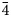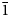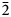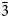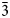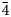Introduction
The discovery of new arsenate mineral species at the Valletta mine dumps, Piedmont, Italy, makes this a significant mineralogical locality. Lombardoite (IMA2016-058, Cámara et al., Reference Cámara, Bosi, Ciriotti, Bittarello, Hålenius and Balestra2016) and aldomarinoite (IMA2021-054, Cámara et al., Reference Cámara, Baratelli, Ciriotti, Nestola and Piccoli2021) belong to the brackebuschite supergroup of minerals and are the fourth and the eighth, respectively, of a series of new As-bearing minerals from Valletta Mine (grandaite, Sr2Al(AsO4)2(OH), Cámara et al. Reference Cámara, Ciriotti, Bittarello, Nestola, Bellatreccia, Massimi, Radica, Costa, Benna and Piccoli2014; braccoite, NaMn2+5[Si5AsO17(OH)](OH), Cámara et al. Reference Cámara, Bittarello, Ciriotti, Nestola, Radica and Marchesini2015; lombardoite, Ba2Mn3+(AsO4)2(OH), Cámara et al. Reference Cámara, Bosi, Ciriotti, Bittarello, Hålenius and Balestra2016; canosioite, Ba2Fe3+(AsO4)2(OH), Cámara et al. Reference Cámara, Bittarello, Ciriotti, Nestola, Radica, Massimi, Balestra and Bracco2017, aldomarinoite Sr2Mn3+(AsO4)2(OH), Cámara et al. Reference Cámara, Baratelli, Ciriotti, Nestola and Piccoli2021; castellaroite, Mn2+3(AsO4)2⋅4.5H2O, Kampf et al., Reference Kampf, Cámara, Ciriotti, Nash, Balestra and Chiappino2016; and rüdlingerite, Mn2+2V5+As5+O7⋅2H2O, Roth et al. Reference Roth, Meisser, Nestola, Škoda, Cámara, Bosi, Ciriotti, Hålenius, Schnyder and Bracco2020). Aldomarinoite represents the fifth Sr-dominant arsenate of the mineral species approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA-CNMNC), after arsenogoyazite, SrAl3(AsO4)(AsO3OH)(OH)6 (Walenta and Dunn, Reference Walenta and Dunn1984), grandaite, Sr2Al(AsO4)2(OH) (Cámara et al., Reference Cámara, Ciriotti, Bittarello, Nestola, Bellatreccia, Massimi, Radica, Costa, Benna and Piccoli2014), kemmlitzite, SrAl3(AsO4)(SO4)(OH)6 (Hak et al., Reference Hak, Johan, Kvaček and Liebscher1969), oberwolfachite, SrFe3+3(AsO4)(SO4)(OH)6 (Chukanov et al., Reference Chukanov, Möhn, Dal Bo, Zubkova, Varlamov, Pekov, Jouffret, Henot, Chollet, Vessely, Friis, Ksenofontov, Agakhanov, Britvin, Desor, Koshlyakova and Pushcharovsky2021), strontiopharmacosiderite, Sr0.5Fe3+4(AsO4)3(OH)4⋅4H2O (Mills et al., Reference Mills, Meisser, Rumsey, Hay, Spratt, Ansermet and Vonlanthen2014), and the second one, after lombardoite, having dominant Mn3+ instead of Al or Fe3+ in the arsenbrackebuschite group.
Samples containing lombardoite were collected by Corrado Balestra and Roberto Bracco, whereas aldomarinoite samples were collected by Gian Carlo Piccoli, on the dumps of a dismissed old mine found close to the Valletta pass at the Valletta Valley (‘Vallone della Valletta’ in Italian) in the Maira Valley, Piedmont, Italy, a small Fe–Mn deposit that has never been studied in detail from a geological or petrological point of view. The name of lombardoite honours Dr. Bruno Lombardo (1944–2014), geologist and petrologist at C.N.R. (National Research Council of Italy), for his extensive and sound contributions on the evolution of orogenic belts worldwide. A fragment of the holotype material is deposited in the mineralogical collections of the Museo Regionale di Scienze Naturali di Torino, Sezione di Mineralogia, Petrografia e Geologia, via Giovanni Giolitti 36, I-10123 Torino, Italy, catalogue number M/U 17111. The name aldomarinoite honours Mr. Aldo Marino, the collector and the founding member of the AMI – Italian Micromineralogical Association, born in Dronero (Cuneo Province) in 1942 who, with Gian Carlo Piccoli, first found the Valletta locality. The holotype material is deposited in the mineral collections of the Museo delle Collezioni di Mineralogia, Gemmologia, Petrologia e Giacimentologia, Dipartimento di Scienze della Terra “Ardito Desio”, Università di Milano, Italy, under the catalogue number MCMGPG-H2021–001.
Geological settings and mineral occurrence
The discovery area of the two minerals is the Valletta mine, ‘Vallone della Valletta’, Canosio, Val Maira, Cuneo province, Piedmont, Italy (44°23'43''N, 7°5'30''E, 2560 m a.s.l.). The area is geologically located in the Briançonnais Zone of the southern Cottian Alps, not far from the French border line. Geological data of the area are limited and a detailed study of the old mine is missing. The information given here is taken mainly from Crema et al. (Reference Crema, Dal Piaz, Merlo and Zanella1971) and Collo (Reference Collo1997). The sequence cropping out at the Valletta mine belongs to the internal units of the Briançonnais Zone (also known as Axial Permian–Carboniferous Zone), and consists of sedimentary detrital deposits and intermediate–acid volcanic activity rocks (Carboniferous–Permian). A quartzitic formation is located above these, comprising quartz conglomerates (Verrucano formation) and arenaceous quartzites. Then the evaporitic sequence (Lower Triassic) and the carbonate platform rocks such as schistose limestones, limestones and dolomites follow. Sedimentation of dolomitic breccias occurs during Upper Triassic followed again by evaporites. Then another transgressive phase occurs, characterised by carbonaceous sedimentation (Cretaceous–Lower Eocene). Finally, the Eocene–Oligocene period consists of calcarenites from detrital and turbidite facies.
In the Valletta mine, the volume of the mineralised body is limited to the surface and the mine dumps are the main remains of the former mining activities (Fig. 1). They consist of yellowish limestones–dolostones and red–brown quartzites and silicoclastic rocks, which contain calcareous, quartzitic and volcanic clasts with variable dimensions from a few millimetres to ~15 centimetres. The quartzitic rocks are composed of quartz conglomerates of the Alpine Verrucano and arenaceous quartzites (Upper Permian–Lower Triassic); the quartzites cover the conglomerates and the fine-grained quartz–feldspar schists derived from the acid volcanism. Above the quartzites, the limestones–dolostones consist of schistose limestones, yellow dolostones and an alternation of limestones and dolostones. From a tectonic point of view, the Valletta area is part of the Marinet anticlinal, or the siliceous Bande de Marinet, the southern tectonic zone of the internal units, and the Sautron unit.

Fig. 1. Image of mine dumps, the main remains of the former mining activities. Rocca la Meja stands in the background.
In the mine dumps, the quartzites and the calcareous rocks are often fractured, and show quartz and calcite veins containing mineral phases rich in As, V, Mn, Ba and Sr, which are also present along the fault planes (Fig. 2). These minerals are probably the result of crystallisation from hydrothermal fluids and oxidising conditions, as suggested by the presence of hematite, braunite and cryptomelane.

Fig. 2. Typical samples of millimetric orange aggregates, associated with quartz, along a fault plane. They are probably As–Mn minerals characteristic in Valletta Mine.
The present new minerals are associated strictly with quartz, aegirine, baryte, calcite, hematite, muscovite and Mn minerals such as cryptomelane, braunite and manganberzeliite. Other minerals that have been observed in the same locality are: adelite, albite, arseniopleite, azurite, bariopharmacosiderite, berzeliite, bosiite, braccoite, canosioite, caryinite, castellaroite, coralloite, diopside, fianelite, fluorapatite, gamagarite, ganophyllite, grandaite, gypsum, hollandite, ilmenite, magnesio-arfvedsonite, magnesio-riebeckite, magnetite, malachite, mimetite, neotocite, opal, orthoclase, oxy-dravite, palenzonaite, phlogopite, piccoliite, pyrobelonite, ranciéite, rhodochrosite, rhodonite, richterite, rüdlingerite, rutile, saneroite, talc, tetrahedrite series minerals, thorianite, tilasite, tinnunculite, tiragalloite, titanite, tokyoite and wallkilldellite.
Appearance and physical properties
Lombardoite (Fig. 3) and aldomarinoite (Fig. 4) occur as subhedral crystals in thin masses, a few cm in size, with uneven fracture, or aggregates of small crystals (<0.5 mm), in white veins of compact quartz or along the fault planes, as a reddish-brown quartzitic masses. Well-formed crystals, typically platy tablets, are very rare. Single crystals of lombardoite are dark red–brown, whereas aldomarinoite crystals are dark orange. Both are translucent, have a yellow–orange streak and a vitreous lustre. Lombardoite and aldomarinoite are optically biaxial (+). Lombardoite has 2Vz(meas.) = 78(4)° and is pleochroic with X = yellowish brown, Y = brown and Z = reddish brown (Z > Y > X); the mean refractive index (n ave) obtained from the Gladstone–Dale relationship (Mandarino, Reference Mandarino1979, Reference Mandarino1981) using the empirical formulae and calculated densities is 1.86, which is far beyond our available experimental range. Aldomarinoite has 2Vz(meas.) = 67.1(1)°, measured using a spindle stage mounted in a Leitz Dialux microscope with polarised light, and with optical calculations made with the Excelibr spreadsheet (Steven and Gunter, Reference Steven and Gunter2018). Aldomarinoite is pleochroic with X = brown, Y = brownish orange and Z = yellowish brown (Z > Y > X) and the calculated mean refractive index is 1.83 (from the Gladstone–Dale relationship). Both minerals are brittle, and no cleavage or parting is observed. Hardness was not measured but assigned by analogy to canosioite (6–6½) for lombardoite, and to tokyoite (4½–5) for aldomarinoite. Density was not measured due to the small crystal size and the frequent micro inclusions of other phases. The calculated density obtained from the empirical formula and unit-cell parameters measured during the single-crystal study is 5.124 g/cm3 for lombardoite and 4.679 g/cm3 for aldomarinoite.

Fig. 3. Picture of lombardoite: reddish-brown veinlet exposed on quartz (Field of view: 5 mm across, collection and photo R. Bracco).

Fig. 4. Picture of subhedral crystals of dark orange aldomarinoite and yellow tilasite within a quartz vein from the holotype, catalogue no. MCMGPG-H2021–001 (photo L. Baratelli).
Micro-Raman spectroscopy
The Raman spectrum of lombardoite (Fig. 5, upper spectrum) was obtained using a micro/macro Jobin Yvon Mod. LabRam HRVIS at the University of Torino, whereas the aldomarinoite spectrum (Fig. 5, lower spectrum) was obtained using a high resolution confocal micro-Raman system (LabRam HR Evolution – Horiba at the University of Milano), both equipped with a motorised x–y stage. The back-scattered Raman signal was collected with a 50× objective and the Raman spectrum was obtained for a non-oriented crystal. The 532 nm line was used as excitation; laser power was controlled by means of a series of density filters. The system was calibrated using the 520.6 cm–1 Raman band of silicon. The spectra were collected with multiple acquisitions (5 to 10 for lombardoite, 3 for aldomarinoite) with single counting times ranging between 10 and 20 s for lombardoite and between 30 and 40 s for aldomarinoite. The spectrum was recorded using the Horiba LabSpec 5 (lombardoite) and LabSpec 6 (aldomarinoite) software packages from 100 to 4000 cm–1 for lombardoite and from 50 to 4000 cm–1 for aldomarinoite, the results of the spectroscopic analysis are reported below.

Fig. 5. Raman spectra of lombardoite and aldomarinoite in the 50–1000 cm–1 region.
The presence of (AsO4)3– is confirmed. Although in many cases it is very difficult to separate the contributions of arsenate from those of vanadate units, a comparative analysis of Raman spectra of other members of the brackebuschite supergroup allows the identification of two main bands in the region of 750–950 cm–1, centred at ca. 818 and 871 cm–1 (plus two weak shoulders at 759 and 914 cm–1) for lombardoite and at ca. 848 and 892 cm–1 (plus three weak shoulders at 776, 816 and 932 cm–1) for aldomarinoite, they are slightly shifted compared to those found for canosioite (838, 862 and 896 cm–1, Cámara et al., Reference Cámara, Bittarello, Ciriotti, Nestola, Radica, Massimi, Balestra and Bracco2017) and grandaite (at 833, 857 and 899 cm–1, Cámara et al., Reference Cámara, Ciriotti, Bittarello, Nestola, Bellatreccia, Massimi, Radica, Costa, Benna and Piccoli2014), although the band at ~850 cm–1 is not clearly resolved in the Raman spectrum of lombardoite. These two main bands that are convoluted in lombardoite can be assigned to the stretching modes of (AsO4)3– and (VO4)3– groups respectively (the 871 cm–1 one is weaker due to the lower content of VO4 in lombardoite). A broad and weak band is observed at 627 cm–1. Similar band frequencies have been observed for canosioite (Cámara et al., Reference Cámara, Bittarello, Ciriotti, Nestola, Radica, Massimi, Balestra and Bracco2017) where multiple bands are shifted to higher frequencies.
In the region 200–600 cm–1 multiple Raman bands are observed at 293, 328 and 356 cm–1 for lombardoite and at 202, 297, 338 and 374 cm–1 for aldomarinoite (As5+–O bending vibrations); further bands are observed at 405, 471 and 505 cm–1 for lombardoite and at 411, 453, 488, 508, 529 and 612 cm–1 for aldomarinoite. Bands lower than 200 cm–1 correspond to M–O and lattice modes (88, 103, 163 and 181 cm–1 for lombardoite and 67, 91, 114, 126 and 160 cm–1 for aldomarinoite).
No bands were observed in the range between 2500 and 4000 cm–1 in the Raman spectrum of lombardoite. The absence of strong bands with frequencies higher than 1000 cm–1 would indicate the absence of OH groups in lombardoite. Same difficulties on observing bands in the range between 2500 and 4000 cm–1 of the Raman spectrum was found in grandaite (Cámara et al., Reference Cámara, Ciriotti, Bittarello, Nestola, Bellatreccia, Massimi, Radica, Costa, Benna and Piccoli2014) and canosioite (Cámara et al., Reference Cámara, Bittarello, Ciriotti, Nestola, Radica, Massimi, Balestra and Bracco2017). However, a broad band centred at ca. 2970 cm–1 was observed in this range in the Raman spectrum of aldomarinoite, which agrees well with the value calculated with the equation 3592−304×109⋅exp(−d(O⋅⋅⋅O)/0.1321) (Libowitzky, Reference Libowitzky1999), i.e. 3108 cm–1.
Infrared spectroscopy
Fourier-transformed infrared (FTIR, Fig. 6) transmission spectra (64 scans) of lombardoite were recorded using a diamond anvil cell (High Pressure Diamond Optics, Inc.). They were obtained with a spectrophotometer Bruker Vertex 70 coupled with a Hyperion 3000 microscope at Centro Conservazione e Restauro “La Venaria Reale” (CCR, Torino, Italy). The instrument is equipped with an MCT detector (Infrared Associates Inc.), working in the spectral range from 4000 to 600 cm–1 with an average spectral resolution of 4 cm–1. The FTIR spectrum obtained for lombardoite shows the presence of a broad weak band at ~2900 cm–1 that could be assigned to the stretching of OH groups. This band agrees well with Raman spectroscopy observations. Thus, in the absence of F in the electron microprobe analysis and on the basis of crystal structure refinement (SREF) data (see below) we also consider the occurrence of (OH) groups in lombardoite. As the FTIR spectrum has no absorption band at ca. 1650 cm–1 (e.g. bending vibration) the presence of H2O groups can be ruled out.

Fig. 6. FTIR spectrum of lombardoite in the 600–4000 cm–1 region.
Optical absorption spectroscopy (OAS)
Polarised optical absorption spectra of double sided polished single-crystal absorbers of lombardoite prepared from X–Y (32 μm thick) and Y–Z (24 μm thick) sections were recorded in the range 9000–34000 cm–1 using an AVASPEC-ULS2048X16 spectrometer attached via a 400 μm UV optic fibre cable to a Zeiss Axiotron UV-microscope. A 75 W Xenon arc lamp was used as a light source and Zeiss Ultrafluar 10× lenses served as objective and condenser. The size of the circular measure aperture was 32 μm in diameter. A UV-quality Glan-Thompson prism with a working range from 250 to 2700 nm (40,000 to 3704 cm–1) was used as the polariser. The recorded spectra (Fig. 7) show three intense and broad absorption bands at 22,900, 22,100 and 18,300 cm–1. An additional broad band of medium intensity occurs at 12,000 cm–1. Observed bands and their intensities, band-widths and energies are typical for spin-allowed electronic d-d transitions observed in spectra of minerals containing strongly elongated or compressed octahedra occupied by Mn3+, for example as observed in piemontite and kanonaite (e.g. Smith et al., Reference Smith, Hålenius and Langer1982). From the observed band energies, the crystal field splitting parameter (10 Dq) for Mn3+ at the M-site of lombardoite is calculated to be 15,100 cm–1. This compares very well with 10 Dq values in the range 14,400 to 15,600 cm–1 for Mn3+ in epidote and andalusite-group minerals, suggesting a mean Mn–O bond distance in lombardoite of ~2.02 Å.

Fig. 7. Polarised single-crystal absorption spectra of lombardoite.
Chemical data
The chemical composition of lombardoite was determined using a Cameca SX-50 electron microprobe in wavelength dispersive spectroscopy (WDS) mode at the Institute of Environmental Geology and Geoengineering (IGAG – C.N.R., Rome) on the same crystal used for structure determination. Major and minor elements were determined at 15 kV accelerating voltage and 15 nA beam current (beam size = 1 μm), with 40 to 20 s counting time on both peak and background. Fluorine, Si, K, Ti, Cr, Ni, Cu and Zn were below the detection limit. Analytical results, standards and spectral line are reported in Table 1. The chemical composition of aldomarinoite was determined by WDS using a Jeol 8200 Super Probe electron microprobe at the University of Milano operating at an acceleration voltage of 15 kV and beam current of 5 nA (beam size = 3 μm). Potassium, Na, Ti, Si and F were below the detection limit of 0.03 atoms per formula unit (apfu). Analytical results, standards and spectral line are reported in Table 1.
Table 1. Analytical data (wt.%) for lombardoite (5 data points) and aldomarinoite (4 data points).

* Calculated by stoichiometry from the results of the crystal structure analysis; **All Fe and Mn is considered as Fe3+ and Mn3+ based on experimental and redox potential arguments; S.D. – standard deviation.
Both the analyses were subject to ZAF-corrections using the PAP routine (Pouchou and Pichoir, Reference Pouchou, Pichoir and Armstrong1985). For both minerals, H2O was calculated based on one OH group per formula unit (pfu) (see Raman and FTIR spectroscopy sections). Manganese was also considered as Mn3+ on the basis of high oxidising environments as well as OAS results. All Fe was considered to be Fe3+ based on the Mn and Fe redox potential arguments and site bond length observed from SREF. The empirical formula, calculated on the basis of 9 O apfu, is, within rounding errors, (Ba1.96Sr0.17Pb0.04Na0.02Ca0.02)Σ2.21(Mn3+0.62Fe3+0.13Al0.06Mg0.11)Σ0.92[(As0.87V0.12P0.01)Σ1.00O4]2(OH) for lombardoite, and (Sr1.93Ca0.21Ba0.04Pb0.01)Σ2.19(Mn3+0.48Al0.35Fe3+0.21Mg0.01)Σ1.05[(As0.92V0.03)Σ0.95O4]2(OH) for aldomarinoite. The ideal formula for lombardoite is Ba2Mn3+(AsO4)2(OH), which requires BaO 49.11, Mn2O3 12.64, As2O5 36.81, H2O 1.44, total 100 wt.%, and for aldomarinoite is Sr2Mn3+(AsO4)2(OH), which requires SrO 40.24, Mn2O3 15.32, As2O5 42.69, H2O 1.75, total 100 wt.%. Lombardoite is unreactive and insoluble in 2 M and 10% HCl and 65% HNO3 and aldomarinoite in 37% HCl and 69% HNO3.
X-ray crystallography
Powder X-ray diffraction
The powder X-ray diffraction pattern of lombardoite was obtained with an Oxford Gemini R Ultra diffractometer equipped with a CCD area detector at CrisDi (University of Turin). Experimental and calculated data are reported in Table 2. The unit-cell parameters refined from the powder data with the software GSAS (Larson and Von Dreele, Reference Larson and Von Dreele1994) are: a = 7.883(2) Å, b = 6.146(1) Å, c = 9.101(2) Å, β = 112.52(1)° and V = 407.3(2) Å3.
Table 2. Powder X-ray diffraction data (d in Å, I in %) for lombardoite and aldomarinoite*.

* Only reflections with I rel > 5%(I rel) are listed; the strongest reflections are in bold.
The powder X-ray diffraction data of aldomarinoite were obtained with a Rigaku XtaLAB Synergy-S, equipped with a microfocus MoKα source and a HyPix-6000HE detector with graphite-monochromatised MoKα radiation (University of Milano). Experimental and calculated data are reported in Table 2. The unit-cell parameters refined from the powder data with the software UnitCell (Holland and Redfern, Reference Holland and Redfern1997) are: a = 7.5414(3) Å, b = 5.9838(2) Å, c = 8.7399(3) Å, β = 112.034(4)° and V = 365.60(2) Å3.
Single-crystal X-ray diffraction
Data for lombardoite were collected using an Oxford Gemini R Ultra diffractometer equipped with a CCD area detector at CrisDi (Università di Torino, Italy), with graphite-monochromatised MoKα radiation. Crystal data and experimental details are reported in Table 3. At room temperature, the unit-cell parameters are: a = 7.8636(1) Å, b = 6.13418(1) Å, c = 9.1197(1) Å, β = 112.660(2)°, V = 405.94(1) Å3, space group = P21/m and Z = 2.
Table 3. Crystal data and summary of parameters describing data collection and refinement for lombardoite and aldomarinoite.

Data for aldomarinoite were collected using a Rigaku XtaLAB Synergy-S at the University of Milano, equipped with a microfocus MoKα source and a HyPix-6000HE detector with graphite-monochromatised MoKα radiation. Crystal data and experimental details are reported in Table 3. At room temperature, the unit-cell parameters are: a = 7.5577(4) Å, b = 5.9978(3) Å, c = 8.7387(4) Å, β = 111.938(6)°, V = 367.43(3) Å3, space group = P21/m and Z = 2. A total of 1755 independent reflections were collected.
The two structure models were refined starting from the atom coordinates of arsenbrackebuschite (Hofmeister and Tillmanns, Reference Hofmeister and Tillmanns1978) for lombardoite and of grandaite (Cámara et al., Reference Cámara, Ciriotti, Bittarello, Nestola, Bellatreccia, Massimi, Radica, Costa, Benna and Piccoli2014) for aldomarinoite, by means of the SHELX set of programs (Sheldrick, Reference Sheldrick2015).
Scattering curves for neutral atoms were taken from the International Tables for Crystallography (Wilson, Reference Wilson1992). Site-scattering values were refined for the cation sites using two scattering curves contributing proportionally and by constraining the sum to full occupancy: As and V were used for the T(1) and T(2) sites; Al and Mn were considered for the M site; Ba and Sr were used for the A(1) and A(2) sites in lombardoite, whereas for aldomarinoite Sr and Ca were used for the A(1) site; and Sr and Ba for the A(2) site. A peak in the difference-Fourier map was found close to O(7) and added to the model as an H atom with fixed coordinates for aldomarinoite and refined coordinates for lombardoite. An isotropic displacement factor of 1.2 times the one observed for the bonded O(7) anion site, and full occupancy were applied. Refinement converged to R 1 = 0.0197 for 2628 observed reflections with F o > 4σ(F o) and 87 parameters for lombardoite and to R 1 = 0.0345 for 1235 observed reflections with F o > 4σ(F o) and 83 parameters for aldomarinoite. Tables 4, 5 and 6 report, respectively, atom coordinates, displacement parameters, and selected bond distances and angles. Bond valence calculations using the parameters of Gagné and Hawthorne (Reference Gagné and Hawthorne2015) are reported in Table 4. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 4. Wyckoff numbers, fractional atom coordinates, and equivalent isotropic displacement parameters (Å2) for lombardoite and aldomarinoite*. Bond valence sums (BVS, in valence units) calculated using the parameters of Gagné and Hawthorne (Reference Gagné and Hawthorne2015).

* The temperature factor has the form exp(–T) where T = 8π2U(sin(θ)/λ)2 for isotropic atoms.
Table 5. Anisotropic displacement parameters (Å2) for lombardoite and aldomarinoite*.

* The temperature factor has the form exp(–T) where T = 2π2Σij(h (i)h (j)U (i,j)a*(i)a*(j)).
Table 6. Selected interatomic distances (Å) and angles (°) for lombardoite and aldomarinoite*.

* Mean quadratic elongation (λ) and the angle variance (σ2, in °^2) were computed according to Robinson et al. (Reference Robinson, Gibbs and Ribbe1971).
Description of the structure
Cation sites
M sites
There is one M site hosting small high-charge cations in octahedral coordination. The observed site scattering value 22.576(3) eps (electrons per site) at the M site of lombardoite and 20.49(7) eps at the M site of aldomarinoite agree with the chemical data confirming a Mn dominance at that site. Those correspond to (Mn3+0.67 + Fe3+0.14 + Mg0.12 + Al0.07)Σ1.00 apfu and 22.50 eps, and (Mn3+0.46 + Al0.33 + Fe3+0.20 + Mg0.01)Σ1.00 apfu and 21.11 eps, respectively.
The observed average bond length at M sites (2.030 Å for lombardoite and 1.981 Å for aldomarinoite, Table 6) is too small for the Fe2+ and Mn2+ ions; the incident bond valence sum at the M site is in agreement with average formal charge being dominantly +3, i.e. considering both Fe3+ and Mn3+ at the M sites, which also agrees with the oxidised nature of the ore.
A sites
There are two non-equivalent sites hosting large divalent cations in eight and eleven coordination: the A(1) and A(2) sites, respectively. Site distribution at the A sites was obtained according to the SREF results that show little difference in scattering values at both sites of lombardoite: 53.44(1) eps at the A(1) site, and 54.93(2) eps at the A(2). Therefore, we assign (Ba0.94Sr0.06) to A(1) and (Ba1.00) to A(2). Also the A site occupancies of aldomarinoite show little difference in the scattering values: 35.05(9) eps at the A(1) site and 38.70(7) eps at the A(2) site. Therefore, we assign (Sr0.81Ca0.19) to A(1) and (Sr0.96Ba0.04) to A(2). Bond valence calculations indicate that both the A(1) and A(2) sites are compatible with the dominance of divalent cations at these sites.
T sites
There are two non-equivalent sites hosting a high charge cation in tetrahedral coordination: the T(1) and the T(2) sites. The refined site scattering values obtained for T sites are 31.26(3) for T(1) and 31.11(4) for T(2) of lombardoite, and 32.9 for T(1) and 32.8 for T(2) of aldomarinoite, are quite similar, whereas average bond lengths and distortion parameters are significantly different (Table 6). The limited quantity of V observed in the chemical analyses (Table 1) and the absence of high-charge cations (Si4+, P5+, S6+) that may potentially occupy the T sites suggests V disordered equally at the T(1,2) sites. The distortion is therefore ascribed to the different A-polyhedron size due to the Ba–1Sr substitution: the smaller A(1)O8 and A(2)O11 polyhedra in aldomarinoite yield a larger distortion of As-centred tetrahedra, in particular of T(1)O4 tetrahedron because of the O(5) atom acting as donor of charge for the hydrogen bond (see below).
The cation part of the structure is represented by [Mn3+(Ba2+,Sr2+)2As5+2]Σ17+.
Anion sites
There are seven anion positions in the structure of lombardoite and aldomarinoite, two of them on general position, accounting for a total of 18 anions per unit cell. Bond valence data (Table 4) show that one out of seven anions sites has bond valence sum close to 2 vu (valence units), whereas the O(7) site has a smaller contribution (1.341 vu for lombardoite and 1.400 vu for aldomarinoite) compatible with the occurrence of monovalent anion at this site. The absence of F in the chemical analyses (Table 1) indicates that the O(7) site must therefore host a hydroxyl group. In fact, a maxima was found in the Fourier difference and added to the model as atom H(7). The hydrogen at H(7) is at 1.64 Å from the O(5) atom, which has a bond-valence sum of 1.503 vu for lombardoite and is at 1.791 Å from the O(5) atom, which has a bond-valence sum of 1.619 vu for aldomarinoite, and so is likely to receive a hydrogen bond. Therefore there is a hydrogen bond with oxygen at O(5) ensuring a further contribution of bond valence to this site (Table 7). The structural model, thus, confirms O–H⋅⋅⋅O = 2.8 Å for lombardoite, in good agreement with IR data, and O–H⋅⋅⋅O = 2.7 Å for aldomarinoite. The anion part of the structure is represented by [O8(OH)]Σ17–.
Table 7. The brackebuschite supergroup, A(1)2+A(2)2+[vıM 3+x vıM 2+1–x][ıv(T 5+xT 6+1–x)O4]2X (references are given in brackets).

Refs: (1) Abraham et al. (Reference Abraham, Kautz, Tillmanns and Walenta1978); (2) Hofmeister and Tillmanns (Reference Hofmeister and Tillmanns1978); (3) Vésignié, J.P.L. (Reference Vésignié1935); (4) Bideaux et al. (Reference Bideaux, Nichols and Williams1966); (5) Zubkova et al. (Reference Zubkova, Pushcharovsky, Giester, Tillmanns, Pekov and Kleimenov2002); (6) Brackebusch et al. (Reference Brackebusch, Rammelsberg, Doering and Websky1883); (7) Clark et al. (Reference Clark, Criddle, Roberts, Bonardi and Moffatt1997); (8) Rammelsberg (Reference Rammelsberg1880); (9) Donaldson and Barnes (Reference Donaldson and Barnes1955); (10) Fanfani and Zanazzi (Reference Fanfani and Zanazzi1967); (11) Foley et al. (Reference Foley, Hughes and Lange1997); (12) González del Tánago et al. (Reference del Tánago J., La Iglesia, Rius and Fernández Santín2003); (13) de Villiers (Reference de Villiers1943); (14) Harlow et al. (Reference Harlow, Dunn and Rossman1984); (15) Basso et al. (Reference Basso, Palenzona and Zefiro1987); (16) Williams (Reference Williams1973); (17) Matsubara et al. (Reference Matsubara, Miyawaki, Yokoyama, Shimizu and Imai2004); (18) Brunet et al. (Reference Brunet, Gebert, Medenbach and Tillmanns1993); (19) Brunet and Chopin (Reference Brunet and Chopin1995); (20) Roth (Reference Roth2007); (21) Pekov et al. (Reference Pekov, Kleimenov, Chukanov, Yakubovich, Massa, Belakovskiy and Pautov2002); (22) Yakubovich et al. (Reference Yakubovich, Massa and Pekov2002); (23) Pekov (Reference Pekov2007); (24) Moore et al. (Reference Moore, Irving and Kampf1975); (25) Rosický (Reference Rosický1912); (26) Busz (Reference Busz1912); (26) Spencer (Reference Spencer1913); (28) Nichols (Reference Nichols1966); (29) Cámara et al. (Reference Cámara, Ciriotti, Bittarello, Nestola, Bellatreccia, Massimi, Radica, Costa, Benna and Piccoli2014); (30) Kampf et al. (Reference Kampf, Adams, Nash and Marty2015); (31) Lafuente and Downs (Reference Lafuente and Downs2016); (32) Cámara et al. (Reference Cámara, Bittarello, Ciriotti, Nestola, Radica, Massimi, Balestra and Bracco2017); (33) Cámara et al. (Reference Cámara, Bosi, Ciriotti, Bittarello, Hålenius and Balestra2016); (34) this study; * recent examination on the holotype specimen (BM1972,194) has confirmed the structural correspondence of heyite and calderónite (Kampf et al., Reference Kampf, Adams, Nash and Marty2015).
Structure topology
The crystal structures of lombardoite and aldomarinoite (Fig. 8) are topologically identical to that of arsenbrackebuschite. Chains of [M 3+(2+)(T 5+O4)2(OH,H2O)] units are connected through interstitial divalent cations. The M 3+O6 octahedron shares edges with other octahedra forming a chain along [010]. The shared edge has one anion that can be an (OH) group or a H2O group, depending on the charge of the M-cation at the octahedron, and the other anion is shared with the apex of a T 5+O4 tetrahedron (the T(1) site). The remaining 4 anions coordinating the octahedron are linked to the edge of another T 5+O4 tetrahedron (the T(2) site) alternating in both sides. Minor V5+ is disordered among the two arsenate tetrahedra. Decorated chains of octahedra link together through bonding with two symmetrically independent interstitial cations at the A(1) and A(2) sites, and hydrogen bonding. In arsenbrackebuschite, Pb occupies both the A sites but is shifted off-centre, as usual, for its lone pair configuration. The small quantity of Pb found in lombardoite and aldomarinoite at the A(2’) site also follows this configuration. The Pb off-centre displacement has been reported previously in the literature [e.g. synthetic Pb2(Pb,K)4[Si8O20]O, Moore et al., Reference Moore, Sen Gupta and Schlemper1985; Pb replacing Ba in hyalotekite, Moore et al., Reference Moore, Araki and Ghose1982 and Christy et al., Reference Christy, Grew, Mayo, Yates and Belakovskiy1998, or Pb replacing REE in lusernaite-(Y), Biagioni et al., Reference Biagioni, Bonaccorsi, Cámara, Cadoni, Ciriotti, Bersani and Kolitsch2013]. The distortion observed for the T(2)O4 tetrahedron along with the low bond valence incidence at O(5) represents a stressed environment for the structure of lombardoite and aldomarinoite.

Fig. 8. Detail of the lombardoite, and aldomarinoite, structure showing the chains of Mn3+ octahedra, AsO4 groups and interstitial large cations, projected onto [010] (a) and [001] (b). Orange: As tetrahedra (T(1) and T(2)); purple: Mn3+ centre M octahedra; cyan: Ba and Sr sites (A(1) and A(2)); pale pink: H sites; red: oxygen sites. O–H⋅⋅⋅O bonds are indicated by dotted black lines. Thermal displacement ellipsoids shown at 95% probability.
Related minerals
Lombardoite, Ba2Mn3+(AsO4)2(OH), is the As-dominant analogue of tokyoite, Ba2Mn3+(VO4)2(OH) and the Ba–As-dominant analogue of brackebuschite, Pb2Mn3+(VO4)2(OH). It is the first Mn–As member of the arsenbrackebuschite group in the brackebuschite supergroup (Table 7).
Aldomarinoite, Sr2Mn3+(AsO4)2(OH), is the Sr-dominant analogue of lombardoite, Ba2Mn3+(AsO4)2(OH), and the Mn3+-dominant analogue of grandaite, Sr2Al3+(AsO4)2(OH). It is the second Sr member of the arsenbrackebuschite group of the brackebuschite supergroup (Table 7).
In the Strunz System (Strunz and Nickel, Reference Strunz and Nickel2001) lombardoite and aldomarinoite fit in subdivision 8.B.G, phosphates, etc. with additional anions, without H2O, with medium-sized and large cations (OH, etc.).
Costin et al. (Reference Costin, Fairey, Tsikos and Guscsik2015) reported an As-rich tokyoite with the formula (Ba1.92Sr0.05Pb0.03)Σ2.00(Mn3+0.98Fe3+0.02)Σ1.00[(As1.050V0.950)Σ2.00O8)](OH) from a drill core obtained from the Postmasburg area in the Northern Cape Province of South Africa. Their observations indicated a possible replacement of tokyoite by a composition richer in As, with an almost complete solid solution from a pure tokyoite towards an As-dominant end-member, reaching compositions of almost 50:50. The grains were associated with K-feldspar, serandite, quartz, witherite, tokyoite and noelbensonite. These authors reported cell parameters obtained by electron backscatter diffraction (a = 9.121 Å, b = 6.142 Å, c = 7.838 Å, α = γ = 90°, β = 112°, monoclinic and P21/m space group), which compare well with the ones we obtained for lombardoite (Table 3). Costin et al. (Reference Costin, Fairey, Tsikos and Guscsik2015) also reported Raman spectra of As-rich tokyoite. The assignment of Raman peaks in As-rich tokyoite was done by similarity with arsentsumebite by Cosin et al. (2015), and suggested a possible ordering of the AsO4 and VO4 tetrahedra. In detail, they observed peaks at 308–340 cm–1, assigned to (AsO4)3– bending modes and at 442–460 cm–1 assigned to (AsO4)3– and (VO4)3– bending modes. Moreover, Cosin et al. (2015) also reported four outstanding peaks between 830 cm–1 and 935 cm–1, a pair between 830–846 cm–1 with a weak band at 730 cm–1 [symmetrical stretching vibrations and asymmetrical stretching vibrations of (AsO4)3–, respectively], and a pair between 906 and 935 cm–1 [symmetrical stretching vibrations and asymmetrical stretching vibrations of (VO4)3–, respectively]. However, the latter two peaks were at higher wave numbers than expected for the VO4 group in brackebuschite, and this was interpreted as due to the Jahn–Teller distortion effects related to Mn3+. Though our samples are almost devoid of V, we observe similar peaks, corresponding to symmetric bending modes for AsO4 at 328 cm–1 and the most intense band corresponding to symmetric stretching modes for AsO4 at 818 cm–1 (with two unresolved shoulders at 871 cm–1 and 759 cm–1) in lombardoite. The wave numbers for the corresponding band in aldomarinoite are shifted to higher wave numbers (339, 773, 849 and 893 cm–1) probably because of the different bond strength with Sr at the A sites.
Acknowledgements
Referees Igor V. Pekov, Fabrice Dal Bo and Oleg Siidra are thanked for useful suggestions. F.C. and L.B. acknowledges financial support by the grant Ricerca Locale 2020, Università di Milano, and from the Italian Ministry of Education (MUR) through the project “Dipartimenti di Eccellenza 2018–2022”. C.B., M.E.C. and G.C.P. acknowledge financial support of AMI – Associazione Micromineralogica Italiana.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.31