The community vector C as a subset of the species pool P. Landscapes, communities and quadrats. Assembly and response. Foundations from Raunkiaer, Major, Mueller-Dombois and Ellenberg. The elements of community ecology: pools, filters, traits, dispersal, time.
Communities
Every ecological community, be it a coral reef, a eucalyptus forest, a tract of prairie, or an alpine lake, is comprised of a set of species. Those with a keen eye for wild nature often see what seem to be the same sets of species recurring, particularly when similar causal factors such as flooding, fire or drought are present. The study of community ecology, therefore, begins with, and is focused upon, one key descriptor: the community vector, which gives the abundance of a set of species in a selected location (Figure 1.1). Examples of communities include the birds found on a tropical island, the fish found in a river delta, the amphibians found in a vernal pond, the plants found in a hectare of Appalachian forest or the beetles occupying an animal carcass.
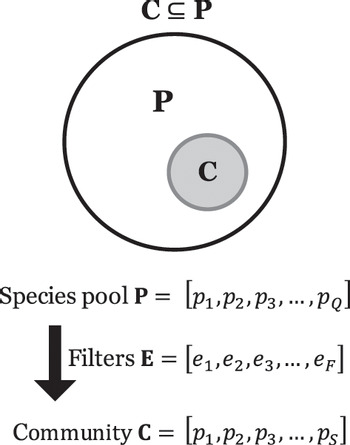
Figure 1.1 The vector C gives the abundance of each species within a specified sample unit, be it an island, a quadrat or a pitfall trap. It is the central data set in community ecology. Each region of Earth has a pool of species, P, each of which might occur in our sample unit, but normally only a subset of this pool, C, is found. The subsetting action is driven by the environmental filters, E.
The scientific literature of ecology, and the unpublished field notes and data files of natural historians and ecologists, are full of such lists of species. In its simplest form, this vector is just a species list, with occurrences scored as either 0 (absent) or 1 (present). Often, too, field observations include more detailed measures of abundance for each species. Probably the best measure of abundance, overall, is biomass. For some organisms, such as birds or beetles, it may be equally acceptable to use the number of individuals.
The habitat that the vector represents can be any selected area of space. At one extreme, the habitat may consist of a discrete and very physical island, as in The Theory of Island Biogeography (Reference MacArthur and WilsonMacArthur and Wilson 1967), or units of landscape such as mountaintops or lakes that have many of the properties of islands. At the other extreme, the vector may represent a rather small sample unit of arbitrary size that is delineated within a larger area of continuous habitat. Such smaller sample units may include the familiar quadrat, as well as the contents of a plankton net or pitfall trap, or the enumerated list of species observed on the ground within a single fallen log or high in the canopy in a water-trapping bromeliad. Sometimes, therefore, the vector represents a quite small and narrowly defined location, whereas other times it represents a larger area.
With such a vector in hand, many of the essential aspects of community ecology emerge. We can ask how many species are in the vector, and why it should be so (e.g., Reference HutchinsonHutchinson 1959, Reference Hutchinson1961). We can ask what causal factors are responsible for the abundances we observe (e.g., Reference GrimeGrime 1979, Reference KeddyKeddy 2010). Note that the term causal factors can include both abiotic factors (flooding, drought, fire) or biotic factors (mutualism, predation, competition) (Reference HilleRisLambers, Adler, Harpole, Levine and MayfieldHilleRisLambers et al. 2012). We can calculate a measure of diversity (e.g., Reference PielouPielou 1975, Reference Magurran and McGillMagurran and McGill 2011). We can also take a set of such vectors, creating a species by sample matrix, and explore patterns among them, taking us into the enormous realm of ordination and classification techniques (e.g., Reference GauchGauch 1982, Reference Digby and KemptonDigby and Kempton 1987). We can also sort the species into groups with similar characteristics, thereby simplifying the vector from S species into a shorter list of functional types (e.g., Reference GrimeGrime 1977, Reference Cummins and KlugCummins and Klug 1979). We could therefore say that community ecology is fundamentally the study of patterns in one or more vectors of species data.
In this book, we do not intend to summarize all the activities that you can carry out with one or more such vectors, as this would require a textbook summarizing all of community ecology (Reference Mittelbach and McGillMittelbach and McGill 2019). Indeed, there is a huge literature on each causal factor that could control a community, with drought and competition being familiar examples. We will focus instead upon two questions that could be considered the central challenges of community ecology. We call them assembly and response rules. Both involve prediction, which ecologists like Rigler and Peters remind us is an essential component of ecology (Reference RiglerRigler 1982, Reference Rigler and PetersRigler and Peters 1995). But first let us digress to put these general principles into a practical context.
A Grounding in Reality
If this discussion all seems somewhat theoretical so far, let us ground it solidly in biological reality. Let us think about these concepts from the practical perspective of how we can wisely manage natural areas such as national parks and ecological reserves. We use this example because protecting a global system of reserves linked by ecological corridors is one of the conservation challenges of modern times and is likely to continue to challenge ecologists in coming generations. Most reserves contain one or more natural communities, each produced by some set of casual factors. You can think about a particular natural area that is important to you, and see if you can find a map of the ecological communities therein. Or, you can enjoy the example in Figure 1.2, Kruger National Park in southern Africa, one of the largest protected areas in Africa (ca. 20,000 km2).

Figure 1.2 The major “habitats” of Kruger National Park in southern Africa. The raw data were the 35 different “landscapes” documented by Reference GertenbachGertenbach (1983). Reference Chirima, Owen-Smith and ErasmusChirima et al. (2012) simplified these “landscapes” into just eight major “habitats,” but did not include the northern 40 km of the park. Our map is expanded from theirs to show the entire park, which necessitated adding two more habitats based on Gertenbach’s map.
In Kruger National Park, Reference GertenbachGertenbach (1983) divided the park into 35 “landscapes” based upon a combination of physical properties, such as soil type, and biological properties, such as the fauna. Thirty-five may seem like rather a large number of different kinds of communities within a single park, and indeed it is larger than the number of types illustrated in Figure 1.2. But in fact, some ecologists might reasonably observe that these landscape types are too large in scale and too variable in composition to be called communities at all. Here is a summary of Gertenbach’s description of the first landscape type.
“Lowveld Sour Bushveld of Pretoriuskop” is a landscape underlain by granite and gneiss, producing acid soils. (Hence, the term “sour.” We shall see another granite and gneiss terrain on a different continent in Chapter 2.) You can see the “sour bushveld” landscape shown as a stippled area on the lower portion of the map (Figure 1.2). The vegetation consists of open tree savanna with low shrubs. There is also a rich herbaceous flora. The relative abundance of woody plants and herbaceous species changes with soil moisture. The animals include Reedbuck, Kudu, Northern White Rhinoceros and Sable Antelope.Footnote 1 There are 530 km2 of this landscape type in the national park.
Within each such landscape, we could indeed look for more narrowly defined sets of habitats with characteristic sets of species. In fact, this raises a difficult question for community ecology: just when do we stop the process of subdividing the landscape into smaller scale units, and how small, and how uniform in composition, do these smaller subdivisions have to be for us to call them a “community?” We are going to deliberately skate around this topic. At first it might seem like a reasonable question to ask. It is also quite possibly the kind of question that causes confusion rather than providing clarity. We might suggest that some of the problems in the scientific development of community ecology have arisen precisely because the wrong questions have been asked. Recall the paradox of Zeno’s tortoise, which turns out to not be very useful if you really want to know how long it takes a tortoise to walk from A to B. Zeno was a Greek philosopher who noted that the tortoise will never actually reach point B if you continue to divide the distance between the two points in half. That is, one ends up dividing a finite distance into an infinite number of small distances, which may be a useful analogy to some of the activity in community ecology, where even grant size may vary inversely with distance covered. It also implies that it is safe to jump off tall buildings, since, like Zeno’s tortoise, you will never quite reach point B, in this case, the pavement. Returning to Kruger National Park (which, by the way, has several tortoise species, including the magnificent leopard tortoise), Gertenbach describes how there is indeed much more fine-scale data on Kruger National Park, including some 1,500 vegetation quadrats collected using the Braun–Blanquet technique. He concludes that the decryption and subdivision into communities at such a fine scale has been of doubtful use to park management at all:
The geographical distribution and size of these plant communities have shown such a complex pattern as to render it impossible to indicate individual plant communities or associations on a map of reasonable scale. Despite intensive research regarding the ecology of these plant communities, no practical management program based upon these communities has been forthcoming. The intensity of these surveys has thus apparently surpassed the practical application of the results.
In our view, for science in general – and community ecology in particular – we think it is necessary to consider practical applications. This is true for two rather different reasons. First, of course, we want to be able to protect wild species and we need practical classifications of communities and habitats to do so. That is partly what motivated both of us to become ecologists in the first place. But there is a more fundamental reason. If we do not demand practical applications from our science, it is easy for that body of science to drift off into intellectual obscurity (Reference RiglerRigler 1982, Reference PetersPeters 1992). You may recall that medieval scholastics were notorious for being able to devote time to arguing over how many angels can dance on the head of a pin. Galileo, in contrast, thought about trajectories of cannonballs rather than angels, which has led us to some remarkable celestial truths. A critic might complain that too much community ecology comes close to being scholastic (e.g., Reference KeddyKeddy 1987). By thinking about real examples, and demanding practical consequences, we force ourselves into a different sort of rigour. Since collecting data costs professional time and money, and since we live in a world with limited amounts of both, and a world with multiple competing demands, we really want to avoid creating situations where the “intensity of our surveys surpasses the practical application of the results” (Reference GertenbachGertenbach 1983).
Here is another example of practical issues in designating communities. If you really need to have a practical classification system, you do have to address the issue of scale. In Ontario, for example, there is an ecological land classification hierarchy: Ontario is divided into ecozones at the provincial scale. These are then further subdivided into ecoregions and ecodistricts (if you flip ahead, you can see a map of ecoregions and ecodistricts in Figure 3.2). These in turn are subdivided further into ecosections, ecosites and ecoelements. Note that these authors do not use the word community at all! Many ecologists would likely apply the word community to either of the two lowest levels. Either level could be reasonably described by a vector of species abundances. The authors themselves (Reference Crins, Gray, Uhlig and WesterCrins et al. 2009) say that ecoelements are more or less equivalent to vegetation types, while ecosites are “Fine-scale landscape areas defined by recurring patterns of ecoelements.”
The important point is this: any particular landscape, or protected area, will have such a nested hierarchy. The community lies toward the lower end of this continuum, whatever term a particular classification system uses. And, as Gertenbach observes, if we move too far down the scale, we reach a level of so much detail that practical application may be obscured.
Composition and Causal Factors Can Both be Mapped
We can consider any protected area as having a set of communities (if we are thinking about it from the point of view of the vector C) or we can talk about a protected area as having a set of habitats (if we are thinking about it from the point of view of the causal factors that produce the vector C). On one hand, this is obvious. We can call an area of our landscape a floodplain community because we have data from the location showing the presence of flood-tolerant trees, or we can call this area a floodplain habitat because we have data showing that the location is under water for several months each spring. Neither is wrong, but each depends upon a different kind of observational data. On the other hand, we have to try to use words carefully. Mapping observed communities is quite different from mapping causal factors. In this book, we shall try to use the word community mostly to refer to the observed species composition (the vector C), while we use the word habitat to refer to a set of causal factors. In general, any location has a set of causal factors that can be ranked in order of their influence upon the community. In wetlands, for example, flooding is the key causal factor, but other factors such as fertility, natural disturbance, grazing, burial and salinity act to modify the effects of flooding and thereby determine the particular kind of wetland that arises (Reference KeddyKeddy 2010). Causal factors can also be biological. In wetlands, again, grazing by animals can be an important causal factor controlling plant composition. And, for many animal species, biotic factors can also be causal. As we shall see in Chapter 2, both amphibian and fish communities may both be strongly affected by predatory fish, in which case predation becomes a causal factor. Similarly, the abundance and structure of woody plants is a well-known causal factor in bird communities: there are clear differences between birds of grasslands and birds of forests, even at quite local scales. We will mostly use the word filter to refer to a single causal factor, and, in the next chapter, we will describe what filters are and how they work in landscapes and communities.
The Language of Samples and Sample Units
Most areas of landscape, particularly those in protected areas like Kruger National Park, have maps showing the patterns that occur, be they “landscapes,” “communities,” “forest types,” “habitats” or “vegetation types.” Sometimes reserve managers have an exhaustive and comprehensive list of all the species in each of these categories. More often, what they have is a set of sample units from each community (or habitat). That is, the communities that you may see on a map of the reserve are documented by only a sample of some number of sample units. This is usually a simple biological and financial reality: there is only so much money and time, so any particular type of habitat is described by collecting data from a set of quadrats, a set of lists from bird counts, a set of data from fish traps and so on. For the purposes of communication, note that people are often careless about the use of the word “sample.” In this book we shall use the word sample to mean a set of observations. A single observation, like a bird list or the contents of a pitfall trap, is a sample unit. The point is that in even relatively small tracts of land, our knowledge of the species composition is derived from a sample, or, that is, a set of sample units. In the case of Kruger National Park, there were 1,500 quadrats (i.e., sample units) documenting vegetation.
Hence, when you visit a particular location within such a protected area, you are viewing one sample unit. There is a larger vector that describes all the sample units combined, that is the community of organisms documented from all the samples collected in that habitat. If we put in enough effort, and collect enough samples, we should end up with an exhaustive and complete list of our species pool. We shall discuss this process further, and give examples, in Chapter 3 on species pools.
More General Principles: Assembly and Response
Now let us move back to general principles for community ecology. We said above that there are two fundamental questions. Our first challenge is to predict the vector C for a given location based upon the environmental filters present at that location, or, in other words, the measurable properties of the habitat. Examples of this challenge include: which birds will be found on this year’s Christmas bird count? Which tree species will be found in a tract of dipterocarp forest in Borneo? Which species of frogs will you find in a vernal pool in prairie parkland? In most cases, we may know the potential list of species, say P, that might be present, from standard documents such as floras and wildlife guides, which provide what we shall call the species pool. The challenge, then, is to predict which set of species C (and their abundances) will actually be found at a specific location (Figure 1.1). That is our primary goal in this book.
There is also a second kind of question (Figure 1.3). Assume that we have a specified location in space, at time t, with a list of abundance of each species present (Ct). Now, assume that a filter is going to be changed: for example, temperature, rainfall, grazing intensity or predation. How will C respond to that changed filter? What will Ct+1 look like? This style of question underlies many current environmental questions, including how forest composition might shift with changing climate, or how fish composition in estuaries might shift with changing fishing pressure, or how forests will respond to logging. Indeed, we could say that this question is the scientific underpinning of environmental impact assessments overall. A wide array of applied questions about the future therefore share a common structure. How can we use knowledge of pools and traits to predict community responses to changing filters? We call this class of problems response rules, as distinct from assembly rules. Although the focus of this book is on assembly rules, our intention is also to consider to what degree response rules may benefit from a similar kind of thinking.
This second challenge, response rules, may appear more difficult, because we are being asked about the response of the set of species to a change in environmental conditions. However, this question also includes one added piece of useful information: the existing state of the system. We are not being asked to predict de novo from a very large number of possible species; we have prior information on the raw material that is already present in the location. So, the extra demands of prediction may be balanced by the prior information on species that are already present, and their traits.
We view the construction of such assembly and response rules as the central, and defining, issue of community ecology. In this book, we will largely focus on assembly rules. We will pursue one particular approach to this problem. It involves knowledge of species pools, functional traits and environmental filters. This is, perhaps, not the only framework for creating assembly and response rules, but it is the one which we believe holds the most promise for unifying community ecology and for providing the vital ability to predict the composition of ecological communities altogether (Reference Weiher, Keddy, Weiher and KeddyWeiher and Keddy 1999b, Reference McGill, Enquist, Weiher and WestobyMcGill et al. 2006).
The scope of the problem is demonstrated by the complexity of the challenge. Earth has over one million species of organisms. If we focus upon plants alone, since they comprise the majority of Earth’s biomass, and provide energy and habitat for nearly all other species, there are still approximately 350,000 species (The Plant List 2013). Anyone who has compared a desert to a wetland, or a lakeshore to a mountaintop, will appreciate that these 350,000 plant species do not occur in random mixtures. Certain groups of species tend to occur as repeating patterns. Indeed, we recognize deserts partly because of dominance of succulents, while we recognize wetlands because of the presence of flood-tolerant plants. Similarly, if you take a canoe trip down a river, or hike along a chain of mountains, you see that certain combinations of species occur again and again.
So, at one scale, it is obvious that certain environmental constraints do indeed select certain vectors of species composition from the global pool of 350,000 species. That, too, is why we can recognize biomes and ecoregions. It is also true, however, that demonstrating patterns in species composition is a challenge at smaller scales, and there is an enormous scientific literature on the challenge of finding recurring patterns in species composition. One might indeed argue that the vast array of papers on patterns in ecological communities has become a distraction of sorts, since there really is no end to asking whether there are patterns in species composition of particular taxa in particular habitats at particular scales. More importantly, the search for such patterns can become an end in its own right, subtly distorting the search for predictive rules based upon cause and effect. Without caution, the organizing theme in ecology can degrade to a simplistic question: is there a pattern? Even if there is a pattern, such information puts one only marginally closer to knowing why, or more importantly, to being able to predict vectors from known causal factors. We will explore this problem more in the next section.
The Study of Pattern Is Not the Study of Community Assembly
Since there seems to be considerable confusion on the topic of pattern in ecological communities, let us look at just how much effort has gone into detecting pattern in plant communities over a period of more than 50 years. Although we begin with the example of plants, let us make it clear we are after the general implications for ecology as a whole. Zoologists have similar problems.
Here is an introduction to pattern in plant communities by Reference Goldsmith, Harrison and ChapmanGoldsmith and Harrison (1976, pp. 113–114):
Non-randomness in vegetation is often referred to as pattern. It may take the form of an aggregation of individuals known as a contagion, or an even distribution known as a regularity. The former is more common than either the latter or randomly distributed individuals. The departure from randomness interests the ecologist because it is a way of characterizing the vegetation or a particular species. Also because it must have a cause and it provides an opportunity to identify the factors that control the distribution of a species.
The most common kind of pattern study (and there were many of them) took data from a large number of quadrats, and asked whether certain species were associated with one another, using simple tests such as chi-squared tests (Reference ColeCole 1949, Reference Greig-SmithGreig-Smith 1952, Reference Greig-Smith1957, Reference KershawKershaw 1973). Usually certain species were found to be associated, and traditionally such papers ended with speculation about what ecological factors might be involved in producing the non-randomness. Reference DaleDale (1999) has provided an overview of this field.
Another group of studies used data collected on zonation patterns. Reference PielouPielou (1975) developed a statistical test for asking whether zonation patterns deviated from null models. Since collecting data on zonation patterns requires considerably more work, there are fewer examples (e.g., Reference Pielou and RoutledgePielou and Routledge 1976, Keddy 1983, Reference KeddyShipley and Keddy 1987, Reference Hoagland and CollinsHoagland and Collins 1997). Again, the results were clear in one way: there is statistically significant deviation from null models. But just as with quadrat data, there are multiple hypotheses for what might cause the non-randomness (e.g., Reference Shipley and KeddyShipley and Keddy 1987). Reference KeddyKeddy (2017, pp. 441–448) has provided an overview of this approach.
Then, of course, there is a vast scientific literature on patterns that can be shown using multivariate methods, with a wide array of techniques including reciprocal averaging and detrended correspondence analysis, to name a few (e.g., Reference OrlociOrloci 1978, Reference GauchGauch 1982, Reference Digby and KemptonDigby and Kempton 1987). These can provide elaborate displays of multidimensional patterns in vegetation (or in other kinds of ecological data), although, unlike simple chi-squared tests, usually do not provide an explicit test for non-randomness. Usually, the non-randomness is assumed, and the techniques are then applied to extract the assumed patterns. Multivariate tools can be useful for describing large data sets, but too often they became an end in their own right, and a subtle justification for collecting large data sets. Paul once overheard a fellow scientist say his career objective was to provide an ordination description of every vegetation type in his province.
The point is that the search for pattern can become an end in itself, and in most of the papers one reads, questions about causal factors are relegated to the end of the paper for speculation in the discussion. Anyone the least familiar with the ecological literature will immediately recognize how many papers fall into this category. After all this work over the past 50 years, we can conclude with certainty that certain species, in some locations, at some scales, show non-random patterns. The problem is that we cannot predict with any confidence which species, which locations or which scales will have which kind of pattern. The plant ecology story is intended to be a cautionary tale, because it would be possible to go through the same process with any other group of organisms, be it birds on islands or fungi on fallen logs or parasites in animal guts, and with probably the same outcome: patterns in some species, at some locations, at some scales.
For progress in ecology, we therefore have to make a concerted effort to move away from simply looking for pattern, and instead start looking for predictive ability (Reference KeddyKeddy 1987, Reference PetersPeters 1992, Reference Weiher and KeddyWeiher and Keddy 1995). That is our goal in this book. Our first task is to illustrate some general steps that provide a logical framework. Otherwise, it might be easy to throw up our hands and give up on prediction, and simply go out and collect more observations. Or, worse still, to host yet another conference on emerging paradigms in community ecology. One is nearly as pointless as the other. Meanwhile, while we entertain ourselves as scholars, we face the prospect that perhaps a quarter of the world’s biota could disappear this century. One might argue that not only is the capacity to predict both the community vector C and changes in C with time one of the most intellectually challenging parts of community ecology, it is simultaneously one of the most significant moral challenges for protecting Earth’s biota.
The design of a global system of protected areas might be seen as one of the most demanding applications of community ecology. Consider the typical questions in the design of a protected areas network. How many species occur in the landscape? Which areas are most important for protecting these species? What are the vectors of C for different habitats and sample areas? What are the key factors that enhance C? What are the key factors causing the vector C to decline in length? How can we restore degraded areas to increase the length of C? All of these fundamental questions involve recurring questions about species pools (P), communities (C), and environmental filters (E).
Now let us turn to some historical context for this inquiry.
Foundations of Community Ecology Laid by Raunkiaer
Consider the work of Raunkiaer on the distribution of plants globally. This work is now over a century old, and yet has several important themes that will recur in this book. First, there is the problem of a very large number of species – that is, a large pool. Second, it is possible to identify functional traits that these species possess. Third, it is possible to sort species into groups based upon these traits. Fourth, there is the challenge of finding patterns. Fifth, and most important, there is the challenge of relating species to environments based upon causal factors. Raunkiaer’s work illustrates all of these themes. Raunkiaer is chiefly remembered for his functional groups, but here we wish to draw attention to the other important aspects of his work, particularly the insight of linking functional traits to environmental filters.
At the time he was working in the early twentieth century, ecologists had become aware that there were vast numbers of species, and that the world was much more complicated than earlier generations of European scientists had understood. To put his work into context, recall that the epic voyage of the Beagle had begun in 1831, while Captain Cook’s voyage to Australia had occurred in just 1770. Clearly the genera recognized by Linnaeus were insufficient for cataloguing the diversity of plants and animals. What generalizations could be drawn? Raunkiaer proposed that the world’s plants could be divided into ten life forms. These were based upon the location of the meristems on the plants (you can have a quick look at them in Figure 6.1). Phanerophytes, for example, are woody plants with buds (meristems) borne above the surface of the ground. He then collected data to show that different environments had different kinds of life forms. He also developed a null model, the “normal spectrum,” long before there were statistical models and computers for the task, by selecting plants from a global list of plants called the Index Kewensis. He showed that the life forms of plants in his data were non-random (Table 1.1). For example, the top row in the table shows that Franz Joseph Land, an archipelago in the Arctic Ocean north of Russia, had just three of 10 life forms (Chamaephytes, Hemicryptophytes and Geophytes). Overall, Raunkiaer concluded that this variation was a consequence of climate, with cold and drought in particular controlling which kinds of life forms could be found.
Table 1.1 The distribution of ten plant life forms in seven locations with different climates (Reference RaunkiaerRaunkiaer 1908). The point of the table is to show non-random patterns in an important life history trait, and a null model (the bottom row, the “normal spectrum”). Selected life forms are illustrated in Figure 6.1. More examples can be found in Reference KeddyKeddy (2017).
| Region | No. species | Percentage distribution of species among life forms | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | E | MM | M | N | Ch | H | G | HH | Th | |||
| Franz Josef Land, Russia | 82° N, 55° E | 25 | – | – | – | – | – | 32 | 60 | 8 | – | – |
| Iceland | 65° N, 19° W | 329 | – | – | – | – | 2 | 13 | 54 | 10 | 10 | 11 |
| Sitka, Alaska | 57° N, 9° E | 222 | – | – | 3 | 3 | 5 | 7 | 60 | 10 | 7 | 5 |
| Death Valley, California | 36° N, 117° W | 294 | 3 | – | – | 2 | 21 | 7 | 18 | 2 | 5 | 42 |
| Ghardaïa, Algeria | 32° N, 4° E | 300 | 0.3 | – | – | – | 3 | 16 | 20 | 3 | – | 58 |
| Aden, Yemen | 13° N, 45° E | 176 | 1 | – | – | 7 | 26 | 27 | 19 | 3 | – | 17 |
| Seychelles | 5° S, 56° E | 258 | 1 | 3 | 10 | 23 | 24 | 6 | 12 | 3 | 2 | 16 |
| Normal spectrum | 1,000 | 1 | 3 | 6 | 17 | 20 | 9 | 27 | 3 | 1 | 13 | |
Abbreviations: S, stem succulent; E, epiphyte; MM, Megaphanerophyte; M, Mesophanerophyte; N, Nanophanerophyte; Ch, Chamaephyte; H, Hemicryptophyte; G, Geophyte; HH, Helophyte and Hydrophyte; Th, Therophyte.
Because this work is a century old, it is easy to assume the ideas are out of date, particularly if you are one of those unfortunate students whose professor told you not to read work more than five years old. Yet, Raunkiaer had shown that plants occur in non-random groups well before all the literature we just reviewed on pattern in ecological communities. He also identified a key causal factor, climate. And he identified an essential functional trait that still remains useful in modern ecology, the position of the meristem. The work still matters. Every time that you see a vast marsh, note that one characteristic is shared by all the dominant marsh species: persistence from buried, not aerial, meristems. Every time you see a tract of forest, note that one characteristic is shared by all those dominant forest species: persistence from aerial meristems. More than 100 years have passed since Raunkiaer’s work, and we might therefore challenge ourselves to find similar relationships between traits and filters in other groups of organisms and at other scales.
A General Framework
We will now propose a general framework that builds on Raunkiaer and incorporates decades of thinking about species pools, filters and traits. We are going to borrow key elements of frameworks presented by Reference JennyJenny (1941) and Reference MajorMajor (1951), who focused, on soil pedogenesis and plant communities, respectively. After some rearranging of terms, their general statement reads:
where o = organisms, c = climate, p = parent soil material, r = relief or topography, and t = time.
This framework suffers from being inordinately focused upon plants, but it is a good starting point, since plants comprise as much as 90 percent of all the biomass on Earth. (We will revisit this particular statement in Chapter 4, particularly in Figure 4.1.) A similar presentation of causal factors can be found in a book chapter entitled “Casual-analytical inquiries into the origin of plant communities,” written by Reference Mueller-Dombois and EllenbergMueller-Dombois and Ellenberg (1974). The chapter begins with the observation that studies of patterns in community composition do not tell us much about mechanisms. Muller-Dombois and Ellenberg begin with a simple but general observation that applies across all taxa: knowledge about mechanisms that underlie patterns in communities can “only be obtained from measurements and experimentation.” It is ironic, and instructive too, that this chapter is 12th in a thick book that spends a great deal of space (11 chapters, 334 pages) talking about patterns in plant communities. This is, of course, part of the problem addressed earlier in this chapter: we have inherited a scientific discipline in which too many people are quite satisfied to focus their attention on description.
Building upon such ideas, let us formalize the various factors that influence the community vector C. We begin with the species pool, P. We will list them first, and then consider each in turn.
P: the species pool, a vector of Q species, P = [p1, p2, p3, …, pQ]
C: the community, a subset of P, a vector of S species, C = [p1, p3, …, pS],
E: environmental filters, a vector of F causal factors, E = [e1, e2, e3, …, eF]
T: functional traits, a vector of K traits, T = [t1, t2, t3, …, tK]
The first three are included in Figure 1.1. We shall add the others into the story later.
The Basic Elements of Community Ecology
Now let us turn to the list presented above, and briefly consider each one by way of introduction to their potential application to assembly rules. Most of the rest of the book will be revisiting these six elements in more detail. What do we mean by P, C, E, T, d and t, and how does each apply to the problem of community assembly?
P: The Species Pool
The species pool P is determined by the location of the community, and its evolutionary history. It is the raw material from which a community is assembled. P varies with location: a fragment of the old continent of Gondwana off the coast of Africa, like the island of Socotra, will have a very different pool from a piece of continental Laurasia in eastern North America. There are evolutionary and geological reasons that pools differ with location (Reference WallaceWallace 1876, Reference TakhtajanTakhtajan 1986, Reference RosenzweigRosenzweig 1995). To keep it simple, for the moment, just think of your favourite local field guide to the fauna or flora as a good first approximation of the species pool. When you have a bird to identify, your local guide gives you the pool to choose from. We devote a later chapter to the nuances of defining a species pool.
C: The Community
The list of species found in any particular community C is the set of species that occur there at time t. It is the list of species, always a smaller subset of P, that have arrived, survived and persisted until the time of sampling (Figure 1.1). C may be the most common element of assembly rules that ecologists measure. We likely have notebooks full of species lists from habitats we have visited. Indeed, if we think small enough and consider the ubiquitous quadrat as a community, then we have an enormous number of estimates of C. We use the word estimate as a humbling reminder that it is always easy to miss species, particularly when they are young or transient, so our list of species is always likely an underestimate of the true C in that location. But this is a minor point, since, when you use biomass as a measure of importance, the unseen species are usually small or visually insignificant, and so are a minor component of biomass in any case, at least in sessile communities. We are aware, of course, that in general, big fierce animals are also rare (Reference ColinvauxColinvaux 1978), and that in any sample, a few species predominate in numbers and mass (Preston 1962). Overall, we have made a good deal of progress in surveying Earth’s biota since the days of Captain Cook. We now have huge data sets giving us C for a wide array of habitats from quadrats to lakes to tropical islands to national parks.
So, P is a list of all possible species that might occur, and C is the list of those that actually occur now. How do we get from P to C? It requires us to know something about the fundamental biology of the species themselves, collectively, and the environmental filters that are acting.
E: Environmental Filters
Every habitat has a hierarchy of environmental filters, which many people also call environmental factors, that control composition. We use the word hierarchy explicitly to emphasize that in any location, some factors are important, and some are unimportant. What are the factors, and how important are they? One would be surprised at just how scarce such information is. Indeed, one might argue that one reason that communities are hard to predict is that too many people study trivial rather than important factors. Filters can be physical or biological in origin. Let us look briefly at three physical filters (climate, moisture and oxygen) and then some biological filters (predation, herbivory and competition). We will explore the topic of filters in more detail in Chapter 2. Here we are just laying down some basic concepts.
Many early ecologists, including the aforementioned Raunkiaer, Jenny and Major, drew attention to climate. Certainly, in our (northern) part of the world, one of the most important factors has to be the low temperature experienced during each winter, usually about −20 °C. This filter is so efficient that it simply eliminates from consideration all woody plants without traits to tolerate cold. Thus many of the tree species that occur in North America (as listed and mapped in the classic book Silvics of Forest Trees of the United States (Reference FowellsFowells 1965)) simply cannot survive in our parts of North America. The pool for northern species is much smaller. Cold weather partly determines the species pool at higher latitudes and altitudes (Reference de Bello, Lavorel and Lavergnede Bello et al. 2013). For example, when Daniel teaches ecology in the Rocky Mountains, he only has to describe a handful of species to capture the majority of trees that occur in the mountains. His brother Andrew, on the other hand, teaches ecology in the warmer, more southerly mountains of Appalachia. This area contains many dozens of tree species. The irony is that even though Andrew is an ornithologist, he spends more time teaching tree identification than Daniel, his botanist brother, all because of the strength of the climatic filter that acts in their respective regions. When we consider the species likely to establish in habitats in wetlands around the Great Lakes in northern North America, we simply do not have to concern ourselves with Baldcypress (Taxodium distichum), nor Sawgrass (Cladium jamaicense), nor with American Alligators (Alligator mississippiensis) nor Wood Storks (Mycteria americana). So, a first generalization would be that one set of environmental filters is usually so powerful and all-pervasive that the filters act at the scale of the pool. These factors create a regional pool. We will return to this topic in Chapter 3.
A second set of environmental factors, or filters, then act upon species that do occur in the regional pool of species. Since all organisms need water, soil moisture is likely a key factor to consider for trees. Another reason that soil moisture matters so much is the reproductive cycle of plants: all must survive the germination stage. Even huge desert plants like the Saguaro Cactus, which have a certain iconic status for the ability to tolerate drought, must none-the-less have water for seedling establishment. Indeed, they may be dependent upon the occasional wet years that occur only a few times each century (Reference Turner, Alcorn, Oli and BoothTurner et al. 1966).
Turning to another group of organisms, and a different habitat, aquatic animals are often controlled by the supply of dissolved oxygen (Reference Lowe-McConnellLowe-McConnell 1975, Reference Junk, Soares, Saint-Paul and JunkJunk et al. 1997). Some aquatic habitats are structured by the lowest levels of oxygen that occur. Low oxygen levels may occur during the winter, when lakes are shut off from the atmosphere by ice. Low oxygen levels may also occur in warm weather, since warm water dissolves less oxygen, and warm aquatic conditions often accelerate decay, in which case oxygen is consumed by microorganisms associated with decay, creating anoxic conditions. In the extreme case, freshwater and marine coastal zones can become dead zones, where oxygen is so depleted that fish are killed (e.g., Reference VallentyneVallentyne 1974, Reference Turner and RabelaisTurner and Rabelais 2003).
Moisture and oxygen are examples of physical filters. As noted above, there are also filters that are biological in origin. In Chapter 2 we will look at several of them in more detail. At this point we can note that predation is a filter in both amphibian and fish communities (e.g., Reference Tonn and MagnusonTonn and Magnuson 1982, Reference Wilbur, Price, Slobodchikoff and GaudWilbur 1984). Grazing is also a key factor in many plant communities (e.g., Reference HarperHarper 1977, Reference KeddyKeddy 2017). And, then, of course, there is competition (e.g., Reference JacksonJackson 1981, Reference SimberloffSimberloff 1984). The relative importance of these biological filters remains a matter of intense discussion in community ecology (e.g., Reference Oksanen, Fretwell, Arruda and NiemeläOksanen et al. 1981, Reference Oksanen, Aunapuu, Oksanen, Gange and Brown1997, Reference HilleRisLambers, Adler, Harpole, Levine and MayfieldHilleRisLambers et al. 2012, Reference Kraft, Adler and GodoyKraft et al. 2015). In general, however, interspecific competition is assumed to play a particularly important role in community assembly by limiting the number of species that share the same set of traits and selecting for species that differ in traits (Reference Pianka and MayPianka 1981, Reference Pianka1983). The observation that similar species are likely to experience the most intense competition goes all the way back to Darwin, and has led to an enormous number of studies addressing the coexistence of similar species (e.g., Reference HutchinsonHutchinson 1959, Reference Hutchinson1961), a familiar example being MacArthur’s classic work on wood warblers (Reference MacArthurMacArthur 1958).
If we consider the direct effects of filters, setting aside competition, we might expect, in a simplistic way, that filters will usually produce a community with an underdispersion of traits. That is, filters will force convergence in traits. Continuing with this perhaps simplistic view, competition is different because the general impact of competition is thought to work in the other direction – to create a community with an overdispersion of traits. If these forces balance one another, the result may appear random. Figure 1.4 gives an overview of how communities might be assembled with regard to filters acting on traits modified by interspecific competition. A good many ecological concepts, particularly the concepts of limiting similarity and resource partitioning, fit neatly into this worldview. Whether this view truly reflects the actual assembly of ecological communities is open to debate (Reference de Bello, Thuiller and Lepšde Bello et al. 2009, Reference Mayfield and LevineMayfield and Levine 2010, Reference Götzenberger, de Bello and BråthenGötzenberger et al. 2012, Reference Spasojevic and SudingSpasojevic and Suding 2012, Reference Kraft, Adler and GodoyKraft et al. 2015, Reference Münkemüller, Gallien and PollockMünkemüller et al. 2020). Let us give two counterexamples.

Figure 1.4 Most filters select a set of species with similar traits, creating trait underdispersion (left), while competition may select for species having different traits, creating trait overdispersion (from Weiher and Keddy Reference Weiher and Keddy1995). Overdispersion appears more common at greater spatial scales. This perspective about trait patterns is still common, yet it is an oversimplification of the processes that generate patterns in ecological communities.
Let us repeat an essential point about filters. Competition is thought to stand out from all the other biological filters in one important way. While predation or grazing may act like physical filters by selecting for a certain subset of species and traits, competition may be expected to have the exact opposite effect from environmental filtering, by selecting for species that differ in traits in order to partition resources (Reference MacArthurMacArthur 1958, Reference Pianka and MayPianka 1981, Reference Pianka1983). In this context, there is a limit to how similar coexisting species can be. This somewhat traditional view of community assembly is widespread, and shown in Figure 1.4. Warblers can partition space on trees, and finches can partition seed resources based on bill size. Given that animals can partition resources, this view may be more directly applicable to animal communities.
Now let us introduce our first counterexample: tall plants in general, but particularly trees. There are some 60,000 species of trees in the world (Reference Beech, Rivers, Oldfield and SmithBeech et al. 2017). In some habitats, competition may cause a less appreciated and possibly contradictory force to that expected from resource partitioning. Competition may drive coexisting species to converge on certain traits, particularly in communities where resources are exploited rather than partitioned, and in communities where space itself is a resource (Reference YodzisYodzis 1978, Reference KeddyKeddy 2001). Consider, for example, plant communities in general, and forests in particular. Here, tall species are often better at exploiting light than are short species. Traits for height have their origins deep within the functional ecology and phylogenetic history of land plants. The evolution of height has been a consistent trend, involving several key traits: apical dominance replaces dichotomous branching, and xylem elements replaces tracheids, while cambium with secondary growth allows for thick woody trunks that support foliage for centuries (e.g., Reference Foster and GiffordFoster and Gifford 1974, Reference Niklas, Tiffney and KnollNiklas et al. 1983, Reference Kenrick and CraneKenrick and Crane 1997). These traits improve access to light, and simultaneously suppress smaller neighbours. In this case, however, competition is driving a convergence in life forms, toward tall species with secondary growth.
More generally, there may be some communities that are structured as competitive hierarchies (a topic we will consider further in Chapter 8). In these communities only a few species tend to dominate the community (Reference Keddy and ShipleyKeddy and Shipley 1989, Reference KeddyKeddy 2001, chapter 5). The dominant species share traits that allow them to suppress most other species. In such situations, coexistence, particularly coexistence of species that are relatively less common, depends upon recurring natural disturbances, such as fire or storms (Reference GrimeGrime 1973a, Reference Grime1979, Reference ConnellConnell 1978, Reference HustonHuston 1979). These disturbances create non-equilibrium communities in which many species coexist within a matrix created by a few dominant species. In such communities, the dominant species frequently share similar traits. We have mentioned plants, where height is an obvious trait, but other examples of competitive hierarchies can be found in animal communities, including corals (Reference YodzisYodzis 1978) and even fruit flies (Reference Gilpin, Carpenter, Pomerantz, Diamond and CaseGilpin et al. 1986).
Now consider our second counterexample to Figure 1.4: aquatic plants. Flooding is a strong environmental filter. We know this because only a small fraction of the world’s plant species can tolerate flooding. So it would be reasonable to expect strong trait convergence in response to this filter. At first, it appears that this pattern is commonly observed: most aquatic plants share a small set of traits, including aerenchyma, floating leaves, turions and rhizomes (Reference SculthorpeSculthorpe 1967, Reference HutchinsonHutchinson 1975). Yet, when we look more closely at actual communities of aquatic plants, we also find some trait divergence. Instead of one life form, one frequently observes multiple growth forms. Yes, there are floating-leaved plants with deeply buried rhizomes, but also emergent plants, submerged rosette plants, and free-floating plants. The number of functional types of wetland plants that occupy flooded sites can include as many as 26 types (Reference HutchinsonHutchinson 1975, table 8)! Alright, you might say, perhaps flooded habitats are unique in some way. Yet, if we turn to the opposite extreme, arid lands, where drought is a powerful filter, we find a similar perplexing situation. Yes, drought produces convergence in plant traits, but then when we look at desert plant communities, we find that desert communities can contain a dozen functional types, each representing a unique solution to establishing and surviving in water-limited environments (more on this in Chapter 2).
The point is that the view presented in Figure 1.4 may make general sense, but it is not sufficient. Competition can apparently produce trait convergence (e.g., trees) in communities, while evolution can apparently produce more than one evolutionary solution (set of traits) in response to even strong filters like drought and flooding.
Returning to the general topic of how filters affect communities, we can conclude that each habitat may have a long list of environmental filters that affect assembly. Some are easy to measure and some less so. Some are more important than others. These filters may also vary with space and time. The challenge for community assembly is to find the shortest list of E key environmental filters that are currently eliminating most species from P and thereby having the greatest impact upon C. This is the problem we focus on in Chapters 5 and 7, where we describe the quantitative tools that are available to test these ideas using real communities. And overall, this remains a principal goal of the book: to explore the methods for assembling communities from data on pools and environments. At the same time, the counterexamples just presented will continue to hover on the horizon, suggesting that the larger picture still has some open questions in ecology and evolution. Thus, Figure 1.4 is a useful starting point, but trees and aquatic plants (and arid land plants and corals) show that this figure is incomplete.
T: The Traits of the Species
Every species has a set of traits that allow it to survive in a particular environment. We can compile these into a matrix of Q species by K traits, which we call a trait matrix. Often, we have rather little information to put in this matrix. Consider the importance of flooding, an environmental factor that creates distinctive communities known as wetlands. Which species might be most tolerant of flooding? It would be very useful to have a nice table showing how long each species can survive inundation. Alas, such data rarely exist. Examples like Table 1.2 are notable for their relative rarity in the scientific literature – and Table 1.2 is small: in the Amazon, there may be a thousand species of trees and little or no information on traits (Reference ParolinParolin 2009). We might turn to physiologists for some assistance, hoping to extract trait matrices from published studies, but physiologists are often constrained in the number of species that can be studied given the difficulty of measuring physiological data. There are thus many papers on flood tolerance of Alnus and Phragmites, but none comparing them simultaneously to other species in the same pool. Hence, these individual measurements on just a few species are of limited value to community ecology. And even for these common species, there is disagreement as to the physiological trait that confers flood tolerance (Reference Armstrong and ArmstrongArmstrong and Armstrong 2005). Hence, one cannot assume that we have, for most areas, a simple table of comparative flood tolerance of trees. Unless, of course, you want to rely on descriptive data, using the tried-and-true logic that species that grow in wet areas must be flood-tolerant, which, while likely true, is completely circular.
Table 1.2 Flooding is a common filter in floodplains. Trees differ in their tolerance to flooding (Reference Crawford, Large, Nobel, Osmond and ZieglerCrawford 1982).
| Species | Survival time (yrs) |
|---|---|
| Quercus lyrata | 3 |
| Quercus nuttalii | 3 |
| Quercus phellos | 2 |
| Quercus nigra | 2 |
| Quercus palustris | 2 |
| Quercus macrocarpa | 2 |
| Acer saccharinum | 2 |
| Acer rubrum | 2 |
| Diospyros virginiana | 2 |
| Fraxinus pennsylvanica | 2 |
| Gleditsia triacanthos | 2 |
| Populus deltoides | 2 |
| Carya aquatica | 2 |
| Salix interior | 2 |
| Cephalanthus occidentalis | 2 |
| Nyssa aquatica | 2 |
| Taxodium distichum | 2 |
| Celtis laevigata | 2 |
| Quercus falcata | 1 |
| Acer negundo | 0.5 |
| Craetagus mollis | 0.5 |
| Platanus occidentalis | 0.5 |
| Pinus contorta | 0.3 |
It is important to distinguish between rankings of tolerances (such as Ellenberg indicator values) as opposed to traits that can be directly measured. Here is where screening an entire group of species for one or more traits is such an important contribution. The study of seed germination characteristics of 403 species of the British flora, compiled by Grime and his co-workers (Reference Grime, Mason and CurtisGrime et al. 1981), using standardized growing conditions, was an essential contribution and a model. Later work expanded the matrix to 67 traits, but in a smaller set of 43 plant species (Reference Grime, Thompson and HuntGrime et al. 1997). Other selected examples include xylem characteristics in 480 species of trees (Reference Choat, Jansen and BrodribbChoat et al. 2012) and a set of 24 traits in 99 European bird species Reference Renner and Hoesel(Renner and van Hoesel 2017). Trait databases for plants now include the European plant trait data set LEDA (Reference Kleyer, Bekker and KnevelKleyer et al. 2008) and the global leaf trait database GLOPNET (Reference Wright, Reich and WestobyWright et al. 2004). The TRY Initiative began compiling plant trait data sets that were scattered across personal computers and journal supplementary material into a searchable global database that now has nearly 12 million records from over 279,000 plant taxa (Reference Kattge, Bonisch and DiazKattge et al. 2020)! We will return to the topic of traits in Chapter 4, where we distinguish between screenings and compilations of traits.
d: Dispersal
We include dispersal as a term, partly for completeness. Neither Reference MajorMajor (1951) nor Reference Mueller-Dombois and EllenbergMueller-Dombois and Ellenberg (1974) include dispersal in their formulations, but they do include time explicitly, which naturally invites consideration of dispersal. Moreover, organisms differ in their dispersal abilities, in which case dispersal traits could be included in the trait matrix. Dispersal is clearly an important process in community assembly, but dispersal limitation likely becomes more important with increasing spatial scale. In one view, community assembly is a goodness-of-fit problem: given the set of species in the pool, and their traits, what is the best set of species to tolerate a given set of filters? In some cases, we may find it convenient to imagine dispersal is the same for all species, even if we know it is not; it is a simplifying assumption.
Dispersal is also a topic that affects the size of a species pool. At the large scale, a species pool exists and can be delineated precisely because there are realistic limits to long-distance dispersal. Prior to globalization, the biota of Earth as a whole did not mix freely, and hence, for example, Reference WallaceWallace (1876) was able to divide the world into six zoological regions: the Palaearctic, Nearctic, Ethiopian, Oriental, Australian and Neotropical. In the same way, Reference TakhtajanTakhtajan (1986) was able to divide the world into six floristic kingdoms and 35 floristic regions (you can see the map in Figure 4.2). Barriers to dispersal explain, in part, why fragments of the old continent of Gondwana are still different from the rest of the world, and even why fragments of Gondwana such as Australia and South America themselves have a different flora and fauna. Since most of the northern hemisphere falls into a single floristic kingdom (the Holarctic Kingdom) with just nine regions, it is easy for northern ecologists to underestimate just how different species pools can be in sub-equatorial regions of Earth. These regions exist largely because of limitations on long-distance dispersal. It is interesting to note that Wallace explicitly discusses dispersal and its limitations, in his words, “barriers to migration” (p. 6). He notes that flightless birds “such as the ostrich, cassowary, and apteryx, are in exactly the same position as mammalia as regards their means of dispersal” (p. 16). He also describes the role of floating rafts of vegetation in dispersing mammals (p. 15). Barriers to migration are an important cause of different biogeographic realms, and therefore help determine the species pool of any particular location. The entire problem of invasive species is driven by humans transporting species across what were formerly ecological boundaries.
Of course, natural long-distance dispersal does occur. Owing to its relative infrequency, naturalists have long been intrigued by examples of long-distance dispersal. The first plant to colonize the new volcanic island of Surtsey, in 1965, was the beach plant called Sea Rocket (Cakile maritima) (Reference Magnússon, Magnússon, Ólafsson and SigurdssonMagnússon et al. 2014). One might argue that this does not qualify as long-distance dispersal since the plant was colonizing within a single floristic region, the circumboreal. Consider the Cattle Egret (Bubulcus ibis), which in the 1930s “began one of the most dramatic and best documented avian range expansions occurring this [meaning the twentieth] century” (Reference ArendtArendt 1988), by crossing the Atlantic Ocean to colonize the New World. Such long-distance dispersal is a rare occurrence, however, and that is what allows us to delineate species pools for different regions. For example, when a tree falls in Algonquin Provincial Park in Ontario, or in Yellowstone National Park in Wyoming, we do not have to consider the possibility that tree ferns will establish in the resulting gap. Such species are not a part of the pool. The odds of them dispersing spores all the way from the Luquillo mountains of Puerto Rico are vanishingly small. That, as we shall see, is why we need to define a pool as a group of species with a reasonable probability of being able to enter a community at a particular site. What “reasonable” might mean is something we will return to later in Chapter 3.
We do need to mention one other aspect of dispersal at this point. Although we can indeed delineate a pool of species, P, it is difficult to avoid the reality that some are more likely than others to arrive at a particular location. The greater the flow of propagules, the greater the likelihood that a species will successfully establish. So, in this way, one could argue that traits that measure dispersal ability are an important part of the trait matrix. Plants with wind-dispersed seeds, for example, are well known for colonizing gaps in vegetation (e.g., Reference HarperHarper 1977, Reference van der Pijlvan der Pijl 1982). Many marine animal species similarly have planktonic larvae for long-distance dispersal (e.g., Reference Jackson and CoatesJackson and Coates 1986, Reference FridFrid 1989). In general, small propagules disperse long distances but many animal-dispersed seeds can be rather large. Many plants and some animals also have clonal growth forms, where there is small-scale movement of adults by clonal growth; this, however, is more of a trait for holding space rather than colonizing new patches of habitat (Reference WilliamsWilliams 1975, Reference GrimeGrime 1979). One exception may be aquatic plants, where vegetative structures like turions and even rhizomes can be dispersed long distances by moving water (Reference SculthorpeSculthorpe 1967). Most clonally spreading species, however, have another means for long-distance dispersal, such as wind-dispersed propagules or planktonic larvae.
If we decide to include dispersal traits, there is a confounding factor to consider. The number of propagules that are available for dispersal will be partly determined by the species composition of adjoining landscapes. Species that are already locally common may therefore be disproportionately represented in the stream of propagules. Here we might wonder if we are entering a zone of circular thinking, along the line that species i is likely to occur because species i is common. This is not circular reasoning so much as accepting that communities and landscapes have a kind of biological inertia. Those species most likely to disperse to a community are indeed generally those that are already the most common. An exception would of course be a common species that did not produce propagules. One way to assess this process in real communities is to create artificial patches and monitor rates of recolonization (Reference HartmanHartman 1988). Another is to experimentally manipulate dispersal (Reference ShurinShurin 2000, Reference Ejrnæs, Bruun and GraaeEjrnæs et al. 2006). It is also possible to use transplanted individuals to measure rates of survival and growth in clearings (Reference BertnessBertness 1991, Reference Geho, Campbell and KeddyGeho et al. 2007). The degree to which community composition is controlled by lack of propagules (“dispersal limitation”) remains an open question. Overall, the greater the efficiency of dispersal in the species, the less dispersal limitation there will be in a community. Thus, it may be important to determine the dispersal traits for members of the species pool.
In conclusion, we may find it convenient to assume that dispersal is not an important factor, and simply approach community assembly as a question of goodness-of-fit in sets of trait matrices. However, it may prove necessary to include dispersal traits in the trait matrix, and perhaps even abundance of those propagules in the surrounding landscape. If dispersal is limiting colonization, then time becomes important in the problem of assembly. We discuss dispersal in relation to species pools in more detail in Chapter 3.
t: Time
You cannot talk about communities without some mention of time. Time was included in Equation 1.1, while, more recently, Plant Ecology (Reference KeddyKeddy 2017) has an entire chapter on the topic, with time scales ranging from 101 to >106 years. Still, our intention is to keep it to a minimum in this book. We could say that our focus in this book is primarily about space, rather than time. We assume that with regard to the central issues of community ecology, we can mostly ignore really long periods of time, at scales greater than 106 years that include continental drift and speciation. We are interested here only in shorter time scales (mostly 101 to 102 years). We do not deny that evolution occurs on ecological time scales. Evolution and biogeography are clearly important. But for the sake of simplicity and devoting attention to our specific goal for the book, we treat the products of evolution and biogeography as essentially fixed quantities.
At the other extreme, we can also ignore many events that happen within relatively short time periods that occur over months and days. Of course, time is inherent in the action of environmental filters. It takes time for a drought to arrive, and one can measure how long it persists. It also takes time for an individual or population to perish from drought, and the time to death will depend both upon traits the organisms possess and the severity of the water deficit. Similarly, it takes time for flooding to kill plants, sometimes just a few days, sometimes a few years (Table 1.2). Having said this, we can, quite conveniently and reasonably, ignore much of this information, since the real point is simply this: is the organism still alive by the time we sample the community? We can, more or less reasonably, ignore most short-term events and focus our attention on those individuals that survive long enough to be sampled. How long other individuals lingered, and the unhappy details of their demise, need not concern us.
This is important enough to deserve a restatement. We have set an upper limit on time scales that is short enough to reasonably ignore evolution, and a lower limit long enough to remove short-term physiological activity in organisms. What we are left with, in particular, is the time involved in events such as succession and dynamics, which is a venerable topic in ecology (e.g., Reference Clements, Weaver and HansonClements et al. 1929), and one that frequently occurs on time scales of about 102 years. For example, a review of old field succession in North America (Reference Wright and FridleyWright and Fridley 2010) found that most fields will be 50 percent covered by woody plants within 50 years, some as few as a decade. Here is where factors like rate of formation of gaps in the vegetation, and rate of dispersal of propagules, do fit within the framework of community assembly. And this is where data on traits are useful, such as whether the species has buried seeds, what stimulates germination and how quickly seeds arrive from adjoining forests. Even here, one could take a longer time view: succession in eastern North America, after all, typically leads to a tree-dominated landscape, so eventually the ability to tolerate competition from other trees, or the ability to regenerate in gaps, will come to predominate the rules for community assembly.
Summary
Here is the central challenge of community ecology: if we have a specified location with a known species pool P, can we use knowledge of the species traits T and the filters E at that location to predict the species composition of the community C? Such a class of rules can be called assembly rules. As we have summarized above, assembly rules involve more than simply asking (once again) whether there is a pattern in a selected set of species at a selected location. We have given an outline of the important elements of this assembly problem above and summarize them in Figure 1.5.

Figure 1.5 A general framework for community ecology. The species pool is the raw material. It increases in size from speciation and immigration and decreases in size from extinction (we discuss reasons why emigration is unlikely to affect pool size in the text). A small set of filters (E) determines which members of the species pool (P) occur in local communities (C). Some filters, like frost and drought, are physical factors. Other filters, like competition and predation, are biotic factors. Community membership increases with dispersal and successful recruitment, and decreases through exclusion and mortality. The actual sample units, shown below, usually reveal only a subset of the actual community.
The upper half of Figure 1.5 shows the logical structure of community ecology viewed through the lens of trait-based community assembly. Species pools are filtered into habitats along environmental gradients based on their functional traits. In this book, we argue that this is the correct lens for community ecology, at least most of the time. In subsequent chapters we will explore the key elements of this figure, with particular attention to filters in Chapter 2, pools in Chapter 3 and traits in Chapter 4. After laying the foundations, we describe quantitative translations of this framework into explanatory and predictive models in Chapters 5 and 7.
A careful examination of Figure 1.5 will reveal an asymmetry between the left and right side of the figure, between the factors that increase the pool on the left, and the factors that reduce it on the right. There are two inputs to the species pool. The first is speciation. Speciation happens slowly enough that P can be considered more or less a constant from the perspective of community assembly. The second input is immigration. It takes only a few individuals to establish a new population in a new ecoregion, so it is reasonable to assume that rates of immigration may be rather higher than rates of speciation. As we have discussed earlier in this chapter, dispersal is a key factor that controls species pools, and has been a topic of discussion since Wallace wrote The Geographical Distribution of Animals in 1876. Reference SavileSavile (1956) has discussed a broad array of examples of plant dispersal that potentially affected the North American species pool: plants that likely crossed the North Atlantic via Greenland, or the Pacific Ocean via Beringia “Whether they were carried by pack ice, blew across solid ice, were accidentally carried by birds or mammals, or were transferred in the mud layer of iced komatik runners is immaterial” (p. 3437). As a result, many northern species have nearly circumpolar distributions (compare this to the southern hemisphere in Figure 4.2, where barriers to immigration are wider).
Now, focusing on the right side of the figure, we have the counterbalancing processes. A symmetrical figure would have two elements on the upper right: extinction and emigration. Extinction is clear enough, and there is evidence for slow but steady rates of extinction through the fossil record, with occasional pulses of extinction owing to dramatic geological events, such as the impacts of the asteroid that caused the extinction of the dinosaurs (Reference Levin and KingLevin and King 2017). Also at the upper right, we come to the issue of whether there could be loss of species from the pool through emigration. Although it would be convenient, from the point of view of symmetry, to imagine that emigration in some way balances immigration, this is unlikely to be the case. There is one obvious reason: for the species pool to increase by one, it is necessary only that a few individuals arrive and successfully establish new populations. Exponential growth will take care of the rest. In contrast with long-distance dispersal, for the species pool to decrease by one, it is necessary for all the members of a population to leave an ecological region. Few examples come to mind. Even if we try to imagine a large population of migratory birds or mammals migrating to a new location, it seems unlikely that such a movement would completely remove a population from the species pool, but would rather leave behind stragglers. We cannot come up with a single recorded example of such an event. Cattle Egrets (Bubulcus ibis), for example, arrived in North America, but left a population behind in the Old World. In this sense, they immigrated to the New World, but did not emigrate from the Old World. Similarly, people may talk carelessly about species “migrating” north with climate change. The reality of the process is that during periods of warming climate, such as occurred at the end of the last ice age, individuals remaining at low latitudes mostly died, while individuals spreading to higher latitudes dispersed and established. The so-called retreat of species from the southern limits of their distribution is usually the result of death, not emigration, particularly in the case of sessile species like trees and corals. Hence, we do not include emigration as a process affecting species pools in Figure 1.5.
The lower half of Figure 1.5 exists mostly to remind us that other uncertainties will influence what we actually discover when we make observations on a particular piece of land. These are potential sources of confusion. Here are five of them:
(1) Any field sampling effort, even a morning excursion in the forest for bird watching, is constrained by the familiar pattern of species accumulation curves. Hence, observations of community composition are always affected by sampling effort (e.g., you may look ahead to Figures 3.8 and 3.9).
(2) The vagaries of dispersal mean that certain species may be absent not because they cannot survive in the habitat, but because they have not yet arrived. This is an old theme going back to the days of Reference WallaceWallace (1876). We shall see in Chapter 3 how field experiments show that dispersal limitation is widespread in nature (e.g., Reference Myers and HarmsMyers and Harms 2009).
(3) Local extinctions can occur from a wide range of phenomena, including biotic factors like competitive exclusion, as well as abiotic factors such as fire or flooding. Many, if not most, communities are in a state of recovery from the most recent natural disturbance (e.g., Reference ConnellConnell 1978, Reference HustonHuston 1979). Hence, what we are observing is one small piece of a large-scale process of patch dynamics. These kinds of natural disturbances will appear several places in this book, particularly in Chapters 2, 4 and 8.
(4) The canonical distribution of commonness and abundance (you may look ahead to Figure 8.5) tells us that in any community, a small number of species will be common and a large number rare, without attributing this pattern to any specific cause. We may not see some species because their populations are just so small that they do not (yet) appear on the accumulation curve. We will have more to say on the canonical distribution in the final chapter.
(5) Finally, we are now living in an era where anthropogenic effects are further changing the state of natural communities, and patterns we see in our sample units may be distorted by new and relatively recent human impacts, be they mining, industrial-scale logging, or commercial overhunting. It is true that a bulldozer, skidder, trawler or herd of domesticated animals can indeed be treated as a kind of filter – and their effects may temporarily override all other factors that have been operating in a landscape for millennia. The lower part of Figure 1.5 is there to remind us that when we observe actual communities, we are frequently finding only a subset of the species that are expected based upon trait-based filtering. We raise these issues now because when we are exploring the basic rules of trait-based community assembly, we need to be respectfully aware of, but not distracted by, these phenomena.
It may help to think back to The Theory of Island Biogeography (Reference MacArthur and WilsonMacArthur and Wilson 1967), where S was the number of species on an island. In this case, we are pursuing a vector C of length S in any specified piece of Earth’s surface, including ponds or lakes, as well as real islands. More importantly, and unlike MacArthur and Wilson, we are explicitly after not just the number of species but the species list for each quadrat or island. In a perfect world, as we said at the beginning of the chapter, we would also want to know abundances of each species in the vector. Since the solution cannot be found on a species-by-species basis, we need to approach it through understanding the traits of the species, and the links between those traits and environmental filters.
We have spent some time on relatively basic topics to emphasize that part of the problem of community ecology has been lack of focus. Yes, ecosystems and communities and populations have frustrating complexities. And most certainly, if we look for complexity, we will find it. And, yes, of course, there are different scales at which C can be measured. And, yes, of course, multiple filters act simultaneously. And, yes, of course, there are rich natural historical details about dispersal and death. But we too often have allowed ourselves to be distracted by detail. Our objective in this chapter has been to introduce a simple logical structure underneath all the rich natural history of wild nature. Insights by scholars including Raunkiaer, Jenny, Major, Mueller-Dombois and Ellenberg all provide a formal structure for our thinking, but too often we have drifted back into detail rather than pursuing this logical structure to its conclusion, hence this book.
Key Points of the Chapter
There are four fundamental elements of community ecology: the species pool vector P, the local community vector C, a vector of environmental filters E and a vector of functional traits T.
The central challenge of community ecology is whether, at a specified location with a known species pool, we can use knowledge of the species traits and the filters at that location to predict the species composition of the community. Many scholars throughout history have approached this challenge.
Local communities (C) are subsets of the regional species pool (P) and have been filtered based on the matching of their traits to the local environmental conditions of the habitat.
Dispersal, time and competition are also important factors in community assembly.
One might argue that not only is the capacity to predict both the community vector C and changes in C with time one of the most intellectually challenging parts of community ecology, it is simultaneously one of the most significant moral challenges for protecting Earth’s biota.









