reference Laksono1, Reference Moss and Griffin2]. It also causes an immunosuppression that can last up to 2 years [Reference Mina3]. This immunosuppression leaves patients with measles susceptible to other pathogens [Reference Moss and Griffin2], particularly in the respiratory tract. Pneumonia, caused by other pathogens or measles virus itself, is the most common fatal complication, occurring in 56–86% of measles-related deaths [Reference Duke and Mgone4].
Worldwide, increased measles vaccination coverage caused a decline in the number of reported measles cases from 8 53 479 in 2000 to 2 54 928 in 2015 [Reference Patel5]. These numbers of reported cases are, however, incomplete: not every infected individual seeks care and not every consultation leads to a reported case [Reference Harpaz6].
The notification of only a fraction of measles cases may suffice to monitor transmission of measles and identify outbreaks [Reference Papania and Strebel7], but it will result in biased estimates for risk of infection and risk of developing severe disease or death upon infection. Incomplete reporting will also result in an underestimate of the true number of infections, which is an essential indicator in the context of measles elimination. If reporting is associated with certain characteristics of cases, under-reporting may result in biased estimates of these.
So far, two approaches have been used to assess completeness of measles reporting. A first approach uses community-based surveys to identify measles cases, and then assess how many of them are reported to a register. This survey approach has been used as early as 1926 in the USA [Reference Sydenstricker8]. Since then a few other community-based surveys have been published worldwide, reporting that notified cases could range from 3% up to 64% of total infections [Reference Harpaz6, Reference Trottier, Carabin and Philippe9]. These surveys, however, all originate from the 1900s [Reference Sydenstricker8, Reference Ewert10–Reference McDonnell and Jorm13] and lack laboratory confirmation of cases. The other approach used to assess the completeness of measles reporting involves comparing the number of cases reported with the number of people projected as susceptible and assuming that almost all are infected [Reference Fine and Clarkson14]. This approach resulted in an estimated completeness of reporting ranging from 7% for a measles outbreak in 1999–2000 in The Netherlands [Reference Wallinga, Teunis and Kretzschmar15], up to 63% for endemic measles in England and Wales in 1946–1979 [Reference Fine and Clarkson14].
We used both approaches to assess completeness of reporting during the most recent measles outbreak in The Netherlands, which took place between May 2013 and March 2014, spread mainly among orthodox Protestant school-aged children [Reference Woudenberg16], and consisted of 2700 cases reported to the national register of notifiable diseases. Orthodox Protestants form a socially and geographically clustered minority group in The Netherlands of about 250 000 individuals among whom vaccination coverage is approximately 60% [Reference Ruijs17]. In addition to measles outbreaks, this group has seen outbreaks of polio (last in 1992), rubella (last in 2004) and mumps (2008). Here, we used a community-based survey including laboratory testing of self-reported cases. In addition, we calculated the number of susceptible individuals in the community and nationally, and compared this with the number of reported cases to the national register.
Methods
National register of notifiable diseases
Measles is a notifiable disease in The Netherlands. Physicians and laboratories are required to report cases to the national electronic web-based register for notifiable diseases (Osiris) through local Municipal Health Services (MHS). Directors of schools and day-care centres are required to report clusters of children with rash in their institutions to MHS. Cases of measles are defined as clinical measles in a person with either laboratory-confirmed measles or epidemiologically linked to a laboratory-confirmed case. Criteria for clinical measles are fever and a maculopapular rash accompanied by at least one of the following symptoms: cough, running nose and red eyes.
Community-based survey
The study population consisted of all children born between 2000 and 2013 and living in the municipality of Rhenen (Fig. 1a). These children were surveyed through a questionnaire in the third trimester of 2014. The survey was limited to this age group because it comprised most (76%) of the reported cases [Reference Woudenberg16], and further, children born during this range of years could not have been infected in the previous outbreak in 1999–2000 [Reference van den Hof, Conyn-van Spaendonck and van Steenbergen18]. The survey took place in the municipality of Rhenen [Reference van Isterdael12], which had 19 116 inhabitants on 1 January 2014 (http://statline.cbs.nl). Rhenen is located in a region characterised by low vaccination coverage (known as the Bible belt), with a measles–mumps–rubella (MMR) vaccination coverage of 80% for the first dose at 14 months of age [Reference van Lier19]. This is substantially lower than the national vaccination coverage, which is approximately 95% in The Netherlands (Fig. 1a).
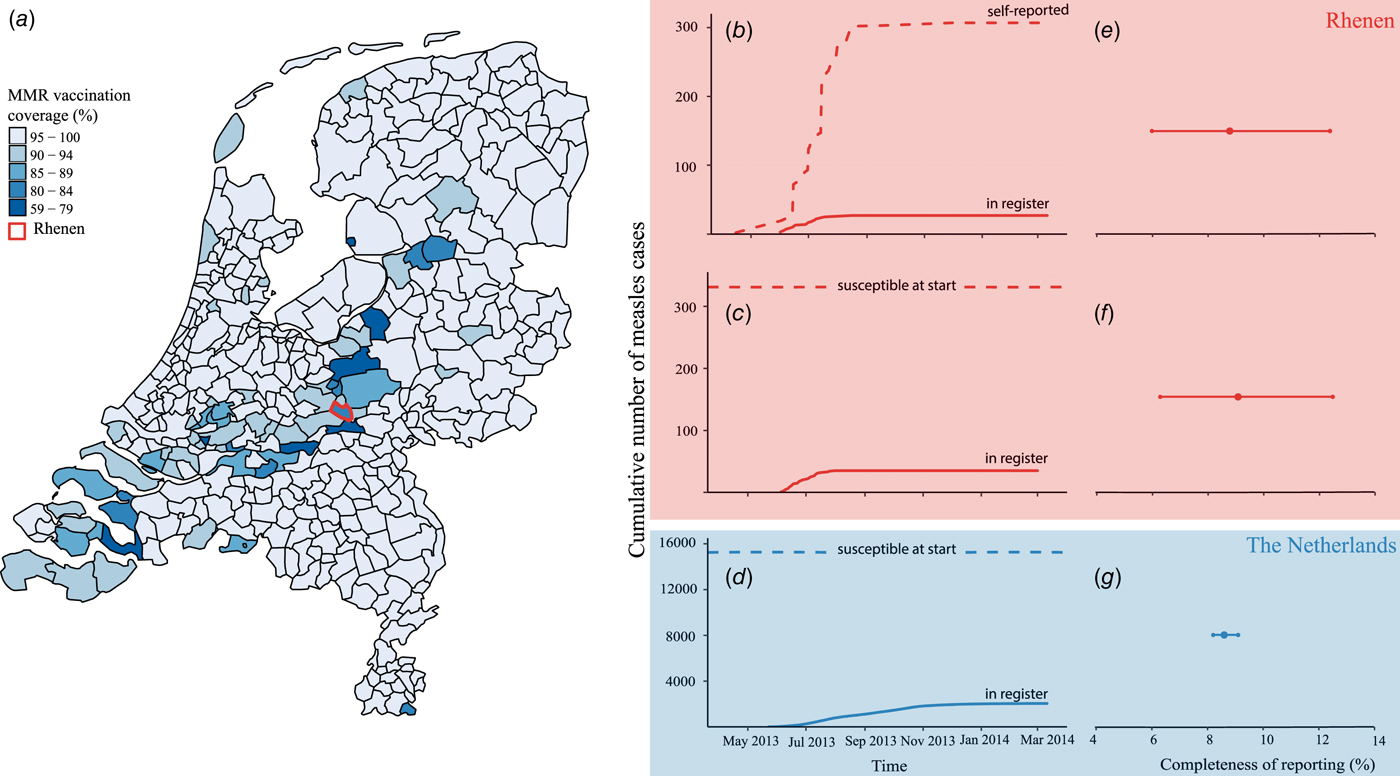
Fig. 1. Completeness of measles notification in The Netherlands, 2013–2014. (a) First dose of measles–mumps–rubella vaccination coverage by municipality, The Netherlands, 2013 and the location of the municipality of Rhenen in the centre of The Netherlands. (b) Cumulative number of self-reported cases from the community-based survey among birth cohort 2000–2013 in Rhenen (dashed line) and the cumulative number of self-reported cases matched to the national register of notifiable diseases (solid line). (c) Estimated number of susceptible orthodox Protestant children aged 4–12 years at the start of the epidemic in Rhenen, The Netherlands (dashed line) and the cumulative number of cases notified in the national register of notifiable diseases of 4–12 years old orthodox Protestants from Rhenen (solid line). (d) Estimated number of susceptible orthodox Protestant children aged 4–12 years in The Netherlands at the start of the epidemic (dashed line) and the cumulative number of orthodox Protestant cases aged 4–12 years notified to the national register of notifiable diseases in The Netherlands (solid line). (e) Completeness of measles notification, with 95% confidence interval in Rhenen as estimated with the community-based survey. (f) Completeness of measles notification with 95% confidence interval in Rhenen as estimated with the reconstruction of the number of susceptible children aged 4–12 years. (g) Completeness of measles notification with 95% confidence interval in The Netherlands as estimated with the reconstruction of the number of susceptible children aged 4–12 years.
In cooperation with the municipality of Rhenen and the MHS, we obtained the children's names and their addresses, where we mailed the questionnaires. Parents were asked to fill in the questionnaire on behalf of their children. The questionnaire could be returned either by regular mail or online. After 3 weeks a reminder was sent. The questionnaire ascertained date of birth, school, any history of measles infection and vaccination status. A measles virus infection was defined as having a red rash on the skin and fever possible accompanied with red watery eyes, coughing or running nose. The school was of interest because The Netherlands has schools of various denominations, including those with an orthodox Protestant denomination. Because there is a relationship between religion and vaccination behaviour, information about school attendance of respondents gives an indication which groups in terms of religion and correspondingly vaccination behaviour will participate in our study.
Parents who reported that their child had a history of measles were requested to answer additional questions about the date of onset (month of first day of illness), general practitioner (GP) consultation, hospitalisation and complications, e.g. otitis media, pneumonia, encephalitis, diarrhoea, other or none. Parents who reported measles symptoms for their child were asked whether they were willing to donate a saliva sample from their child to test its immune status against measles.
Completeness of reporting and determinants
We matched the cases found in the questionnaire survey and those found in the national register by name, address and date of birth at the MHS. We divided the matched cases by the total number of cases found in the questionnaire survey to estimate the completeness of reporting. We calculated a binomial proportion confidence interval using the Wilson score method.
We also assessed determinants of reporting: GP consultation and hospitalisation, and variables such as birth cohort, date of onset and sex that could provide insight as to the actual epidemiology of reported measles cases compared with unreported measles. The study participants were categorised by year of birth in three groups: 2000–2004, 2005–2008 and 2009–2013. Date of onset was dichotomised into groups of equal size. To discover whether reported cases were different from unreported cases, we used either Pearson's χ 2 test or Fisher's exact test to compare proportions between groups. For all the analyses, we used R (version 3.2.0).
Laboratory testing
Parents who were willing to have their child's saliva tested received a measles saliva test kit and an additional questionnaire. They were instructed to collect a saliva sample of their child by gently rubbing a swab (a small sponge on a stick) in the subgingival area for about 1 min. The sponge absorbs approximately 0.5 ml of crevicular fluid during this period. The swab was then sealed in a tube and transported at ambient temperature by posting the reply-paid envelope to the laboratory at the National Institute for Public Health and the Environment.
Saliva specimens were tested for measles-specific IgG antibodies using a measles-specific IgG capture enzyme immunoassay developed by Microimmune Ltd. This assay has been reported to show good concordance with serum IgG results in detecting measles-specific IgG antibodies in both vaccinated populations [Reference Kremer and Muller20, Reference Vainio21] as well as in largely unvaccinated populations [Reference Nokes22]. This assay does not distinguish between measles-specific IgG antibodies from a natural infection and those from a measles vaccination.
The additional questionnaire sent along with the measles saliva test kit consisted of one additional question assessing whether the participant was a first or subsequent measles case in the household.
Reconstruction of susceptible school-aged children in the community
Our second approach to assess completeness of reporting was by estimating the number of susceptible individuals in the most affected group prior to the outbreak, assuming that almost all will be infected, and to compare this number with the number of reported cases from this group. Those most affected during the outbreak in the municipality of Rhenen were from a group of unvaccinated orthodox Protestant school-aged children. From the questionnaire survey, we derived the number of susceptible school-aged children in orthodox Protestant schools based on the number of children and vaccination coverage of these schools. Subsequently, the number of susceptible school-aged children in orthodox Protestant schools was compared with the number of school-aged children reported to the national register from the municipality of Rhenen.
Reconstruction of susceptible school-aged children in The Netherlands
We conducted a similar assessment at the national level. Those most affected during the outbreak in The Netherlands were from a group of unvaccinated orthodox Protestant school-aged children. The number of orthodox Protestant school-aged children in The Netherlands can be estimated using data about the number of children by age per school with orthodox Protestant denomination, which are publicly available in The Netherlands (https://duo.nl/open_onderwijsdata/databestanden/).
The number of susceptible orthodox Protestant school-aged children can then be estimated using the vaccination coverage, which was approximately 60% among orthodox Protestants [Reference Ruijs17]. Because the previous outbreak among orthodox Protestants occurred 14 years earlier in 1999–2000 [Reference van den Hof, Conyn-van Spaendonck and van Steenbergen18], unvaccinated orthodox Protestant children born after 1999 were assumed to be susceptible prior to the outbreak of 2013. Exposure to measles during the outbreak of 2013 is highly likely for unvaccinated orthodox Protestant children given the infectiousness of measles and high transmission within these schools with low vaccine uptake [Reference Woudenberg16]. We can therefore make a comparison between the number of susceptible school-aged orthodox Protestant children registered in schools and those reported to the national register of notifiable diseases.
Having both the estimate from Rhenen and that of the entire country, we could assess whether the completeness of reporting estimate could be generalised to the national population.
Ethical considerations
Data concerning names, addresses and dates of birth of children with measles cases reported to the national register were only available at the local MHS. After the matching, respondent names and addresses were erased from the data. Measles surveillance data obtained at the national registry are anonymised. Ethical approval was given by a medical ethics review committee (METC Noord Holland, M014-030).
Results
National register of notifiable diseases
In the national register of notifiable diseases, we found 2700 measles cases for The Netherlands reported during 27 May 2013 and 12 March 2014, of which 39 measles cases were reported from the municipality of Rhenen. Of these, 35 were born between 2000 and 2013 (the age range of the questionnaire survey). Of these, 30 were orthodox Protestant. A total number of 1312 reported cases in The Netherlands were born between 2000 and 2013 and were orthodox Protestant.
Community-based survey
In total, 3422 questionnaires were sent to all parents of all children born between 2000 and 2013 in the municipality of Rhenen, of which we received 2077 responses (response rate 60.7%). Of those 2077 respondents, 1067 were boys (51%) and the median age was 7 years (IQR 3–10). The responders did not differ from the non-responders in terms of sex (P = 0.61) and age (P = 0.48).
Overall, 307 respondents were reported to have had measles during the course of the outbreak of 2013–2014 (Fig. 1b). Of the 307 outbreak-related cases, 171 patients were boys (56%) (Table 1). Nearly all cases were unvaccinated (n = 296, 96%), 2% (n = 6) were vaccinated once and 1% (n = 2) were vaccinated twice. The majority of cases (n = 236, 77%) did not consult a GP. In five cases hospitalisation was reported. Almost a quarter of the cases (n = 69, 23%) reported at least one complication. Diarrhoea (44 cases, 14% of all cases) was reported most commonly, followed by otitis media (n = 21, 7%), pneumonia (n = 12, 4%) and dehydration (n = 1, 0%). We found no record of measles-related deaths in Rhenen.
Table 1. Completeness of reporting stratified by case characteristics for cases in Rhenen, The Netherlands, 2013
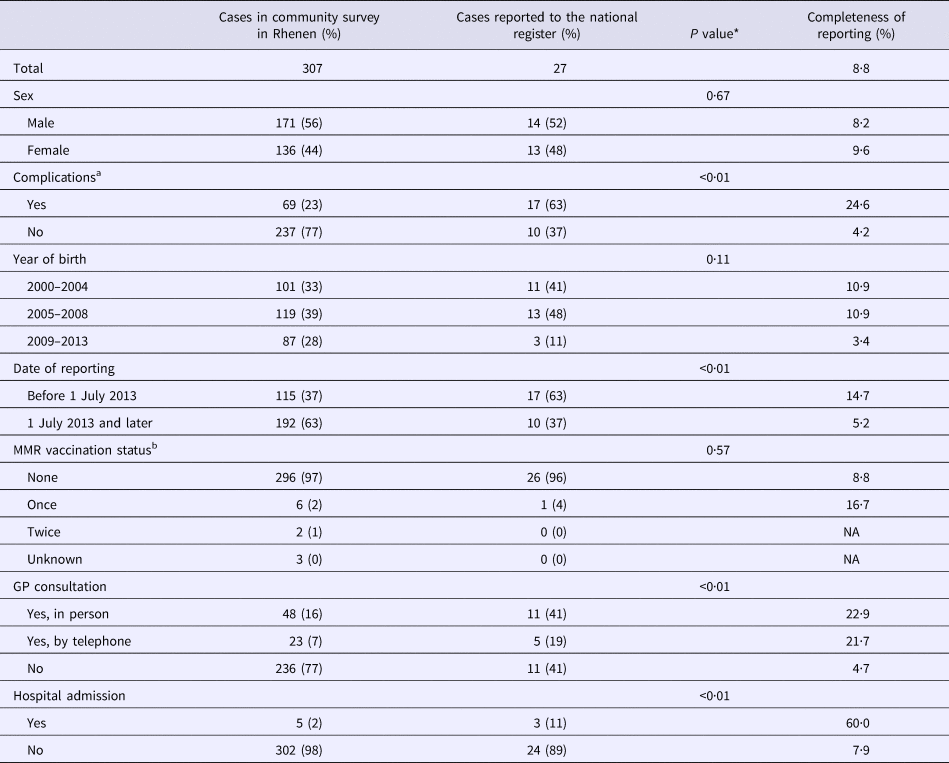
*The P value estimated by χ 2 or Fisher's exact test indicates whether reported cases were different from unreported cases.
a One missing for complications.
b Two missing for vaccination status.
Completeness of reporting and determinants
Of the 307 measles cases found in the survey, 27 were reported to the national register (Fig. 1b). Thus, the completeness of reporting was 8.8% (95% CI 6.0–12.4%) (Fig. 1e).
In Table 1, estimates of completeness of reporting are stratified by case characteristics. Cases of measles in children born in 2000–2008 were about three times more likely to be reported than cases in children born in 2009–2013 (P = 0.11). Cases with complications were six times more frequently reported than cases without complications (P < 0.01). Cases occurring before July 2013 were also more likely to be reported (14.7%) than those occurring in July 2013 or later (5.2%) (P < 0.01). Cases in children whose parents sought health care were more likely to be reported (P < 0.01).
Laboratory testing
Of the 307 measles cases identified in the survey whose parents were invited to submit saliva samples, we received samples of 126 children. Among these, four sent insufficient material to be tested. Five out of the 122 samples with sufficient levels of saliva were vaccinated and their samples tested positive. Of the remaining 117 saliva samples from unvaccinated children, all but one were positive for measles antibodies (n = 116, 99%). Thus, the positive predictive value of self-reported measles in unvaccinated individuals with a completed test was 99%. Those whose saliva was sampled were comparable in terms of sex (P = 0.3), age (P = 0.9), complications (P = 0.7) and GP consultation (P = 0.3) with those who opted out from laboratory testing.
On the basis of information from the additional questionnaire (n = 126), which was sent along with the saliva test kit, we found that young children born in 2009–2013 and children with complications were more likely to be a subsequent case in the household (Table 2).
Table 2. Case characteristics from children self-reported to have had measles and send in a saliva sample to test (n = 118)a, stratified by order of infection in the household

†P value estimated either by χ 2 or Fisher's exact test indicates whether primary cases were different from secondary cases.
a Eight out of the 126 from whom we received a sample of saliva and an additional questionnaire lacked information as to whether it was the first or subsequent case in the household.
Representativeness of survey respondents
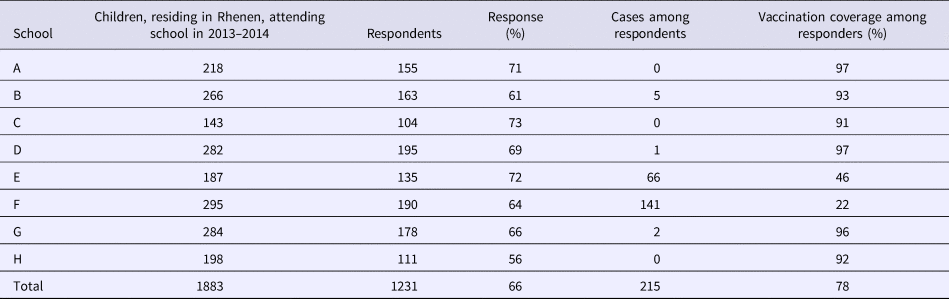
With regard to the survey, 1304 out of 2077 respondents were enrolled in an elementary school. Of those, 1231 children attended one of the eight elementary schools in Rhenen (Table 3). The majority of the measles cases found in the survey were reported from two schools with an orthodox Protestant denomination. For each school, we compared the total number of children enrolled in school year 2013–2014 with the number of respondents. The percentage of respondents was similar among the different schools with different denominations, supporting that our study sample was representative of the community in terms of vaccination uptake.
Table 3. Distribution of measles cases and respondents among elementary schools with different denominations with different vaccination coverage in Rhenen, The Netherlands, 2013
Reconstruction of susceptible school-aged children in the community
In Rhenen, 588 children were enrolled in two orthodox Protestant schools, of who 482 were residents of Rhenen. The vaccination coverage among the children who were Rhenen residents in these schools was 22% and 46% (Table 3). Therefore, 331 children were estimated to be susceptible before the outbreak (Fig. 1c). Thirty cases of orthodox Protestant school children from Rhenen, aged 4–12 years old, were reported to the national register. This means that the percentage of susceptible children in the orthodox Protestant schools in Rhenen that were reported during the outbreak was 9.1% (95% CI 6.3–12.5%) (Fig. 1f).
Reconstruction of susceptible school-aged children in The Netherlands
In orthodox Protestant elementary schools in The Netherlands, 38 131 children were registered in 2014. With a vaccination coverage of 60%, 15 252 children can be assumed to be susceptible prior to the outbreak (Fig. 1d). During the outbreak, 1312 orthodox Protestant cases of children with measles 4–12 years of age were reported to the national register. If all the children who were assumed to be susceptible became infected in this outbreak, then only 8.6% (95% CI 8.2–9.1%) of these children were reported to the national register (Fig. 1g).
Estimating the number of measles infections in the 2013–2014 measles outbreak in the community
The estimated reporting rate for measles of 9.1% as found in the calculation of the susceptible population for the municipality of Rhenen and the reported number of 35 measles cases for this community, suggest that during the outbreak approximately 384 (95% CI 280–555) individuals were infected with measles virus in the community of Rhenen.
Estimating the number of measles infections in the 2013–2014 measles epidemic in The Netherlands
The estimated reporting rate for measles of 8.6% for The Netherlands and the reported number of 2700 cases [Reference Woudenberg16] suggest that the epidemic encompassed approximately 31 388 (95% CI 29 670–32 926) measles virus infections.
Discussion
During a large measles outbreak among predominantly orthodox Protestants in The Netherlands, only 8.8% of the measles cases in Rhenen were reported. Thus, for every reported case to the national register, there were approximately 10 other unreported cases. The congruity in the estimates between the community-based approach (8.8%) and the nationwide reconstruction method (8.6%) lends support to the credibility of these values.
Previous estimates of completeness of reporting in The Netherlands date back almost 15 years. A community-based survey found that 15 out of the 164 measles cases found (9%) were reported to the national register [Reference van Isterdael12]. A reconstruction method estimated that 7% of the infections were reported to the national register during a previous measles epidemic in 1999–2000 [Reference Wallinga, Teunis and Kretzschmar15]. These two estimates resemble our estimates from this study, despite a transition from paper-based to internet-based reporting. This suggests that the reporting rate is not much affected by the reporting system.
A major benefit of our community-based survey is that it allowed us to investigate the factors that affect the completeness of reporting. We found that having a complication and being infected early in the outbreak increased the likelihood of a given case being reported. While complications tend to be more common among children younger than 4 years of age [Reference Moss and Griffin2], cases in children <4 years old were not as likely to be reported than those in older children. This observation might result from parents becoming accustomed to measles due to a first case in the household, most likely a school-aged child, and are then less inclined to seek health care for a subsequent case in the household.
Main factor of the incompleteness of reporting is for the most part due to the large number of measles cases who did not seek health care. Measles is a familiar disease in the orthodox Protestant community, and after the first cases were diagnosed within a school or community, more infections were expected. Most infected individuals refrained from seeking health care, unless they were among the first to be infected, and unless there were complications. The infected individuals that did seek health care could have exceeded the capacity for reporting as the measles cases were highly clustered in space and time.
Our survey had a high response rate. This response rate was high among respondents from schools with an orthodox Protestant denomination as well as in schools with other denominations. The equally high response rate among respondents from schools with an orthodox Protestant denomination is reassuring in estimating the completeness of reporting, as this group was the most affected during the outbreak of 2013–2014 [Reference Woudenberg16]. Another strength of our study relative to previous studies is that we offered laboratory testing to a subset of the self-reported measles cases. The positive predictive value of cases that had a laboratory test result was almost 100%, which decreases the possibility of misclassification bias due to self-reporting of measles cases in the survey.
A limitation of the community-based survey is the restriction to one location (municipality of Rhenen) and one time period (the course of the outbreak in Rhenen took place mainly in June and July 2013). The close resemblance between the estimates from the community-based survey (8.8%) and the estimates from the reconstruction method for the study population (9.1%) strengthens our confidence that the estimate does not depend on the estimation approach. Furthermore, the close resemblance between the estimates for the reconstruction method for the study population (9.1%) and the national population (8.6%) suggests that the findings can be generalised beyond the single community. The outcomes of these various estimation approaches, when taken together, suggest that the completeness of measles reporting during the outbreak in The Netherlands was very close to 9%.
The 2700 reported measles cases in The Netherlands over the 2013–2014 epidemic represent just the tip of the iceberg of the true number of measles infections. Our findings show that the measles epidemic in The Netherlands in 2013–2014 consisted of approximately 30 000–33 000 individuals infected with measles virus. The completeness of reporting varies with case characteristics. Epidemiological analyses on severity using only reported cases should be viewed in light of this knowledge.
Further, this study shows that our assessment of the number of susceptible children prior to an outbreak closely approximated the estimate from a community-based survey. An assessment of the susceptible population prior to an outbreak may therefore be sufficient to assess the true number of infections of a future outbreak in The Netherland but also in other high-income countries that have an overall high but heterogeneous vaccination coverage with pockets of communities with lower coverage. The epidemiological pattern of having periodic and sometimes large outbreaks has been seen recently in, for example, Brazil [Reference Leite, Barreto and Sousa23], Canada [Reference De Serres24] and in the USA [Reference Hall25]. Calculating the expected number of susceptible individuals in the groups at risk could help to estimate the completeness of reporting and to assess the true extent of the measles outbreak in those populations. An important condition, however, is to have accurate census data and data on vaccination uptake.
That reported cases represent only a very small proportion of the actual incidence emphasises the difficulty in achieving measles elimination. Having accurate estimates of the number of measles virus infections allows us to calculate the risk of complications upon infection with measles virus, to measure the health burden of measles and to assess the possibility of breaking the chain of transmission to eliminate measles.
Acknowledgments
We thank all the participants from Rhenen, The Netherlands for their participation in this study. We thank Margreet te Wierik for enabling data collection through the Public Health Service region Utrecht.
Financial support
This work was supported by the Dutch Ministry of Health, Welfare and Sports.
Conflict of interest
None.






