During the last decades, the prevalence of allergic diseases has dramatically increased(Reference Bach1). Mast cells (MC) are key effector cells in allergy and play a pivotal role in initiating and maintaining allergic reactions and inflammation by the release of numerous inflammatory mediators(Reference Metz and Maurer2–Reference Metcalfe, Baram and Mekori4). Upon activation, MC immediately release a plethora of preformed mediators such as histamine and proteases that are stored in secretory cytoplasmic granules. Arachidonic acid (AA)-derived eicosanoids and multiple pro-inflammatory chemokines and cytokines, such as allergy-driving IL-4 and IL-13, are generated de novo (Reference Metz and Maurer2, Reference Stone, Prussin and Metcalfe5). These mediators are important in the pathogenesis of allergic responses and can increase the susceptibility to develop allergic disease and enhance allergic symptoms(Reference Chai, Han and Lee6–Reference Brandt, Munitz and Orekov10).
PUFA can modulate immune responses. In general, n-6 long-chain (LC) PUFA such as AA (20 : 4n-6) are considered to be pro-inflammatory and the n-3 LC-PUFA EPA (20 : 5n-3) and DHA (22 : 6n-3) protect against inflammation(Reference Calder11–Reference Prescott and Calder14). Decreased dietary intake of n-3 LC-PUFA from fatty fish together with the high intake of n-6 PUFA from mainly vegetable oils in the Western diet is likely to contribute to the increased incidence of allergic and inflammatory disease in humans over the last decades(Reference Prescott and Calder14–Reference Black and Sharpe17). Dietary supplementation with n-3 LC-PUFA has been studied to some extent in allergic disease, focusing on prevention. Human pregnancy studies revealed a reduction in infant atopy when women were supplemented with fish oil during pregnancy and lactation(Reference Lauritzen, Kjaer and Fruekilde18–Reference Kankaanpaa, Nurmela and Erkkila23).
n-3 LC-PUFA compete with n-6 LC-PUFA for incorporation into the cell membrane. After release from the membrane, AA can be metabolised by cyclo-oxygenases (COX) and lipoxygenases, resulting in the formation of 2,4-series eicosanoids which support inflammatory responses. By contrast, exchange of AA by n-3 LC-PUFA results in the production of 3,5-series eicosanoids which are less potent(Reference Calder11, Reference Stulnig13, Reference Simopoulos15). Besides modification of the generation of eicosanoids, LC-PUFA may change intracellular signalling pathways and may affect the activation of nuclear transcription factors and consequently gene transcription(Reference Calder11, Reference Miles and Calder24). Dietary EPA and DHA have been shown to inhibit the release of pro-inflammatory cytokines (IL-1, IL-6, IL-8 and TNF-α) by macrophages and mononuclear cells(Reference Calder11, Reference James, Gibson and Cleland25, Reference Kelley26); however, little is known about the effects of LC-PUFA in MC.
In MC, cytokine secretion is under the regulation of intracellular reactive oxygen species (ROS), mitogen-activated protein kinases (MAPK) and NF-κB signalling pathways(Reference Kim and Ro27, Reference Swindle and Metcalfe28). The pathophysiological importance of ROS such as superoxide generated from MC has not been fully elucidated. At high levels, ROS are involved in the innate immune response while at low levels they are involved in cell signalling(Reference Swindle and Metcalfe28). It has been described that n-3 LC-PUFA may suppress ROS generation by targeting eicosanoids, cytokines and protein kinase C(Reference Calder11, Reference Stulnig13). These molecules control enzymes involved in ROS formation such as NADPH oxidase(Reference Calder11, Reference Swindle and Metcalfe28). Wong et al. (Reference Wong, Kwon and Choi29) reported that DHA inhibited NADPH oxidase and ROS generation in macrophages.
Downstream of ROS MAPK extracellular signal-regulated kinase (ERK), p38 and c-Jun N-terminal kinase (JNK) and transcription factors including NF-κB become activated, resulting in cytokine production(Reference Kim and Ro27, Reference Suzuki, Yoshimaru and Matsui30). Precise signalling pathways are still unclear but activation of these routes induces transcriptional activity of cytokine genes in MC.
Although the anti-inflammatory effects of EPA and DHA have been studied in neutrophils and mononuclear cells, the effects on MC function have not been fully addressed. Modulation of MC mediator release, for example by modification of membrane lipid composition, may have an impact on the allergic outcome. In view of the steadily increasing prevalence of allergies and inflammatory diseases, there is a need for (novel) treatment or prevention strategies. Therefore, we studied the effects of AA, EPA and DHA on MC degranulation and cytokine production. In particular, the involvement of ROS, MAPK and NF-κB signalling in cytokine secretion and the modulation of the MC phenotype by LC-PUFA was addressed.
Materials and methods
Cell culture
All chemicals and inhibitors were derived from Sigma-Aldrich, unless otherwise stated. Human leukaemic mast cell line HMC-1 was kindly donated by the Mayo Clinic and used with permission(Reference Nilsson, Blom and Kusche-Gullberg31). These non-adherent cells were cultured in Iscove's modified Dulbecco's medium (Gibco; Invitrogen) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml) and 10 % (v/v) fetal bovine serum (HyClone, Perbio; see Table S1 for fatty acid composition, available online) at 37°C with 5 % CO2 in a humidified atmosphere. HMC-1 were passaged once a week.
LAD2 cells were grown in StemPro-34 serum-free complete medium (serum-free medium plus nutrient supplement; Gibco) plus l-glutamine, recombinant human stem cell factor (100 ng/ml; ProSpec), penicillin (100 U/ml) and streptomycin (100 μg/ml).
Long-chain PUFA incubation
For modification of cell lipids, MC were pre-incubated with either AA, EPA or DHA for 24 h. Cells were collected and resuspended at a concentration of 1 × 106 cells/ml in fresh medium containing l(+)-ascorbic acid (75 μm; Merck) in order to prevent LC-PUFA oxidation. Stock solutions in ethanol of AA, EPA or DHA were further diluted in fetal bovine serum or the StemPro-34 nutrient supplement containing the antioxidant α-tocopherol. Then, 5 % fetal bovine serum or 2·5 % nutrient supplement was added to the cells (final concentration of ethanol ≤ 0·1 % (v/v)). The final concentration of α-tocopherol was 20 μm. Control wells also included ethanol (0·1 % (v/v)). Membrane fatty acid composition in HMC-1 (0, 10, 25, 50 and 100 μm) and LAD2 cells (0, 50 and 100 μm based on HMC-1 results) was assessed by GC as previously described by Bligh & Dyer(Reference Bligh and Dyer32, Reference Willemsen, Koetsier and Balvers33).
IgE-mediated β-hexosaminidase release assay
LAD2 cells were incubated for 24 h with solely AA, EPA or DHA (0, 25, 50 and 100 μm chosen from membrane fatty acid analysis) in a ninety-six-well culture plate (Costar; Corning Incorporated) at 1 × 105 cells/100 μl per well. Stempro-34 medium without the nutrient supplement but supplemented with l(+)-ascorbic acid was used. The nutrient supplement enriched with α-tocopherol or α-tocopherol+LC-PUFA was added to determine the degranulation of LC-PUFA-supplemented cells. After 4 h, human purified IgE (Chemicon; Millipore) was added at a concentration of 0·5 μg/ml and incubated for an additional 20 h at 37°C, to prime the cells with IgE. The cells were washed two times by Tyrode's buffer (10 mm-HEPES (Acros Organics) buffer, pH 7·2, Tyrode salts (Gibco) 9·5 g/l, 0·1 % sodium bicarbonate (Merck), 0·1 % (w/v) bovine serum albumin (fraction V; Roche)). Next, α-human IgE–fluorescein isothiocyanate (KPL) was added to a final concentration of 10 μg/ml and incubated for 1 h at 37°C to induce degranulation. Degranulation was determined by the amount of β-hexosaminidase released in the cell-free supernatant. To determine the total amount of β-hexosaminidase release present in untreated cells, Triton X-100 (1 %) was used to lyse the cells. Supernatants were diluted 1:1 with 160 μm-4-methyl umbelliferyl-N-acetyl-β-d-glucosaminide in 0·1 m-citrate buffer (pH 4·5) and incubated for 1 h at 37°C. This reaction was terminated by the addition of glycine buffer (15 g glycine (MP Biochemicals), 11·7 g NaCl/l (Merck), pH 10·7). Fluorescence was measured within 1 h at an excitation of 360 nm and an emission of 460 nm by a fluorescence measurement system (Millipore, CytoFluor 2350; B&L Systems). Average fluorescence of unstimulated cells (background) was subtracted from all values. Background release did not differ between the groups. The amount of β-hexosaminidase release was calculated as a percentage of total β-hexosaminidase in Triton X-100 cell lysates (maximal release).
Mast cell mediator release and cell viability
After pre-incubation with AA, EPA or DHA, in some experiments, inhibitors were used before MC stimulation. HMC-1 were incubated for 30 min with 1–10 μm of COX inhibitors (indomethacin or NS398) or for 10 min with a ROS inhibitor (superoxide dismutase; 20 or 100 U/ml (equivalent to 7 or 35 μg/ml) or 1,3-dimethyl-2-thiourea; 30, 40, 50 and 60 mm), a MAPK inhibitor (ERK inhibitor PD98059; 50 and 100 μm, p38 inhibitor SB203580; 20 and 50 μm, JNK inhibitor SP600125; 10 and 20 μm) or an NF-κB inhibitor (Bay117082; 10 and 30 μm). The inhibitors were diluted in medium (final concentration of dimethyl sulphoxide ≤ 0·2 % (v/v), except for PD98059 with 0·5 % dimethyl sulphoxide (v/v)). After pre-incubation with LC-PUFA and/or inhibitors, the cells were stimulated with 1 μm-ionomycin plus 16 nm-phorbol 12-myristate 13-acetate (PMA) (both diluted in medium, final concentration of dimethyl sulphoxide 0·1 % (v/v)) and incubated at 37°C. Optimal doses of ionomycin and PMA were chosen after pilot experiments. To assess inflammatory mediator production in the culture supernatants of HMC-1, supernatants were collected 30 min (PGD2), 4 h (TNF-α and IL-8) and 24 h (IL-4 and IL-13) after stimulation. Secreted cytokine concentrations were determined by ELISA cytoSet kits according to the manufacturer's instruction (BioSource International, Inc.). PGD2 was measured by the Enzyme Immunoassay Prostaglandin D2-MOX kit (Cayman Chemical).
After 24 h stimulation, cell proliferation/viability reagent WST-1 (Roche) was added to the cells to assess cell viability (mitochondrial activity). Absorbance at 450 nm was determined using a Bio-Rad Benchmark Microplate Reader (Bio-Rad Laboratories). In separate experiments, cell death (necrosis) was examined by light microscopy with trypan blue exclusion
Intracellular reactive oxygen species production
Following 24 h incubation with 0, 25 or 100 μm-AA, EPA or DHA, ROS production by HMC-1 was measured by means of flow cytometry. Cells were washed and resuspended in 200 μl fluorescence-activated cell sorter buffer (5 % fetal bovine serum in PBS (BioWhittaker) in a V-shaped ninety-six-well culture plate (Cellstar, Greiner bio-one, TC-plate). Subsequently, the cells were incubated with 5 μm-2′,7′-dichlorofluorescein diacetate for 10 min at 37°C followed by ionomycin and PMA, as described earlier. After 30 min, ROS production was determined as the potency to oxidise 2′,7′-dichlorofluorescein to fluorescent dichlorofluorescein on a FACSCalibur flow cytometer (BD Biosciences), as described previously(Reference Kim and Ro27, Reference Nakano, Nakao and Uchida34). Generation of intracellular ROS was determined by counting of 10 000 cells in channel FL1 (excitation 488 nm and emission 530 nm) of the fluorescence-activated cell sorter.
Statistical analysis
Graphs were made by statistical software GraphPad Prism (GraphPad Prism for Windows, version 4; GraphPad Software, Inc.). Data are presented as means with their standard errors of the different experiments under the same conditions. Differences between the groups were assessed by one-way ANOVA and post hoc Dunnett's test for multiple comparisons. To compare the effects between the different LC-PUFA, one-way ANOVA and post hoc Bonferroni's test for multiple comparisons or paired Student's t test was used. SPSS version 15 software (SPSS, Inc.) was used for these analyses. Pearson's correlation coefficients were calculated by GraphPad Prism. P< 0·05 was considered as statistically significant.
Results
Fatty acid composition of human mast cells
Membrane fatty acid composition of HMC-1 and LAD2 cells was significantly altered after AA, EPA or DHA incubation. These LC-PUFA did incorporate dose-dependently in the cellular membranes of HMC-1 and LAD2 cells (Table 1). Furthermore, incubation with EPA or DHA significantly reduced AA in the cell membrane of HMC-1 and LAD2 cells, while EPA and DHA were substituted significantly by the addition of AA in HMC-1 only due to the lack of EPA and low DHA in LAD2 cells under basal conditions. In addition to exchanges in LC-PUFA membrane composition, AA, EPA or DHA supplementation competed with 18 : 1n-9 and 16 : 0 for incorporation (data not shown).
Table 1 Membrane fatty acid composition of HMC-1 and LAD2 cells after 24 h long-chain PUFA incubation (Mean values with their standard errors; n 4 independent experiments)
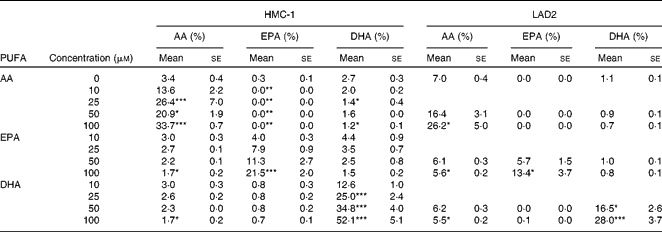
AA, arachidonic acid.
Mean values were significantly different from the control group (0 μm) after incubation with AA, EPA or DHA: * P< 0·05, ** P< 0·01, *** P <0·001 (one-way ANOVA with post hoc Dunnett's multiple comparison test).
Effect of arachidonic acid, EPA or DHA on IgE-stimulated degranulation of LAD2 cells
β-Hexosaminidase release was used as a marker for MC degranulation. LAD2 cells were stimulated by sensitisation with IgE followed by cross-linking with α-IgE. AA at 100 μm increased FcɛRI-mediated β-hexosaminidase release of LAD2 cells while EPA and DHA did not affect MC degranulation (AA 100 μm: 36·4 (sem 6·7) v. 49·9 (sem 5·6) %, n 3, P< 0·05; Fig. 1(a)). Degranulation after incubation with AA did not differ significantly from EPA or DHA. The solvent of LC-PUFA (ethanol, 0·1 % (v/v)) did not affect β-hexosaminidase release (data not shown).

Fig. 1 (a) IgE-mediated degranulation of LAD2 cells 1 h after α-IgE stimulation incubated with different concentrations of arachidonic acid (AA, ![]() ), EPA (
), EPA (![]() ) and DHA (■). Degranulation was determined by the amount of β-hexosaminidase release as a percentage of Triton X-100-treated cells. Values are means of three independent experiments, average background release is subtracted, with their standard errors represented by vertical bars. Effect of LC-PUFA on ionomycin/phorbol 12-myristate 13-acetate (iono/PMA)-induced release of (b) PGD2 after 30 min (n 3), (c) TNF-α after 4 h (n 5), (d) IL-4 after 24 h (n 6) and (e) IL-13 after 24 h (n 6) by HMC-1. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from those of the control group (O+): * P< 0·05, ** P< 0·01 (one-way ANOVA followed by Dunnett's test for multiple comparisons). Mean values were significantly different between the LC-PUFA: † P< 0·05, †† P< 0·01 (one-way ANOVA followed by Bonferroni's multiple comparison test).
) and DHA (■). Degranulation was determined by the amount of β-hexosaminidase release as a percentage of Triton X-100-treated cells. Values are means of three independent experiments, average background release is subtracted, with their standard errors represented by vertical bars. Effect of LC-PUFA on ionomycin/phorbol 12-myristate 13-acetate (iono/PMA)-induced release of (b) PGD2 after 30 min (n 3), (c) TNF-α after 4 h (n 5), (d) IL-4 after 24 h (n 6) and (e) IL-13 after 24 h (n 6) by HMC-1. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from those of the control group (O+): * P< 0·05, ** P< 0·01 (one-way ANOVA followed by Dunnett's test for multiple comparisons). Mean values were significantly different between the LC-PUFA: † P< 0·05, †† P< 0·01 (one-way ANOVA followed by Bonferroni's multiple comparison test).
Modulation of mediator release of HMC-1 by arachidonic acid, EPA or DHA
To determine the effects of modification of cell membrane fatty acid composition on cytokine production, HMC-1 were used. To study dose-dependency of MC mediator release, HMC-1 were incubated with 0, 1, 10, 25 and 100 μm-AA, EPA or DHA because this was expected to be more sensitive to changes in cell membrane fatty acid composition than degranulation. As shown in Fig. 1(b), the production of PGD2 increased dramatically in a dose-dependent manner after incubation with AA (13·4 (sem 6·3) v. 71·6 (sem 24·1) ng/ml, 25 μm-AA, n 3, P< 0·01); in contrast, EPA and DHA inhibited PGD2 production (13·4 (sem 6·3) v. 3·5 (sem 1·9) ng/ml, 25 μm-DHA, n 3, P< 0·05). AA (25 μm) was also found to enhance TNF-α secretion by HMC-1 (4·4 (sem 1·0) v. 10·1 (sem 3·5) ng/ml, n 5, P< 0·05); no effects were observed for EPA and DHA (Fig. 1(c)). IL-8 secretion was not affected by AA, EPA or DHA (data not shown). However, 24 h pre-incubation with AA, EPA or DHA resulted in a decrease in ionomycin/PMA-induced IL-4 and IL-13 release (Fig. 1(d) and (e)). The effects of DHA on IL-13 production were most pronounced since DHA already reduced IL-13 secretion at 25 μm (300·1 (sem 94·5) v. 104·6 (sem 25·8) pg/ml, n 6, P< 0·01). In addition, this was significantly lower than AA-treated cells (P< 0·05). AA as well as EPA and DHA reduced IL-13 secretion at a concentration of 100 μm (AA 86·8 (sem 19·7) pg/ml; EPA 38·5 (sem 13·7) pg/ml; DHA 44·0 (sem 14·6) pg/ml, n 6, P< 0·01); however, the EPA and DHA treatments resulted in significantly lower IL-13 secretion than AA (P< 0·01). At the highest concentration used, EPA and DHA were able to reduce IL-4 secretion (119·8 (sem 50·6) v. 35·7 (sem 10·2); 32·8 (sem 8·1) pg/ml respectively, n 6, P< 0·05). The solvent of LC-PUFA (ethanol, 0·1 % (v/v)) and ionomycin/PMA (0·1 % (v/v) dimethyl sulphoxide) did not affect mediator release (data not shown).
Pre-incubation for 30 min with indomethacin (general COX inhibitor; 10 μm) as well as NS398 (COX-2 inhibitor; 1 and 10 μm) effectively inhibited COX as PGD2 release was blocked by more than 90 % (data not shown). However, COX inhibitors did not affect TNF-α or IL-13 secretion; hence, these cytokines are not regulated by a COX-dependent mechanism (data not shown).
Arachidonic acid, EPA and DHA do not affect cell viability
AA, EPA and DHA (0, 1, 10, 25 and 100 μm) incubation up to 48 h did not affect cell viability of stimulated HMC-1 as determined by WST-1 assay. Ionomycin (1 μm) and PMA (16 nm) stimulation for 24 h slightly tended to reduce cell viability when compared with non-stimulated HMC-1 (data not shown). EPA tended to induce a slight increase in mitochondrial activity and the WST-1 signal was significantly higher with 100 μm-AA (n 6, P< 0·05). Cell viability was also studied by the trypan blue exclusion test. The addition of AA, EPA or DHA did not induce cell death at the concentrations 25 and 100 μm (data not shown).
Generation of intracellular reactive oxygen species and modulation by arachidonic acid, EPA or DHA
ROS are known as second messengers and are related to different inflammatory diseases(Reference Kim and Ro27). HMC-1 showed a slight increase in ROS generation upon ionomycin/PMA stimulation (Fig. 2(a)). HMC-1 cells have high basal ROS levels (mean fluorescence intensity (MFI)) and due to limitations in the sensitivity of the method used, additional ROS production upon stimulation was not higher than ROS production in unstimulated cells (MFI 394·8 (sem 95·3) v. 424·9 (sem 53·7), NS) (Fig. 2(b)). To study the effect of AA, EPA or DHA on ROS production in ionomycin/PMA-stimulated HMC-1, cells were incubated with 25 and 100 μm based on the differences between 25 and 100 μm in IL-4 and IL-13 secretion. Intracellular ROS generation is dose-dependently reduced by AA, EPA or DHA in stimulated HMC-1. This effect was most pronounced for DHA, which showed a significant reduction in ROS generation at 25 μm (MFI 325·1 (sem 47·9), P< 0·05) when compared with basal ROS generation after stimulation. This also was significantly lower than the AA-treated cells (P< 0·05). At a concentration of 100 μm-AA, EPA and DHA were all able to reduce ROS generation (AA: MFI 278·2 (sem 22·6), P< 0·01; EPA: MFI 267·6 (sem 47·1), P< 0·001; DHA: MFI 215·6 (sem 32·1), P< 0·001). ROS generation after stimulation (mean of n 3 for each condition, used from Fig. 2(b)) was found to correlate positively with IL-4 and IL-13 secretion (mean of n 5/6 for each condition, used from Fig. 1(d) or (e), respectively) in HMC-1 (P= 0·006, r 0·897 and P= 0·005, r 0·907, respectively) (Fig. 2(c) and (d)).

Fig. 2 (a) Generation of reactive oxygen species (ROS) in HMC-1 increased by stimulation with ionomycin/phorbol 12-myristate 13-acetate (Iono/PMA) as compared with unstimulated HMC-1 cells (left). Long-chain PUFA (LC-PUFA) reduced ROS generation in stimulated HMC-1 in a dose-dependent manner (shift to left) as shown in this example for DHA (25 and 100 μm) (right). ![]() , Unlabelled − iono/PMA;
, Unlabelled − iono/PMA; ![]() , unlabelled+iono/PMA;
, unlabelled+iono/PMA; ![]() , 0 − iono/PMA;
, 0 − iono/PMA; ![]() , 0+iono/PMA;
, 0+iono/PMA; ![]() , 25 μm+iono/PMA;
, 25 μm+iono/PMA; ![]() , 100 μm+iono/PMA. (b) ROS generation (n 3) by iono/PMA-stimulated HMC-1 after LC-PUFA incubation in mean fluorescence intensity (MFI). Values are means, with standard errors represented by vertical bars.
, 100 μm+iono/PMA. (b) ROS generation (n 3) by iono/PMA-stimulated HMC-1 after LC-PUFA incubation in mean fluorescence intensity (MFI). Values are means, with standard errors represented by vertical bars. ![]() , AA;
, AA; ![]() , EPA; ■, DHA. ROS (mean of n 3 per data point) were found to correlate positively with (c) IL-4 (mean of n 6; P= 0·006, Pearson's correlation coefficient (r) 0·897) and (d) IL-13 (mean of n 6; P= 0·005, r 0·907) secretion in HMC-1. Mean values were significantly different from those of the control group (0+): * P< 0·05, ** P< 0·01, *** P< 0·001 (one-way ANOVA followed by Dunnett's test for multiple comparisons). Mean values were significantly different between the LC-PUFA: † P< 0·05 (one-way ANOVA followed by Bonferroni's multiple comparison test).
, EPA; ■, DHA. ROS (mean of n 3 per data point) were found to correlate positively with (c) IL-4 (mean of n 6; P= 0·006, Pearson's correlation coefficient (r) 0·897) and (d) IL-13 (mean of n 6; P= 0·005, r 0·907) secretion in HMC-1. Mean values were significantly different from those of the control group (0+): * P< 0·05, ** P< 0·01, *** P< 0·001 (one-way ANOVA followed by Dunnett's test for multiple comparisons). Mean values were significantly different between the LC-PUFA: † P< 0·05 (one-way ANOVA followed by Bonferroni's multiple comparison test).
Effect of reactive oxygen species inhibition on IL-4 and IL-13 secretion
Since AA, EPA and DHA were able to suppress ROS and IL-4 and IL-13 secretion, it was assessed whether these allergy-related cytokines were under the regulation of ROS in HMC-1. Pre-incubation with 1,3-dimethyl-2-thiourea at the highest concentrations (40–60 mm) resulted in a decrease in IL-4 secretion of ionomycin plus PMA-stimulated HMC-1 (354·1 (sem 122·7) v. 100·4 (sem 55·2) pg/ml, 60 mm, P< 0·01) (Fig. 3(a)). IL-13 release was dramatically and dose-dependently decreased by 1,3-dimethyl-2-thiourea at all dosages (129·7 (sem 32·8) v. 7·1 (sem 2·9) pg/ml, 60 mm, P< 0·001) (Fig. 3(b)). The addition of superoxide dismutase only resulted in the reduction of the secretion of IL-4 (136·0 (sem 68·8) pg/ml, 100 U/ml (35 μg/ml), P< 0·01) but not of IL-13 (Fig. 3(c) and (d)). Incubation with ROS inhibitors did not reduce mitochondrial activity or increase cell death as shown by the WST-1 assay and trypan blue exclusion, respectively (data not shown).

Fig. 3 Effect of incubation with the general reactive oxygen species (ROS) inhibitor 1,3-dimethyl-2-thiourea (DMTU) on (a) IL-4 and (b) IL-13 secretion. Contribution of superoxide on (c) IL-4 and (d) IL-13 release was assessed by determining the effect of the specific ROS inhibitor superoxide dismutase (SOD). Values are means, with standard errors represented by vertical bars. Mean values were significantly different from those of the control group (0+): * P< 0·05, ** P< 0·01, *** P< 0·001 (one-way ANOVA followed by Dunnett's test for multiple comparisons). Iono/PMA, ionomycin/phorbol 12-myristate 13-acetate.
Effects of mitogen-activated protein kinases and NF-κB inhibitors on IL-13 release
ROS have been described to operate upstream in the signalling cascade. MAPK and NF-κB are known to contribute to cytokine secretion by MC. To determine the involvement of MAPK and NF-κB signalling in the secretion of IL-13 and the possible effects of LC-PUFA on these pathways, we examined the effects of the ERK inhibitor PD98059, the p38 inhibitor SB203580, the JNK inhibitor SP600125 and the NF-κB inhibitor Bay117082 on IL-13 release by HMC-1 in the absence or presence of pre-incubation with 25 μm-LC-PUFA since at this concentration, the differences between AA v. EPA or DHA on the suppression of IL-13 secretion were most pronounced. The solvents did not have an effect on mediator release (data not shown).
At the concentration of 25 μm, DHA reduced ionomycin/PMA-induced IL-13 secretion by HMC-1 by 30 % (316·1 (sem 60·2) v. 218·6 (sem 44·8) pg/ml, P< 0·05; Fig. 4). SB203580 (20 μm), SP600125 (10 μm) and PD98059 (50 μm) inhibited IL-13 secretion by more than 50 % (139·6 (sem 33·6), 114·2 (sem 61·9) and 147·3 (sem 38·9) pg/ml, respectively). At higher concentrations, the p38 or JNK inhibitor was even more effective in the suppression of IL-13 secretion (SB203580, 50 μm: 60·9 (sem 15·1) pg/ml; SP600125, 20 μm: 27·0 (sem 23·9) pg/ml) but not the ERK inhibitor (PD98059, 100 μm: 200·0 (sem 56·3) pg/ml). In contrast, Bay117082 (10 μm) did not inhibit IL-13 release, whereas at the concentration of 30 μm, it strongly reduced cell viability. Simultaneous treatment of the MAPK or NF-κB inhibitor with DHA induced a further inhibition in IL-13 release using suboptimal SB203580 and SP600125 incubation (41·7 (sem 8·3) and 34·2 (sem 12·7) pg/ml, P< 0·05 and P= 0·07, respectively). At the concentration of 25 μm, EPA was able to further reduce IL-13 release in combination with SB203580 when compared with the inhibitor alone (67·7 (sem 10·8) pg/ml, P< 0·05), while AA did not add to the effect of the MAPK or NF-κB inhibitor used (data not shown). The MAPK and NF-κB inhibitors did not enhance cell death in HMC-1. Trypan blue exclusion showed that at the end of the experiment, cell viability was >80 % (data not shown).

Fig. 4 Effect of the extracellular signal-regulated kinase inhibitor PD98059 (50 μm), the p38 inhibitor SB203580 (20 μm), the c-Jun N-terminal kinase inhibitor SP600125 (10 μm) and the NF-κB inhibitor Bay117082 (10 μm) in the presence or absence of DHA on ionomycin/phorbol 12-myristate 13-acetate (Iono/PMA)-induced IL-13 release by HMC-1. Values are means, with standard errors represented by vertical bars. Mean values were significantly different from those of the control group (0+): * P< 0·05, ** P< 0·01 (one-way ANOVA followed by Dunnett's test for multiple comparisons). Mean values were significantly different: † P< 0·05 (paired Student's t test). □, 0; ■, DHA.
Discussion
Upon activation, MC initiate and maintain allergic inflammation due to the release of various inflammatory mediators. MC-derived mediators such as PGD2, TNF-α, IL-4 and IL-13 increase the susceptibility to develop allergic disease and enhance allergic symptoms(Reference Chai, Han and Lee6–Reference Brandt, Munitz and Orekov10). The present study shows the differential effects of the n-6 LC-PUFA AA v. the n-3 LC-PUFA EPA or DHA on mast cell phenotype.
IgE-mediated MC degranulation was enhanced upon 24 h pre-incubation with AA. The LAD2 cell line is the only human analogue that can degranulate in an IgE-dependent manner. Teshima et al. (Reference Teshima, Amano and Nakamura35) found AA and other n-6 PUFA to increase degranulation in rat basophilic leukaemia (RBL-2H3) cells, while n-3 PUFA including EPA had no effect. Nakano et al. (Reference Nakano, Nakao and Uchida34) reported that AA significantly increased β-hexosaminidase release upon IgE-antigen stimulation and EPA showed the same tendency. An increase in the content of PUFA in membrane phospholipids is accompanied by an increase in membrane fluidity (decrease in microviscosity)(Reference Calder, Yaqoob and Harvey36, Reference Stubbs and Smith37). MC degranulation may occur more easily when the membrane is more fluid, which may explain the significant increase in degranulation after AA incubation. The same trend was shown for EPA and DHA. In addition to membrane fluidity, LC-PUFA may affect events in signal transduction and MC mediator release. IgE-mediated MC activation involves recruitment of tyrosine kinase, linker for activation of T cells and Syk, as well as Ca mobilisation(Reference van den Elsen, Garssen and Willemsen38). Nakano et al. (Reference Nakano, Nakao and Uchida34) have shown that supplementation of RBL-2H3 cells with AA or EPA augmented the activation of linker for activation of T cells and Syk when compared with control cells. In addition, AA-supplemented cells had increased intracellular Ca concentration(Reference Nakano, Nakao and Uchida34, Reference Teshima, Amano and Nakamura35).
Besides LAD2 cells, HMC-1 is often used as a human MC line to circumvent costly isolation procedures for human tissue MC. They lack a functional IgE receptor but can be cultured in large quantities and produce sufficient amounts of mediators for analysis. To simulate IgE receptor signalling, the cells are stimulated by ionomycin (Ca ionophore) and PMA (activating protein kinase C). FcɛRI signalling in MC also leads to the simultaneous activation of Ca and protein kinase C, by inositol triphosphate and diacylglycerol, respectively(Reference Gilfillan and Tkaczyk39). Hence, similar downstream signalling pathways are activated and these pathways act synergistically to provide exocytosis.
Supplementation of LC-PUFA to HMC-1 or LAD2 cells readily resulted in effective AA, EPA or DHA membrane incorporation in a dose-dependent manner. LC-PUFA incorporation seems to be slightly less efficient in LAD2 cells when compared with HMC-1, which may be the result of serum-free culturing of these cells. In both MC lines, EPA and DHA incorporate at the cost of AA and vice versa, which results in changes in membrane n-6:n-3 LC-PUFA and EPA:DHA ratios due to alterations in membrane composition. In addition, 18 : 1n-9, followed by 18 : 0 and 16 : 0, were exchanged for the supplemented AA, EPA or DHA (data not shown), enabling efficient incorporation of high amounts of LC-PUFA having implications for the biological function of MC.
The differential effects of n-3 v. n-6 LC-PUFA on cytokine secretion by HMC-1 were demonstrated in the present study. The n-6 LC-PUFA AA increased TNF-α and PGD2 secretion by HMC-1, while the n-3 LC-PUFA EPA and DHA dose-dependently reduced PGD2 release and were most effective in suppressing allergy-driving IL-4 and IL-13 secretion. These MC mediators are important in the initiation and persistence of the allergic response(Reference Chai, Han and Lee6–Reference Brandt, Munitz and Orekov10). Besides their role in allergic disease, MC and their products can regulate the adaptive (acquired) immune response via the effects on the maturation, function and migration of B cells, T cells and dendritic cells(Reference Galli, Nakae and Tsai3, Reference Stelekati, Orinska and Bulfone-Paus40).
The pro-inflammatory effect of AA is clearly demonstrated by the dramatic induction of PGD2 release. In contrast to AA, EPA and DHA reduced PGD2 secretion by activated HMC-1. EPA has been shown to reduce IgE-mediated PGD2 generation by cultured human MC as well(Reference Obata, Nagakura and Masaki41). PGD2 is the main prostanoid secreted by activated MC and associated with allergic diseases. It can decrease IL-12 secretion by dendritic cells and promote Th2 polarisation(Reference Faveeuw, Gosset and Bureau42, Reference Gosset, Bureau and Angeli43). Furthermore, PGD2 is important in the MC-dependent activation of Th2 lymphocytes, eosinophils and basophils via chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2)(Reference Pettipher, Hansel and Armer7). Recently, CRTH2 antagonists have shown to be promising in the treatment of asthma and related disorders. A randomised, double-blind comparison of a CRTH2 antagonist and placebo in a population with moderate persistent asthma showed beneficial effects of the CRTH2 antagonist, including an improvement in lung function and asthma symptoms(Reference Barnes, Pavord and Chuchalin44). These results provide evidence for an important role for PGD2 in asthma and other allergic disorders, which implicate a beneficial effect of EPA and DHA by reducing PGD2 generation in these patients.
Nakano et al. (Reference Nakano, Nakao and Uchida34) reported that AA dose-dependently augmented TNF-α release using RBL-2H3 cells, similar to what was shown in these experiments in human MC. MC-derived TNF-α has recently been incriminated to worsen allergic symptoms via the induction of adhesion molecules, enabling influx of inflammatory cells resulting in, for example, airway inflammation and the development of airway hyperresponsiveness(Reference Chai, Han and Lee6). However, studies using anti-TNF-α treatment have not been consistent, showing marked heterogeneity in responses(Reference Berry, Brightling and Pavord45, Reference Corren46). This makes the involvement of TNF-α-dependent pathways in LC-PUFA effects less likely.
The present study showed that EPA and DHA, in particular DHA, most effectively inhibit IL-4 and IL-13 secretion from human MC. This has not been reported previously. AA was also able to reduce IL-13 secretion but less effectively than EPA or DHA, while enhancing TNF-α and PGD2 secretion. IL-13 is produced by Th2 cells, MC, eosinophils and basophils, and is critical in the induction and persistence of allergic disease. In allergic asthma, IL-13 is required for the induction of clinical symptoms(8–Reference Brandt, Munitz and Orekov10). Furthermore, cytokines including IL-4 and IL-13 affect B-cell development and induce IgE isotype switching(Reference Weise, Hilt and Milovanovic47). Recent studies reporting about fish oil supplementation during pregnancy have shown inhibition of IL-13 release by neonatal mononuclear cells in response to allergens as well as reduced levels of IL-13 in cord blood plasma(Reference Dunstan, Mori and Barden19, Reference Dunstan, Mori and Barden48).
LC-PUFA are known to affect signal transduction cascades, leading to the transcription and production of cytokines. A variety of tissues and cells, including MC, produce ROS such as superoxide and H2O2 upon stimulation which are upstream regulators of signal transduction pathways(Reference Kim and Ro27, Reference Suzuki, Yoshimaru and Matsui30). Although the effect of ionomycin/PMA stimulation on ROS production by HMC-1 was small in the present experiments, the reduction in ROS generation after LC-PUFA supplementation appeared to be specific for activated MC. Furthermore, within these experiments, IL-4 and IL-13 secretion by HMC-1 after stimulation was found to correlate positively with the amount of ROS. This is in agreement with a study in bone marrow-derived MC in which IL-4 and IL-13 secretion after IgE-mediated activation was associated with increased ROS generation(Reference Cho, Seo and Lee49). Unfortunately, no sensitive method for measuring intracellular ROS generation upon cell activation is currently available, while only minor changes in ROS upon activation are required to activate intracellular signalling cascades(Reference Swindle and Metcalfe28). Similar to the present in vitro results, fish oil has been shown to decrease ROS production in several animal studies and in healthy human volunteers(Reference Fisher, Levine and Weiner50–Reference Thompson, Misso and Passarelli54). By contrast, other animal studies have reported contradictory results for ROS production in macrophages after fish oil supplementation(Reference Berger, German and Chiang55, Reference Yaqoob and Calder56). Studies assessing the impact of LC-PUFA on ROS production in MC are rare. Nakano et al. (Reference Nakano, Nakao and Uchida34) found that AA and EPA enhance ROS production in stimulated RBL-2H3 cells. The canine mastocytoma cell line C2 was incubated with 18 : 2n-6 (linoleic acid), AA, 18 : 3n-3 (α-linolenic acid) and EPA, which all increased ROS production. However, this was possibly due to lipid peroxidation since antioxidant supplementation resulted in a lower increase in ROS production(Reference Schmutzler, Bachmann and Fuhrmann57). LC-PUFA, in general, are oxidised easily because of their high degree of unsaturation, and thereby form oxygen radicals in many cell types(Reference Kim and Ro27, Reference Zhang, Dong and Ren58). In the present experiments in HMC-1, the antioxidants l(+)-ascorbic acid and α-tocopherol were used, which act as free radical scavengers and protect LC-PUFA from harmful lipid peroxidation and neutralise the free radicals formed. This may explain some of the discrepancies observed in the effects of LC-PUFA on ROS generation in in vitro studies. As suggested by others, the number of double bonds present may be important in the anti-inflammatory effects generated by LC-PUFA. It may explain the most potent inhibition of ROS generation by the fatty acid with the highest degree of unsaturation, namely DHA. This possibly results in the inhibition of IL-4 and IL-13 secretion by AA < EPA < DHA(Reference De Caterina, Bernini and Carluccio59). The present results imply that EPA and DHA act via similar mechanisms in the suppression of IL-4 and IL-13 secretion from MC, DHA just being slightly more effective than EPA. Probably the anti-allergic effects of AA are overruled since AA also enhances pro-inflammatory PGD2 and TNF-α secretion. Indeed, it has been shown that high maternal intake of margarine and vegetable oils rich in n-6 PUFA during the last 4 weeks of pregnancy is associated with enhanced occurrence of atopic eczema in offspring(Reference Sausenthaler, Koletzko and Schaaf60); by contrast, fish oil supplementation during pregnancy and lactation reduces the susceptibility of developing allergic disease in the neonates(Reference Lauritzen, Kjaer and Fruekilde18–Reference Kankaanpaa, Nurmela and Erkkila23).
Use of ROS inhibitors confirmed the involvement of ROS in the IL-4 and IL-13 secretion pathway in MC. ROS consist of a number of different mediators and although both IL-4 and IL-13 secretion could be blocked using the general ROS inhibitor 1,3-dimethyl-2-thiourea, use of superoxide dismutase showed that IL-4 but not IL-13 secretion is under the regulation of superoxide. LC-PUFA may be less able to affect this superoxide cascade in relation to other ROS mediators since IL-13 was suppressed more effectively by n-3 LC-PUFA than IL-4. ROS generation by MC can contribute to the secretion of inflammatory cytokines via NF-κB and/or MAP kinase signalling(Reference Hundley, Prasad and Beaven61). Previous studies have shown that IL-13 secretion by RBL-2H3 cells is regulated by JNK and p38(Reference Hirasawa, Izumi and Linwong62). However, as revealed using inhibitors of these pathways, the MAPK ERK, p38 and JNK were all involved in IL-13 secretion by activated HMC-1, while the NF-κB inhibitor Bay117082 did not reduce IL-13 release. This suggests ROS generation to be upstream of the MAPK signalling cascade.
Recently, a study with anti-IL-4/IL-13 demonstrated an improvement in asthma endpoints in patients with severe, uncontrolled asthma(Reference Corren, Busse and Meltzer63), suggesting a possible role for dietary supplementation with the n-3 LC-PUFA EPA and DHA in allergic disease. However, even though some studies have reported modest improvement of atopic dermatitis, there is no convincing evidence yet for dietary n-3 LC-PUFA supplementation alone for the treatment of those with established atopic disease(64, 65). Thus, although AA, EPA and DHA suppress allergy-related mediator release by MC, the effects are moderate and may not be strong enough for treatment purposes. However, when MC were treated with suboptimal doses of DHA and MAPK inhibitors, the suppression of IL-13 secretion by the p38 inhibitor SB203580 (20 μm) and the JNK inhibitor SP600125 (10 μm), but not the ERK inhibitor PD98059 nor the NF-κB inhibitor Bay117082, was further supported by DHA. The addition of DHA to p38 and JNK inhibitors was as effective as the higher inhibitor doses tested (50 and 20 μm, respectively). In addition to DHA, EPA was able to support SB203580 in the suppression of IL-13 secretion, while AA did not enhance the efficacy of any of the inhibitors. Hence, the combination of n-3 LC-PUFA with other drugs seems to be promising in reducing allergic type mediator release of MC. EPA and more prominently DHA, but not AA, added to the inhibitory effect of MAPK inhibitors on IL-13 secretion. Dietary n-3 LC-PUFA may therefore be able to optimise the efficacy and/or safety of novel strategies to treat allergies using drugs aiming to suppress IL-4, IL-13 and/or PGD2.
In conclusion, the n-6 LC-PUFA AA promotes the allergic cascade by enhancing degranulation and TNF-α and PGD2 secretion by activated MC. In contrast, the n-3 LC-PUFA EPA and DHA suppress PGD2, IL-13 and IL-4 secretion as well as ROS generation most effectively. Hence, LC-PUFA differentially modulate the MC phenotype. MC are involved in the initiation and perpetuation of allergic disease, and the suppression of allergy-related mediators by dietary n-3 LC-PUFA may contribute to reduced susceptibility to develop or sustain allergic disease.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114512003959
Acknowledgements
This study was financially supported by a grant from the Nutricia Research Foundation. The authors' responsibilities were as follows: L. E. M. W. and L. W. J. v. d. E. designed the study with input from F. A. R., E. F. K. and J. G.; L. W. J. v. d. E. and Y. N. performed the experiments under the supervision of L. E. M. W.; F. A. R. provided LAD2 cells; M. B. analysed the mast cell membrane composition; L. W. J. v. d. E. and L. E. M. W. analysed the data; L. W. J. v. d. E. drafted the manuscript and all authors contributed to and approved the final version of the manuscript. None of the authors has any financial or personal conflict of interest to report.







