CVD, in particular CHD and stroke, is a major cause of mortality worldwide(1). A number of genetic and environmental factors play a role in the initiation and progression of CVD, and one of the primary dietary risk factors for CVD is the consumption of diets high in saturated fat(Reference Hu and Willett2). Saturated fat is believed to increase cardiovascular risk through its effects on atherosclerosis, ‘endothelial dysfunction’ and ultimately hypertension(Reference Glasser, Selwyn and Ganz3), all prognostically relevant events in CVD(Reference Vogel4). Endothelial dysfunction is characterised by a number of physiological changes, including the decreased bioavailability of endothelium-derived vasodilators, primarily NO (NO∙) and the increased plasma levels of endothelial-derived contracting factors(Reference Bonetti, Lerman and Lerman5). Such changes may result from the dysfunction of endothelial NO synthase, as deficiencies in endothelial NO synthase have been linked to the development of atherosclerosis in animals(Reference Kuhlencordt, Chen and Han6, Reference Naruse, Shimizu and Muramatsu7) and hypertension in human subjects(Reference Celermajer, Adams and Clarkson8). Indeed, hypertension is the primary clinical diagnostic risk factor for CVD(Reference Lawes, Bennett and Lewington9). In rats, hypertension has been associated with an impairment of NO-dependent endothelial function(Reference Kim, Akoh and Lee10).
Epidemiological and medical anthropological investigations suggest that flavonoid-rich foods exert cardiovascular health benefits(Reference Knekt, Kumpulainen and Jarvinen11–Reference Hertog, Kromhout and Aravanis13) and their intake has been shown to lower blood pressure (BP) in both hypertensive(Reference Taubert, Berkels and Roesen14, Reference Grassi, Necozione and Lippi15) and normotensive individuals(Reference Fraga, Actis-Goretta and Ottaviani16, Reference Taubert, Roesen and Lehmann17). The beneficial vascular effects of flavonoids are probably mediated by the ability of absorbed flavonoids and/or their circulating metabolites to increase the bioavailability of NO(Reference Fisher, Hughes and Gerhard-Herman18–Reference Leikert, Rathel and Wohlfart20). In support of this, in vitro studies have indicated that flavonoids are capable of directly activating endothelial NO synthase(Reference Schroeter, Heiss and Balzer21, Reference Steffen, Jung and Klotz22). Blueberries (BB) are a rich source of flavonoids, in particular anthocyanins and flavanols(Reference Prior and Gu23, Reference Wu, Beecher and Holden24), and have been shown to induce improvements in cognitive performance(Reference Williams, El Mohsen and Vauzour25, Reference Andres-Lacueva, Shukitt-Hale and Galli26), to inhibit oxidative stress and inflammation(Reference Giacalone, Di Sacco and Traupe27) and to promote beneficial vascular effects(Reference Norton, Kalea and Harris28–Reference Del Bo, Kristo and Kalea32). Furthermore, in a randomised controlled human intervention study, favourable changes in platelet function, HDL-cholesterol and BP were found after consumption of berries for 8 weeks in elderly individuals with CVD risk factors(Reference Erlund, Koli and Alfthan33). In animal models of hypertension, BB supplementation has been shown to lower BP(Reference Shaughnessy, Boswall and Scanlan31, Reference Elks, Reed and Mariappan34), and also to affect endothelium-mediated vasorelaxation(Reference Kalea, Clark and Schuschke30, Reference Del Bo, Kristo and Kalea32). However, to our knowledge, no studies have investigated the effect of a BB-enriched diet on vascular function in rats fed a high-fat/high-cholesterol diet, reflective of a typical Western diet.
In the present study, we examined the effect of BB supplementation on BP in both normal and high-fat/high-cholesterol-fed animals. Furthermore, we investigated the impact of 10 weeks of BB consumption on the health of the vasculature by assessing the responsiveness of isolated aortic rings (from fed animals) to l-phenylephrine (Phe; endothelium-dependent contraction), acetylcholine (Ach; endothelium-dependent vasodilation) and sodium nitroprusside (SNP; endothelium-independent vasodilation).
Materials and methods
Materials
All chemicals were purchased from Wako Pure Chemical Industries unless otherwise stated.
Animals and diets
A total of thirty-two male Wistar rats (180–200 g, 8 weeks of age) were obtained from Japan SLC. Animals were housed individually in a temperature-controlled room (25°C) with a 12 h light–12 h dark cycle. Body weights (BW) were measured weekly and food intake was measured daily. Rats were randomly assigned to one of four diet groups (n 8 per group): a control chow diet, a control chow diet plus 2 % (w/w, dry weight) freeze-dried BB powder, a high-fat and high-cholesterol diet consisting of the control chow diet plus 10 % (w/w) of lard and 0·5 % (w/w) cholesterol and a high-fat and high-cholesterol diet plus 2 % (w/w) freeze-dried BB powder. The control chow diet was purchased from the Oriental Yeast Company Limited. The composition of the control chow diet (per 100 g diet) was: water, 7·8 g; protein, 17·9 g; fat, 3·3 g; fibre, 8·6 g; energy, 1218 kJ (291 kcal); cholesterol, 1 mg; and vitamin E, 9·1 mg. The fatty acid composition of the control chow diet was: 16 : 0, 15·9 %; 18 : 0, 2·2 %; 18 : 1, 13·5 %; 18 : 2, 40·7 %; 18 : 3, 18·7 % and others less than 1 %. The fatty acid composition of the lard was: 16 : 0, 27 %; 16 : 1, 2·5 %; 18 : 0, 13 %; 18 : 1, 46 %; and 18 : 2, 8 %. The composition of the diets is shown in Table 1.
Table 1 Composition of the diets used in the present study: control chow diet (control diet), control chow with 2 % (w/w, dry weight) BB (2 % BB diet), control chow with 10 % lard and 0·5 % cholesterol (high fat diet) and control chow with 2 % (w/w, dry weight) BB, 10 % lard and 0·5 % cholesterol (2 % BB+high-fat diet)*

* The amount of each type of fat is expressed in percentage of total fat in the diets. The amount of total measured flavonoids is expressed in mg/100 g of diet.
Highbush BB of unknown variety were purchased from a local supplier (Axons of Southampton) and immediately frozen at − 20°C. Frozen BB were freeze-dried ( − 25°C; 6 mbar vacuum) in an Edwards MFD 01 freeze drier (Edwards) and ground to a fine powder using an Apex Comminuting Mill (Apex Processing Technology). The freeze-dried BB powder was kept at − 20°C and shipped from the UK to Japan, where the animal experiments were conducted.
The freeze-dried BB powder was analysed for anthocyanin and procyanidin content according to the established methods in our laboratory, as previously described(Reference Rodriguez-Mateos, Cifuentes-Gomez and Tabatabaee35). Diets were prepared fresh and were stored at 4°C for a maximum of 2–5 d following preparation. All rats were provided with water and food ad libitum for the duration of the experiment. All animal procedures were in accordance with the institutional guidelines for the care and use of laboratory animals of the University of Tokushima.
Blood pressure measurements
Systolic BP was measured in all rats at weeks 1, 2, 4, 6, 8 and 20 via the tail-cuff method, using an oscillometric method (TK-370C, UNICOM). Before measurement, conscious rats were placed in a holding device for 10 min prior to BP monitoring. The mean of triplicate measurements was recorded. All measurements were taken at the same time of the day for all rats ( ± 1 h).
Preparation of aortic rings and tension measurements
Tension measurements on aortic rings were performed according to the established procedures with some modifications(Reference Wu, Harada and Nakamura36). At the culmination of the 10-week supplementation, rats were anaesthetised using diethyl ether and the thoracic aortas were dissected (free of connective tissue) and then cut into ring segments, 3–4 mm in length. Each ring was placed in a 3 ml organ bath (Micro Easy Magnus, Kishimoto Medical) and mounted on two stainless steel wires, one of which was fastened to the bath and the other connected to a force transducer for the measurement of isometric tension. The bath was filled with Krebs–Ringer bicarbonate buffer solution at 37°C and bubbled with a mixture of 95 % CO2 and 5 % O2. The Krebs–Ringer bicarbonate buffer contained (in mmol/l) 118 NaCl, 4·6 KCl, 2·5 CaCl2, 24·8 NaHCO3, 1·2 MgSO4, 1·2 KH2PO4 and 5·6 glucose. Each aortic ring was equilibrated for 60 min under a resting tension of 1·5 g and the Krebs–Ringer bicarbonate solution was changed at 30 min intervals. Cumulative concentration–response curves to the endothelium-dependent vasodilator Ach and endothelium-independent vasodilator SNP were performed. To test the relaxation responses of Ach and SNP, the aortic rings were pre-contracted with the α-adrenergic agonist, Phe (10− 6m; 10 min), until the contraction curve reached a plateau. Following Phe-induced contraction, cumulative applications of Ach (10− 9 to 10− 5·5m) or SNP (10− 8·5 to 10− 5m) were applied for 5 min, during which maximum aortic ring relaxation was achieved. For quantification, the relaxant effect to each Ach or SNP dose was expressed as percentage relaxation relative to the initial Phe pre-contraction.
Determination of plasma TAG and cholesterol
Blood samples were collected from the abdominal aorta. Plasma was obtained by immediately centrifuging heparinised blood at 3000 g for 15 min at 4°C and stored at − 80°C until required for analysis. TAG, total and HDL-cholesterol were determined using enzymatic assay kits (Wako Pure Chemical Industries). The content of LDL-cholesterol was calculated by subtracting HDL-cholesterol from total cholesterol.
Statistical analysis
Analyses were carried out in the software package SAS version 9.1.3 (SAS Institute). For the BP results, a mixed model was fitted to take into account the repeated measurements across time. The four diet treatments, the week of the experiment and the interaction between them were included as fixed effects in the final model. A compound symmetry matrix for the random effect was used, which is a pattern chosen for modelling the variance–covariance matrix of the random effect. It assumes the same variance within time for all subjects and the same correlation between each pair of time points for each subject. When possible, orthogonal contrasts were calculated to understand the nature of the significant interaction.
For the aortic ring experiments, a two-way ANOVA model was fitted with dose concentration, treatment group and the interaction between them as covariates. Dose concentration was considered as categorical variable. Orthogonal contrasts were carried out to detect differences between groups and between doses. Significance was defined as P< 0·05. All models were validated plotting standardised residuals v. predicted values to test the assumption that residuals follow a normal distribution centred in zero and with a constant variance. quartile-quartile (QQ) plots were also used to assess the normality of the data.
Results
Food intake and weight
All animals gained weight during the 10 weeks feeding period, although no significant differences were recorded in mean BW between animals following the four dietary groups. Initial mean BW were 184 (sem 10) g, whilst at the end of the 10-week period, it was 362 (sem 17) g for the animals following the control chow diet, 356 (sem 17) g for the animals following the BB diet, 354 (sem 11) g for the animals following the high-fat diet and 360 (sem 20) g for the animals following the BB plus high-fat diet. Rats that followed the high-fat dietary regimen were observed to consume significantly less food during the 10 week feeding period (P< 0·05); however, BB intervention did not significantly influence food intake. On average, rats fed with the control chow diet, with or without BB, had a daily food intake of 20 (sem 3) g, whereas animals following the high-fat diet, with or without BB, had a daily intake of 18 (sem 2) g.
Flavonoid intake
The highbush variety of BB used in the present study was analysed for anthocyanin and procyanidin (monomers to decamers) content (Table 1). Considering that the percentage of freeze-dried BB powder used in the present study was 2 % (w/w), and that the average food intake was approximately 19 g of food per d, the daily total measured flavonoid intake was approximately 3·85 mg of total flavonoids. Considering an average rat weight of 290 g throughout the 10 weeks of supplementation, average daily intake per kg of BW was 13·3 mg total flavonoids/kg BW (7·7 mg anthocyanins/kg BW, 5·5 mg procyanidins/kg BW, 0·4 mg of flavanol monomers/kg BW and 1·17 mg of flavanol dimers/kg of BW). Flavonols, phenolic acids and hydroxycinnamate esters were not quantified in the present study, but previous reports have shown that these compounds are also present in BB(Reference Hakkinen, Karenlampi and Heinonen37, Reference Taruscio, Barney and Exon38).
Effect on systolic blood pressure
Initial statistical analysis indicated a significant interaction between group and week (P= 0·0001). BB intervention (2 % (w/w)) along with the control chow diet significantly reduced SBP at 8 (11 %) and 10 (14 %) weeks, compared to the control chow diet alone (week 8 SBP: 107·5 (sem 4·7) v. 122·2 (sem 2·1) mmHg, P= 0·018; week 10 SBP: 115·0 (sem 3·1) v. 132·7 (sem 1·5) mmHg, P< 0·0001; Fig. 1(a)). For rats fed the high-fat/cholesterol diet, BB also significantly reduced SBP by 14 % at week 10 (118·2 (sem 3·6) v. 139·5 (sem 4·5) mmHg, P< 0·0001; Fig. 1(b)). High fat consumption itself was observed to elevate SBP after 1 and 4 weeks of intervention, relative to those fed with the control chow diet, independently of whether they were consuming BB (SBP week 1: 112·3 (sem 3·2) v. 104·2 (sem 2·6) mmHg, P= 0·009; SBP week 4: 138·1 (sem 5·1) v. 120·4 (sem 5·0) mmHg, P< 0·0001). At weeks 6, 8 and 10, difference in SBP between rats fed the control chow diet or high-fat diet were not significant.
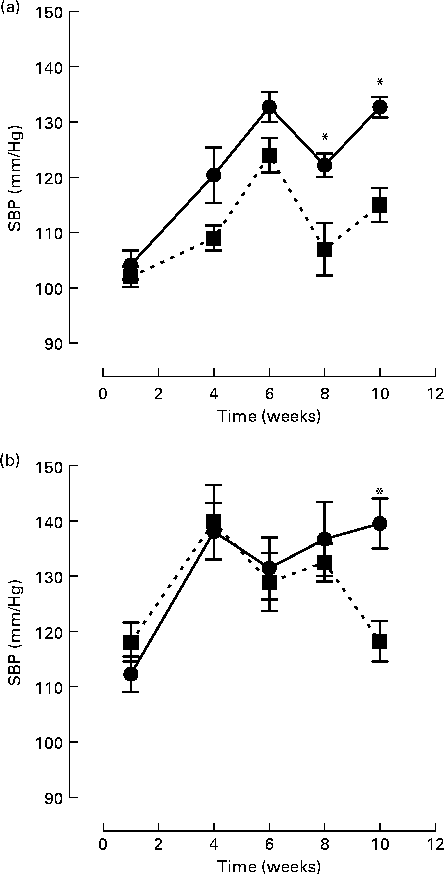
Fig. 1 Systolic blood pressure (SBP) of rats fed with (a) a control chow diet (control, ![]() ) or a control chow diet with 2 % blueberry (BB;
) or a control chow diet with 2 % blueberry (BB; ![]() ) and (b) a high-fat diet consisting of control chow diet plus 10 % lard and 0·5 % cholesterol (high fat,
) and (b) a high-fat diet consisting of control chow diet plus 10 % lard and 0·5 % cholesterol (high fat, ![]() ) or a high-fat diet with 2 % BB (
) or a high-fat diet with 2 % BB (![]() ) for 10 weeks. A total of eight rats were fed the respective diet and SBP was measured via the tail-cuff method at weeks 1, 4, 6, 8 and 10. The mean of triplicate blood pressure readings was recorded for each rat. Values are means, with their standard errors represented by vertical bars. Statistical comparison between groups was performed using a mixed model with contrasts. * Mean values were significantly different with respect to the control group (P< 0·05).
) for 10 weeks. A total of eight rats were fed the respective diet and SBP was measured via the tail-cuff method at weeks 1, 4, 6, 8 and 10. The mean of triplicate blood pressure readings was recorded for each rat. Values are means, with their standard errors represented by vertical bars. Statistical comparison between groups was performed using a mixed model with contrasts. * Mean values were significantly different with respect to the control group (P< 0·05).
Effects of blueberry supplementation on aortic ring constriction
When aortic rings were treated with the selective α-adrenergic receptor agonist, Phe (10− 6m), the maximum force developed was significantly greater for the rats fed with the control chow diet compared to those supplemented with 2 % (w/w) BB (0·79 (sem 0·11) v. 0·59 (sem 0·07) g; P< 0·05; Table 2). This was also the case for aortic rings isolated from rats fed the high-fat diet, where BB-fed rats showed significantly less contractile response compared to high fat only animals (0·79 (sem 0·08) v. 0·67 (sem 0·08) g; P< 0·05; Table 2). No significant differences in contractile response were observed between animals fed the control diet or the high-fat diet in response to Phe.
Table 2 Maximum force developed when aortic rings of rats fed with a control chow diet (control diet), a control chow diet with 2 % (w/w, dry weight) BB (2 % BB diet), control chow diet with 10 % lard and 0·5 % cholesterol (high-fat diet) or a high-fat diet with 2 % BB (2 % BB+high-fat diet) for 10 weeks were treated with the selective α-adrenergic receptor agonist, l-phenylephrine (10−6m) (Mean values with their standard errors)

a,bMean values with unlike superscript letters are significantly different (P≤ 0·05).
Effects of blueberry supplementation on endothelium-dependent and independent relaxation
Cumulative dose–response curves indicated a concentration-dependent relaxation of pre-contracted aortic rings in response to Ach and SNP (Fig. 2). Both main effects (dose and treatment group) covariates were significant (P< 0·0001); however, the interaction between dose and treatment group was not significant (P= 0·999). Orthogonal contrasts were carried out to detect differences between groups and between doses and indicated a marked reduction in vasorelaxation of pre-contracted aortic rings to Ach in high-fat-fed animals compared to control chow-fed animals (P= 0·0001). BB supplementation had a significant impact on the aortic dilatory response in animals that followed the high-fat diets (P= 0·0001; Fig. 2(b)), although it did not influence the dilatory potential of aortic rings from animals fed the control chow diet (P= 0·926; Fig. 2(a)). Furthermore, supplementation of high-fat-fed animals with BB was observed to restore vasorelaxation to the levels observed for animals fed with the control chow diet (no significant difference between control chow and high fat+BB; P= 0·621; Fig. 2(a) and (b)). The maximum vasorelaxation in response to Ach was also greater for the aortic rings of the BB-fed animals compared with their respective controls, although this was only significant for the high-fat-fed animals (P< 0·05; Fig. 2(b)). Relaxation responses for aortas stimulated with SNP were not significantly different between the control and high-fat-fed ones (P>0·05), and BB supplementation had no significant impact on this relaxation (P>0·05; Fig. 2(c) and (d)), despite there being a trend for an increase in relaxation in animals following both diets at higher concentrations of SNP (Fig. 2(c) and (d)).

Fig. 2 (a) Acetylcholine (Ach)-induced relaxation of aortic rings from rats fed with a control chow diet (control diet, ![]() ) and a control chow diet with 2 % blueberry (BB) (2 % BB diet,
) and a control chow diet with 2 % blueberry (BB) (2 % BB diet, ![]() ) for 10 weeks. (b) Ach-induced relaxation of aortic rings from rats fed with a high-fat diet consisting on control chow diet plus 10 % lard and 0·5 % cholesterol (high-fat diet,
) for 10 weeks. (b) Ach-induced relaxation of aortic rings from rats fed with a high-fat diet consisting on control chow diet plus 10 % lard and 0·5 % cholesterol (high-fat diet, ![]() ) and a high-fat diet with 2 % BB (2 % BB high-fat diet,
) and a high-fat diet with 2 % BB (2 % BB high-fat diet, ![]() ) for 10 weeks. (c) Sodium nitroprusside (SNP)-induced relaxation of aortic rings from rats fed with a control diet (
) for 10 weeks. (c) Sodium nitroprusside (SNP)-induced relaxation of aortic rings from rats fed with a control diet (![]() ) and a control diet with 2 % BB (
) and a control diet with 2 % BB (![]() ). (d) SNP-induced relaxation of aortic rings from rats fed with a high-fat diet (10 % lard plus 0·5 % cholesterol,
). (d) SNP-induced relaxation of aortic rings from rats fed with a high-fat diet (10 % lard plus 0·5 % cholesterol, ![]() ) and 2 % BB high-fat diet (
) and 2 % BB high-fat diet (![]() ) for 10 weeks. The graph represents relative relaxation in response to Ach or SNP (n 8 per group) in aortic rings. Ach-induced relaxation was reduced in the high-fat fed group in comparison with the other groups. Aortic rings of rats fed a high-fat diet showed marked reduction in their response to Ach compared to rings from control or BB-fed rats. No significant changes in SNP-induced relaxation were observed for any of the groups. * Mean values were significantly different with respect to the control group (P< 0·05).
) for 10 weeks. The graph represents relative relaxation in response to Ach or SNP (n 8 per group) in aortic rings. Ach-induced relaxation was reduced in the high-fat fed group in comparison with the other groups. Aortic rings of rats fed a high-fat diet showed marked reduction in their response to Ach compared to rings from control or BB-fed rats. No significant changes in SNP-induced relaxation were observed for any of the groups. * Mean values were significantly different with respect to the control group (P< 0·05).
Plasma cholesterol and TAG
Rats fed with the high-fat diet had significantly higher levels of total cholesterol, LDL-cholesterol and TAG levels and significantly lower levels of HDL-cholesterol in comparison with control chow-fed animals after 10 weeks of supplementation (Table 3). BB supplementation had no significant impact on any of these plasma markers, either in control or high-fat-fed animals (Table 3).
Table 3 Plasma total cholesterol (TC), LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C) and TAG in plasma of rats fed with a control chow diet (control diet), a control chow diet with 2 % (w/w, dry weight) BB (2 % BB diet), control chow diet with 10 % lard and 0·5 % cholesterol (high-fat diet) or a high fat diet with 2 % BB (2 % BB+high-fat diet) for 10 weeks (Mean values with their standard errors)

a,b,c,d,e,f,g,hMean values with unlike superscript letters are significantly different (P≤ 0·05)
Discussion
Evidence from human(Reference Taubert, Roesen and Lehmann17, Reference Schroeter, Heiss and Balzer21, Reference Hooper, Kroon and Rimm39), animal(Reference Shaughnessy, Boswall and Scanlan31, Reference Elks, Reed and Mariappan34) and cell studies(Reference Lodi, Jimenez and Moreno40) suggests that flavonoids may exert benefits on endothelial function through their ability to improve NO bioavailability and lower BP. In the present study, we show that dietary supplementation with BB, which is rich in both anthocyanins and procyanidins(Reference Prior, Lazarus and Cao41), resulted in a significant lowering of BP after 8 and 10 weeks of consumption in normotensive animals fed with a control chow diet and after 10 weeks consumption of a high-fat/high-cholesterol diet (Fig. 1). In addition, BB supplementation improved aortic constriction in animals fed both diets (Table 2) and Ach-induced vasorelaxation in animals fed a high-fat/high-cholesterol diet (Fig. 2). With regards to the BP changes, the present data agree with previous findings that a high-fat dietary regimen does not induce significant modification of BP(Reference Kim, Akoh and Lee10, Reference Knight, Quigley and Yuan42). An increase in BP from week 1 to week 4 was observed for animals fed the high-fat/high-cholesterol diet, and from week 1 to week 6 for animals fed the control chow diets. We are unable to explain this increase in BP; however, increases in SBP with age have been reported previously using the tail-cuff method for measurement of BP in normotensive animals(Reference Shaughnessy, Boswall and Scanlan31). The present results also agree with data indicating that supplementation for 4–12 weeks with BB (2–3 % w/w) induces a reduction in systolic BP in spontaneously hypertensive rats(Reference Shaughnessy, Boswall and Scanlan31, Reference Elks, Reed and Mariappan34). However, in contrast to these studies, we also observed a significant reduction in BP following BB supplementation in normotensive, control chow-fed animals (Fig. 1). A possible explanation for this difference in efficacy in normotensive animals may relate to the total flavonoid dose delivered in the various interventions. Although the percentage of BB intervention and the length of the supplementation were comparable to those used in the present study, it is difficult to compare interventions precisely for flavonoid delivery, as the previous studies did not report the flavonoid content and profile of their BB interventions. It is known that polyphenol levels in BB are strongly influenced by variety/genotype(Reference Rodriguez-Mateos, Cifuentes-Gomez and Tabatabaee35, Reference Kalt, Ryan and Duy43), environmental growing conditions(Reference Jin, Wang and Wang44–Reference Sellappan, Akoh and Krewer46) and extraction and analytical methods used among others(Reference Khanal, Howard and Prior47). Differences in animal weight and daily food intake can be another source of variation between studies. For example, in a previous study, a 2 % (w/w) diet was reported to lead to a daily flavonoid intake of 10·5 mg of flavonoids (anthocyanins and procyanidins)(Reference Williams, El Mohsen and Vauzour25), whereas, in the present study, an intake of 3·8 mg of anthocyanins and procyanidins per d was observed. Indeed, the flavonoid content of the BB variety used, the average animal weight and, thus, the daily food intake of the animals were all considerably lower in the present study.
Changes in BP induced by flavonoid-rich foods, such as BB and cocoa, are postulated to occur through the interactions of flavonoid/metabolites with the endothelium post-absorption(Reference Elks, Reed and Mariappan34, Reference Gomez-Guzman, Jimenez and Sanchez48, Reference Grassi, Desideri and Necozione49). We observed that BB supplementation in control chow- and high-fat-fed animals for 10 weeks induced a reduction in the contractile response of isolated aortas in response to Phe. Previous studies support this observation, with BB capable of attenuating the contractile response to Phe at concentrations above 10− 7m(Reference Norton, Kalea and Harris28, Reference Kalea, Clark and Schuschke29). Such effects were suggested to be endothelium dependent, in that there were no effects in aortas where the endothelium was denuded or when NG-monomethyl-l-arginine acetate salt (l-NMMA), the endothelial NO synthase inhibitor, was co-administered(Reference Norton, Kalea and Harris28, Reference Kalea, Clark and Schuschke29).
The present high-fat dietary regimen (a mix of saturated fats (approximately 40 %; palmitic and stearic acid), monounsaturated fat (approximately 50–60 %; oleic acid) and 0·5 % cholesterol) was reflective of a typical Western diet and led to a significant reduction in endothelium-dependent vasorelaxation, and increases in the plasma levels of cholesterol and TAG, as has been previously reported(Reference Kim, Akoh and Lee10, Reference Mawatari, Kakui and Harada50). In human subjects, hypercholesterolaemia has been associated with atherogenesis and with the impairment of endothelium-dependent vasodilatation(Reference Henry51, Reference Quyyumi, Mulcahy and Andrews52), primarily due to SFA that are known to reduce endothelium-dependent vasodilation(Reference Edirisinghe, McCormick Hallam and Kappagoda53–Reference Giannattasio, Zoppo and Gentile56). Unlike BP, BB intervention was only observed to improve aortic dilation in animals that consumed the high-fat diet (Fig. 2). The lack of effects of BB on aortic relaxation in animals on the control chow diet is supported by previous data, where animals fed as much as 8 % BB (w/w) along with a standard chow diet induced no effect on Ach-induced vasorelaxation(Reference Kalea, Clark and Schuschke29). However, it has been reported that BB improves Ach-induced relaxation in spontaneously hypertensive rats, although an increase in relaxation was only observed at Ach doses between 10− 9 and 10− 7m, whereas higher Ach concentrations induced no or even reduced relaxation in BB-fed rats(Reference Kalea, Clark and Schuschke30, Reference Kristo, Kalea and Schuschke57). In addition, the maximum relaxation in response to Ach in BB-fed animals has been observed to be lower or not significantly different with respect to the control in spontaneously hypertensive rats(Reference Kalea, Clark and Schuschke30, Reference Kristo, Kalea and Schuschke57). However, spontaneously hypertensive rats have complex vascular beds and an imbalance between vasorelaxants and vasoconstrictors(Reference Taddei, Ghiadoni and Virdis58); thus, this could explain the differences observed in Ach-induced relaxation in the present study, where normotensive Wistar rats were used. A significant difference between the present study and those previously conducted relates to the total amount of BB used in interventions. In previous studies conducted by Klimis-Zacas et al. mentioned earlier(Reference Norton, Kalea and Harris28–Reference Kalea, Clark and Schuschke30, Reference Del Bo, Kristo and Kalea32), BB was incorporated into the diet of the animals at the 8 % (w/w, dry weight) level, significantly higher than that used in the present study (2 % BB diet (w/w), dry weight). This is relevant as an amount of 2 % (w/w) delivers approximately 1·3 g freeze-dried BB/kg BW per d (based on average BW and food intake over the 10 week supplementation), which relates to an approximate 450 g intake per d in a 70 kg person. Thus, although high, the present data better reflect an achievable level of intake in human subjects. We speculate that supplementation with higher doses of BB might be less effective than lower doses, as a 2 % BB diet (w/w, dry weight) was more effective than a 4 % dose in lowering plasma cholesterol in pigs(Reference Kalt, Foote and Fillmore59). In addition, higher doses of anthocyanins have been shown to diminish cardioprotection and induce cardiotoxicity in an isolated in vitro heart model of ischaemia–reperfusion(Reference Ziberna, Lunder and Moze60). Furthermore, a recent meta-analysis has reported that a non-linear dose–response relationship exists between the effects of flavanol-rich foods and endothelial function, with higher flavanol concentrations leading to less potent vascular effects(Reference Shrime, Bauer and McDonald61).
Although it is difficult to scale these intakes to human subjects, previous human interventions report that consumption of 350 g of BB for 8 weeks is capable of reducing systolic and diastolic BP by about 6 and 4 %, respectively, in individuals with the metabolic syndrome(Reference Basu, Du and Leyva62). In addition, consumption of 100 g of berries and a small glass of a berry drink per d for 8 weeks was observed to reduce systolic BP by 1·5 mmHg (from 1227·8 to 126·3 mmHg) in unmedicated patients with at least one risk factor of CVD(Reference Erlund, Koli and Alfthan33). Furthermore, a recent prospective study has shown an inverse association between anthocyanin consumption and hypertension, mainly due to BB and strawberry consumption(Reference Cassidy, O'Reilly and Kay63).
Regarding the effect of BB supplementation on plasma lipids, no changes were observed in TAG and in total, LDL- and HDL-cholesterol in the BB-enriched groups compared with the respective controls (Table 2). This is in agreement with previous studies on mice and ApoE-deficient mice(Reference Wu, Kang and Xie64, Reference Prior, Wilkes and Rogers65). However, other studies with different animal models focusing on obesity-prone rats and pigs have reported a decrease in plasma lipids after supplementation with 1, 2 or 4 % BB diet(Reference Kalt, Foote and Fillmore59).
The possible involvement of NO–cyclic GMP system in the mechanism of action of absorbed BB flavonoids is reinforced by the fact that, in the present experiments, the effects were only observed to be Ach dependent. In support of this, when purified anthocyanins were delivered orally to hyper-cholesterolaemic subjects, increases in Flow-mediated dilatation were paralleled by an increase in cyclic GMP and HDL-cholesterol concentrations(Reference Zhu, Xia and Yang66). Furthermore, in the presence of NO–cyclic GMP inhibitors, the effects of anthocyanins on endothelial function were suppressed in human volunteers after intravenous administration of l-NMMA, a NOS inhibitor, and in vitro, using a rat aortic ring model in the presence of l-NMMA and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), a guanylate cyclase inhibitor(Reference Zhu, Xia and Yang66). Chronic administration of anthocyanin-rich and/or procyanidin-rich foods have also been shown to improve endothelial function and to lower BP via a NO mechanism in rat models of hypertension(Reference Gomez-Guzman, Jimenez and Sanchez48, Reference Poudyal, Panchal and Brown67, Reference Quiñones, Muguerza and Miguel68). Alternatively, the athero-protective effects of BB may be linked to an up-regulation of antioxidant enzymes(Reference Wu, Kang and Xie64) or an inhibition of scavenger receptors, CD36 and scavenger receptor class A expression, involved in the binding and uptake of oxidised LDL into macrophages and, therefore, the production of foam cell formation(Reference Xie, Kang and Chen69).
In summary, we show that consumption of dietary quantities of BB may lower BP and improve endothelial dysfunction induced by a high fat, high cholesterol-containing diet.
Acknowledgements
The present research was supported by a fellowship awarded to A. R.-M. by the Japanese Society for the Promotion of the Science. The authors responsibilities were as follows: A. R.-M., J. T., J. P. E. S. and K. M. designed research; A. R.-M. and A. I. conducted research and analysed data; A. R.-M. and J. P. E. S. wrote the paper; and A. V.-D. performed statistical analysis of the data. All authors read and approved the final manuscript. None of the authors declared a conflict of interest.







