Introduction
Research coordinators (RCs) are a vital component of the clinical research enterprise within academic health centers (AHCs). RCs serve as the liaison between investigators and human research subjects, clinical care providers, regulatory bodies, sponsors, and numerous others involved in the research process. RCs also play a pivotal role in protecting human research subjects through the numerous activities and responsibilities delegated to them [Reference Speicher1].
RCs are essential to the scientifically sound and ethical conduct of clinical research, and so by supporting RCs, an institution invests and advances its human subjects research capabilities. For this reason, it is imperative that investigators and AHCs together ensure that RCs receive adequate training and support to carry out each of their delegated tasks [Reference Calvin-Naylor2]. In the context of this paper, “support” refers to training, resources, and services provided to the RC workforce beyond salary support.
As part of the Research Coordinator Taskforce of the Clinical & Translational Science Award (CTSA) consortium, the authors sought to understand the ways in which CTSA-funded AHCs support RCs, believing the existence of a RC network to be a primary indicator of support. Institutions have organized research nurses utilizing networks [Reference Ecklund3] but given the absence of a prior systematic inquiry into such RC networks in clinical translational research, in 2013 the authors surveyed CTSA institutions to identify existing RC networks, and where none existed, to characterize how RCs are supported, if at all.
Background
In 2006, the National Institutes of Health’s National Center for Research Resources launched the CTSA program to support a national consortium of medical research institutions designed to transform how biomedical research is conducted. The CTSA aims to speed the translation of laboratory discoveries into treatments for patients, engage communities in clinical research efforts, and train a new generation of clinical and translational researchers. At that time, a variety of working groups known as key function committees and taskforces were created. The RC Taskforce was established as part of the Regulatory Knowledge group of the Clinical Research Innovation key function committeesFootnote 1 and focused on enhancing the capability of AHCs to support and train RCs by recommending best practices and providing relevant tools. RC Taskforce initiatives have included the development of core competencies in research coordination, professionalization of the RC role through defined career ladders and job descriptions, and the pursuit of a means to increase the support of RCs at AHCs [Reference Speicher1, Reference Needler, Christy and Cowan4]. In 2013, the RC Taskforce distributed a CTSA-wide survey to examine the ways CTSA-funded AHCs provide support and services to RCs and, specifically, to identify whether institutions have an RC network.
Objectives of the Survey
The objectives of the survey were to characterize existing RC networks and, in the absence of an RC network, to determine what other supports for RCs might exist within a CTSA AHC. From the institutions with a RC network, we specifically sought to gather information on how networks were established and to identify the network’s key characteristics and activities. This article summarizes findings and recommendations for how AHCs can support RCs.
Institutional Review Board (IRB) approval was received for this survey and was either determined to require no review, meet the criteria for exemption from IRB review or, be considered Not Human Subjects Research at the authors’ respective institutions.
METHODS
The web-based survey, developed by a small working group of the RC Taskforce, was reviewed by the Taskforce, the Dana Faber/Harvard Cancer Center (DF/HCC) Survey Data Management Core (SDMC)Footnote 2 ; endorsed by the Regulatory Knowledge Key Function Committee members; and approved by the CTSA Consortium Executive Committee.
The survey was designed, and data collected, using Research Electronic Data Capture (REDCap)Footnote 3 data collection. The iterative development and testing process included beta testing at 4 Harvard Catalyst institutions, and resulted in an improved data collection strategy and questionnaire design. As no survey of its kind was available to use as a reference, the list of questions and wording was decided by consensus of the working group.
The final survey was comprised of 2 sections (online Supplementary Appendix 1):
(1) The first section focused on the demographics of respondents, their institutions, and the characteristics of RCs and RC support services/activities offered at those institutions.
(2) The second section provided additional questions targeted to institutions that reported having a RC Network.
On May 28, 2013, the finalized online survey was disseminated to the Regulatory Knowledge Committee members at each of the participating CTSA institutions through the National CTSA Regulatory Knowledge listserv and posted on the CTSA website. Survey completion reminders were sent out at regular intervals. The survey link was disabled on August 16, 2013. Data collection was completed on October 29, 2013.
The survey received a 93% response rate (57 of 61 CTSA institutions). As many CTSAs are comprised of multiple institutions, more than 1 response per CTSA was possible and the survey was designed to allow for individual responses from each of the primary CTSA institutions as well as responses from their affiliated institutions and community partners. From the 57 participating CTSAs, 79 responses were received (57 from primary institutions, 22 from affiliate institutions). Of the 79, 73 were complete and 6 partially completed. Of the 6 partially completed responses, 1 response was omitted from analysis because the respondent did not provide any survey data beyond identification of the CTSA institution. Thus, the survey sample size is 78. Almost 3-quarters (71.8%) of respondents self-identified as an “administrator/manager/director of RCs.”
Frequency distributions and summary statistics were used to describe the participant characteristics. χ2 Tests and Fisher’s exact tests were used to examine the associations between 2 categorical variables. All statistical analyses were conducted using Excel and the statistical analysis software SAS v9.4 (SAS Institute Inc., Cary, NC, USA). The significance level for all tests were set at 5%.
Results
Results indicated 4 key areas of RC support: (1) identification of RCs, (2) professionalization of the RC position, (3) dissemination of information to RCs, and (4) offering of services to RCs. In addition, characteristics of RC networks were captured.
Support Mechanism 1: Identification of RCs
To inform RCs of services and resources available AHCs must have the ability to identify the individuals that make up the RC workforce. The majority of institutions (55/77, 71.4%) had a database for identifying existing RCs. Of those, 25.5% (14/55) had a complete database and 60.0% (33/55) had a fairly complete database. In order to identify newly hired RCs, approximately half of the institutions had a database (or combination of databases). Of institutions that did have a database for identifying newly hired coordinators, 37.1% (13/35) had a complete database and 40.0% (14/35) had a fairly complete database.
Importantly, 96% of respondents indicated that RCs at their institutions operated under titles other than “RC.” The survey defined “RC” as:
A research professional who, manages and oversees one or more aspects of human subject research studies under the immediate supervision and direction of the principal investigator and/or sub-investigators. The role of the research coordinator can include such titles as Clinical Research Coordinator, Research Assistant, Project Manager, Clinical Research Nurse, etc.
Based on survey responses, other commonly used titles at participating AHCs included: research nurse, project coordinator/manager, research associate, clinical RC/specialist, grant specialist, research administrator, consent form administrant, quality management coordinator, study coordinator, regulatory manager, and protocol development specialist.
Support Mechanism 2: Professionalization of the RC Position
Another mechanism by which institutions can support RCs is through institutional support for, or endorsement of, professional development, such as national certification (i.e., Association of Clinical Research Professionals and the Society of Clinical Research Associates). Through such ongoing development and certification, RCs gain knowledge and expertise that serve not only to advance their careers but to enhance their professional performance and skills, ultimately improving the conduct of research at their institutions [Reference Speicher1]. A majority (53/77) of survey respondents reported their institutions either informed their RCs of national certification opportunities (15/77, 19.5%), encouraged (31/77, 40.3%), or required (7/77, 9.1%) such certification. Some (39.6%) of these institutions also reported offering financial assistance for RCs to obtain national certification(s).
Several respondents (28/76, 36.8%) reported offering formalized, local education programs covering research management and Good Clinical Practice competencies while an additional 31.6% (24/76) were in the process of adding such an offering. The completion of such a program could prepare RCs to sit for a national certification exam and/or promote career advancement.
Support Mechanism 3: Dissemination of Information to RCs
Having adequate communication mechanisms in place enables an AHC to appropriately relay to RCs (in a timely and efficient manner) the information necessary for RCs to fulfill their responsibilities (e.g., information about educational opportunities, institutional policy or federal regulations). For this reason, the survey examined whether institutions shared such information with their RCs, and if so, how. The majority of the respondents had more than 1 method of disseminating pertinent research-related information to their RC workforce. The most commonly used means were use of email (85.9%), training events (76.9%), meetings (75.6%), content on Web sites (70.5%), and listservs (53.9%) (Fig. 1).
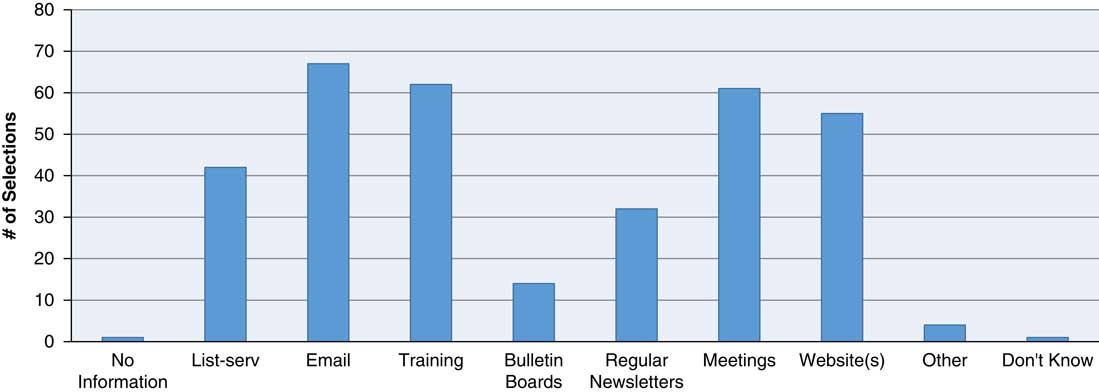
Fig. 1 How does pertinent research-related information get disseminated to/exchanged with research coordinators? Methods by which pertinent research-related information is disseminated to/exchanged with research coordinators includes email, training, meetings, websites, and listservs, newsletters and bulletin boards with email being used the greater percentage of time (85.9%). Respondents were instructed to select “all that apply.”
In terms of the types of information disseminated to RCs at responding institutions, the majority of institutions disseminated information about educational events (74/78, 94.9%) and information about institutional policies (68/78, 87.2%). A high percentage (49/78, 62.8%) also disseminated federal regulation announcements (Fig. 2).

Fig. 2 What pertinent research-related information is disseminated to/exchanged with research coordinators? Research-related information disseminated to/exchanged with research coordinators includes educational events, institutional policies, federal regulation announcements, mentoring information, and job opportunities. Educational events were most frequently shared (94.9%). Respondents were instructed to select “all that apply.”
Support Mechanism 4: Offering of Services to RCs
Institutions reported a varied range of support services offered to RCs. The majority of respondents provided workshop/education events (66/78, 84.6%) and training (57/78, 73.1%) specific to the RC role and related responsibilities. Many institutions also reported offering coordinator orientation (41/78, 52.6%). Only 2 respondents indicated their institution did not offer any services or activities to RCs (Table 1).
Table 1 Research coordinator (RC) networks: services provided and communication methods

* Respondents were instructed to select all that applied.
Nearly 80% (60/77) of institutions facilitate collaboration and education of RCs through at least 1 method of systematic information exchange, such as listservs, lunch series, meetings, trainings, newsletters, and RC networks (Table 1).
RC Networks Described
Believing the existence of an RC network to be a strong indicator of institutional support for the RC professional, we sought to determine specific characteristics of these networks and the institutions that provide them. The survey defined RC networks as: a supportive system of information exchange and/or services provided to facilitate collaboration and education of RCs. Of 77 respondents, 29 (37.7%) indicated that their institution had an RC network. On average, the RC networks had been in existence for 5 years, though the minimum was 1 year, and the maximum 16 years.
Respondents that indicated having a network at their institutions were asked additional questions about the network’s formality, institutional recognition, and financial support. The survey also collected respondents’ feedback with regards to the strengths of their networks, as well as any challenges encountered in network establishment.
Formal RC networks were characterized by the existence of one or more of the following: a governing body (defined as a formal group of individuals who oversee the activities of the network), formal mission statements or a schedule of formal monthly/weekly professional meetings. Approximately half (53.6%) of the RC networks were managed by a governing body, 39.3% had “a formal mission statement,” 28.6% a formal charter, and 67.9% had a “Chair/Leader,” generally chosen on a voluntary basis (42.1%) or appointed by institution leadership (31.6%). Respondents were instructed to select “all that apply.”
Of the institutions that indicated having a RC network, approximately half have a formal institutional or department/program-wide organization that is officially recognized by the institution, and 37.9% have an informal institution-wide organization that is not endorsed by administration, division, or department. Approximately a third (34.5%) have a system in which RCs must join/sign-up/register to become members (Table 2).
Table 2 Research coordinator networks—structures and models
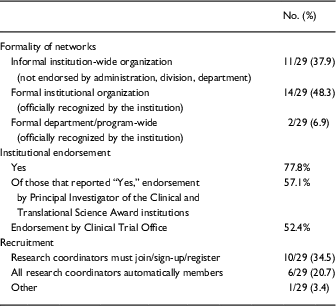
Of the institutions with RC networks, 77.8% reported leadership at the institution officially “endorsed” the RC network. Of those institutions with official leadership endorsement, most endorsers were the PI of the CTSA (57.1%) and Clinical Trials Office (52.4%).
Most respondents indicated their institutions recruit RCs to their network via several methods including: word-of-mouth (86.2%), email (72.4%), and through a website (51.7%). Other methods for recruitment included human resource or IRB databases or other research or institutional listservs or newsletters. Respondents noted network information was also shared through orientation, meetings, or other educational sessions.
In terms of membership, of the 29 respondents that reported having a RC network at their institution, the average participation rate was 49.9%. The average respondent-reported attendance at RC network activities/events was 55.0%.
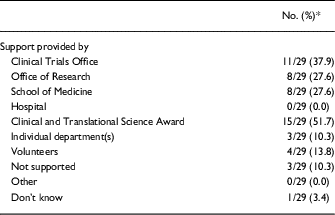
Nearly half of respondents indicated that the CTSA was involved in the creation of the RC network at their institution. The CTSA’s institution was also the most frequently cited source of financial support for the ongoing operations of the network, with clinical trial offices as the second most-cited department providing financial support for RC networks (Table 3).
Table 3 Research coordinator networks—financial support
* Respondents were instructed to select all that applied.
In contrast, respondents indicated that the informal RC networks arose by means of voluntary efforts, such as brown bag lunches, informational sessions on “hot topics,” and peer-to-peer networking over listservs. These sorts of activities were reported as being funded by the offices of research, schools of medicine, individual departments, and volunteers, through direct financial support as well as through indirect support, such as the provision of lunches or offering of in-kind staff resources.
When asked what have been the strengths/successes of the RC network at their institutions, respondents most commonly cited: (1) providing assistance in new procedure implementation; (2) enhancing communication and connecting, and information sharing; (3) providing educational workshops, formalized education program, improved professionalism of coordinators.
In terms of establishing and maintaining a RC network, respondents indicated (selected all that applied) that the most common challenges were lack of RC time to attend events (43.9%), lack of infrastructure (34.1%), and financial costs (29.3%).
The respondents from institutions without an RC network (48/77, 62.3%) cited 3 main challenges to establishing a network: (1) lack of infrastructure (29.3%), (2) lack of RC time (41.5%), and (3) lack of institutional leadership’s endorsement (14.6%).
Discussion
With this survey the authors sought to identify institutional traits or characteristics that suggested support of RCs. In particular, we hypothesized the existence of a RC network would be a primary indicator for strong support for, and communication pathways with, RCs.
Our findings indicate having a RC network is in fact significantly associated with information dissemination which occurs through training activities, content on a website, and with having a database to identify existing coordinators (Table 4).
Table 4 Research coordinator network versus information dissemination

Further, our findings indicate that institutions with a RC network are marginally more likely to encourage or inform their RCs about national certification than those without a network (marginally significant at p=0.06). Among the institutions that have RC network, 58.6% (17/29) either require or encourage RCs to be certified (Table 5) whereas of the institutions that identified not having a RC network, only 39.0% (16/41) require or encourage certification.
Table 5 Research coordinator network versus national certification endorsement

Although institutions with a RC network are marginally more likely to endorse national certification, the existence of networks does not appear to be a significant factor in receipt of financial assistance to enable certification. In fact, only 42.1% of the institutions with a RC network provide financial assistance for certification, while 48.0% of institutions without a network do.
One reason institutions with a RC network may not provide financial support for national certification may be because these institutions and their RC networks tend to be slightly more likely to provide services/activities to their RCs.
While RC networks appear to increase the likelihood of some information dissemination modalities and having a database of existing coordinators, our findings suggest that institutions support RCs in many different ways, beyond (and/or outside of) the existence of an RC network. While important in the professionalization of the position (in terms of endorsement of certification), RC networks are just 1 way institutions can provide RCs with support and foster communication.
An analysis of the data indicates that, while RC networks provide support, the ability to first identify and communicate with RCs appears to be the largest gap for institutions to fill in order to then provide support mechanisms to the RC community. Even institutions with RC networks cite word-of-mouth as being the leading way that RCs learn about the existence of the RC network. This suggests that RC networks are not sufficiently connected to the other programs/bodies that disseminate information throughout the institution or to the RC workforce, in particular.
Conclusion
With the underlying assumption that the presence of an RC network implies support of RCs within an institution, we sought to identify the presence and nature of RC networks as well as other types of RC support services offered at AHCs, if any. In particular, we identified the support of RCs as: (1) ability to identify RCs within the institution, (2) endorsement or support for professionalization of the RC role, (3) dissemination of information to the RC workforce, and (4) the provision of support services, including (but not limited to) RC networks.
The subset of the institutions (29/77) with RC networks described via open text field diverse origins and configurations of these networks, with some formed through grass roots endeavors, others introduced via a top-down approach, some formal, others informal, and each varied in methods of operation and support. Regardless of how the network originated, some level of institutional support or endorsement appears to be a component of RC networks within the CTSA consortium. More than half of the institutions with RC networks indicated the institution’s CTSA to be a source of financial support for the ongoing operations of the RC network, which raises some concerns regarding the ongoing viability of these networks should an institution lose CTSA funding. While the data show institutions with RC networks to be marginally more likely to encourage (in some capacity) the professionalization of the RC role through certification, such support also occurs at institutions without RC networks as well (in fact, these institutions are slightly more likely to offer financial support for such certification).
What became most clear in our review and analysis, however, is that a major gap exists in the ability to identify, and disseminate information to, RCs. More than the presence of an RC network, being able to identify, communicate with, and disseminate relevant information to RCs, are strong indicators of an institution’s ability to support their RC workforce. Of those surveyed, fewer than half of responding institutions have a complete (or “fairly complete”) database for contacting RCs, and 96% indicated that RCs operate under many different titles. This diversity of roles is an important factor to consider when identifying individuals performing the tasks of a RC. An institution simply cannot support those fulfilling the functions of an RC if such individuals cannot be identified.
Further, although RC networks may have started informally and without explicit authorization from executive management, RC networks reported the importance of management endorsement and/or involvement, especially in the management of financial support for national certification of RCs, official recognition of networks, infrastructure commitments (i.e., creation of databases for networking/communication); and institutionalization of policies and procedures.
In conclusion, while a variety of factors contribute to supporting the RC workforce, the results of this survey suggest that institutions should take steps to first identify the individuals within their RC workforce; only then can an institution effectively communicate the available support services and specific training opportunities to their RCs. An AHC that wishes to tailor research training and services for its RCs will need such identification and communication structures in place before targeted training and support can be effectively implemented.
Acknowledgments
The authors wish to thank Rebecca Betensky at Harvard Catalyst and Dongmei Li at the University of Rochester for their biostatistical consultations, the CTSA Central.org staff for assisting in this project and the other members of the CTSA Networking subgroup: Donna Adelman (Stanford University); Jennifer Albrigo Niemeyer (Ohio State University); Tammy Anderson (Virginia Commonwealth University); Claudia Christy (University of North Carolina); Virina Dejesus (University of California, Davis); Lisa Disiderio (University of Pennsylvania); Marsha Fox (University of Massachusetts); Barbara Longmire (University of North Carolina); Sabrena Mervin-Blake (University of North Carolina); Sheila Noone (University of Massachusetts); Gerri O’Riordan (Stanford University); Colleen Pellegrini (University of Pennsylvania); Mary-Tara Roth (Boston University); Lisa Speicher (Children’s Hospital of Philadelphia); Veronika Testa (Tufts University); Clare Tyson (Medical University of South Carolina).
Financial Support
This work was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 1UL1 TR001102-01 (S.W., M.J.G.) and financial contributions from Harvard University and its affiliated academic health care centers. The project described in this publication was supported by the University of Rochester CTSA award number 1 UL1 TR000042 (N.A.N.) from the National Center for Advancing Translational Sciences of the National Institutes of Health. The project described in this publication was supported by the Boston University CTSA award number 1UL1TR001430 (S.B.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
The authors have no conflicts of interest to declare.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cts.2017.309









