Introduction
Malaria is a kind of infectious disease mainly caused by four species of protozoan parasites of the genus Plasmodium, including Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae [Reference Tangpukdee1]. Pregnant women are at higher risk of malaria infection than the non-pregnant, but the susceptibility may decline with successive pregnancies due to the acquisition of immunity [Reference Lagerberg2]. Malaria during pregnancy generally has nonspecific and flu-like symptoms [Reference Lagerberg2, Reference Zhou3]. It may lead to anaemia, spontaneous abortion, preterm delivery, stillbirth and maternal death [Reference Maestre and Carmona-Fonseca4–Reference Desai8]. This undoubtedly increases the mortality rate, health and financial burden.
It has been identified that red blood cell polymorphisms and genetic variants of the host are contributors of malaria infection, and the ABO blood group is possibly one of the genetic factors [Reference Wahlgren, Goel and Akhouri9–Reference Cserti and Dzik12]. The specificity of blood group antigens is determined by a simple Mendelian inheritance pattern. ABO antigens are essentially carbohydrate and their differences are determined by the terminal sugar [Reference Greenwell13]. Based on the presence or absence of the blood-group antigens A and B on the surface of erythrocytes, the host may have blood group A, B, O or AB [Reference Franchini and Bonfanti14, Reference Hosoi15].
ABO blood group is hypothesised to be one factor affecting malaria infection. Understanding the effect of the ABO blood group on clinical manifestations of malaria infection may help to identify corresponding pathogenesis. The association between the ABO blood group and malaria has been reported a lot in recent years [Reference Degarege16, Reference Degarege17]. Some studies focused on pregnant women and reported the susceptibility of malaria in primiparae and multiparae with different ABO blood group. However, the results and conclusions of these studies were inconsistent. Therefore, the present study systematically reviewed literature and knowledge of this topic. A meta-analysis was conducted to clarify the risk of malaria in primiparae and multiparae with different ABO blood group.
Methods
Literature search strategy
All articles available in MEDLINE and PubMed were searched using the terms (‘ABO’ OR ‘blood group’ OR ‘blood groups’ OR ‘blood type’ OR ‘blood typing’) AND (‘helopyra’ OR ‘malarial fewer’ OR ‘malaria’ OR ‘impaludism’ OR ‘ague’ OR ‘plasmodium’). The search cut-off date was 30 November 2021. Only studies published in English were included. These studies were then screened with inclusion and exclusion criteria to determine whether it could be included for further analysis.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) Study population was pregnant women and was divided into primiparae and multiparae. (2) The Number of malaria positive and negative cases both in primiparae and multiparae was available. (3) Diagnosis of malaria was confirmed by whichever method including blood smears and microscopy, histology, rapid diagnostic test, or polymerase chain reaction (PCR). The study would be included in the meta-analysis only when it satisfied all these criteria.
Data collection
Data of included studies was collected as below: first author, publication year, country of origin, type of study, blood group typing and malaria diagnostic methods, the number of malaria positive and negative cases in primiparae and multiparae.
Data synthesis and analysis
For the meta-analysis, odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess the association between the ABO blood group and the risk of malaria during pregnancy. Statistical heterogeneity between included studies was evaluated using Cochran Q and I2 statistics, P value in Q statistics <0.05 means heterogeneity [Reference Leucht, Kissling and Davis18]. Substantial heterogeneity was considered when I 2 > 50%. The random-effects model was applied to the statistical analysis in the light of the conservative characteristic [Reference DerSimonian and Laird19]. P < 0.05 was considered to be statistically significant. All statistical analyses were carried out using Review Manager (RevMan, Version 5.3 for Windows, Oxford, England, The Cochrane Collaboration, 2014).
Results
Study selection
Study selection was carried out by using the flow-chart based on PRISMA requirements (Fig. 1). Of the 6921 articles identified from MEDICINE and PubMed, 1787 were excluded as duplicates. 4975 articles were then excluded by screening records, among which 4646 lacked comparisons and 329 studied other blood group systems. 159 full-text articles were then assessed for eligibility. 154 of them were excluded, among which 127 studied and compared other populations, while 27 lacked data of primiparae and multiparae. Finally, five studies satisfied the inclusion criteria and were included in the meta-analysis [Reference Adam20–Reference Senga24].
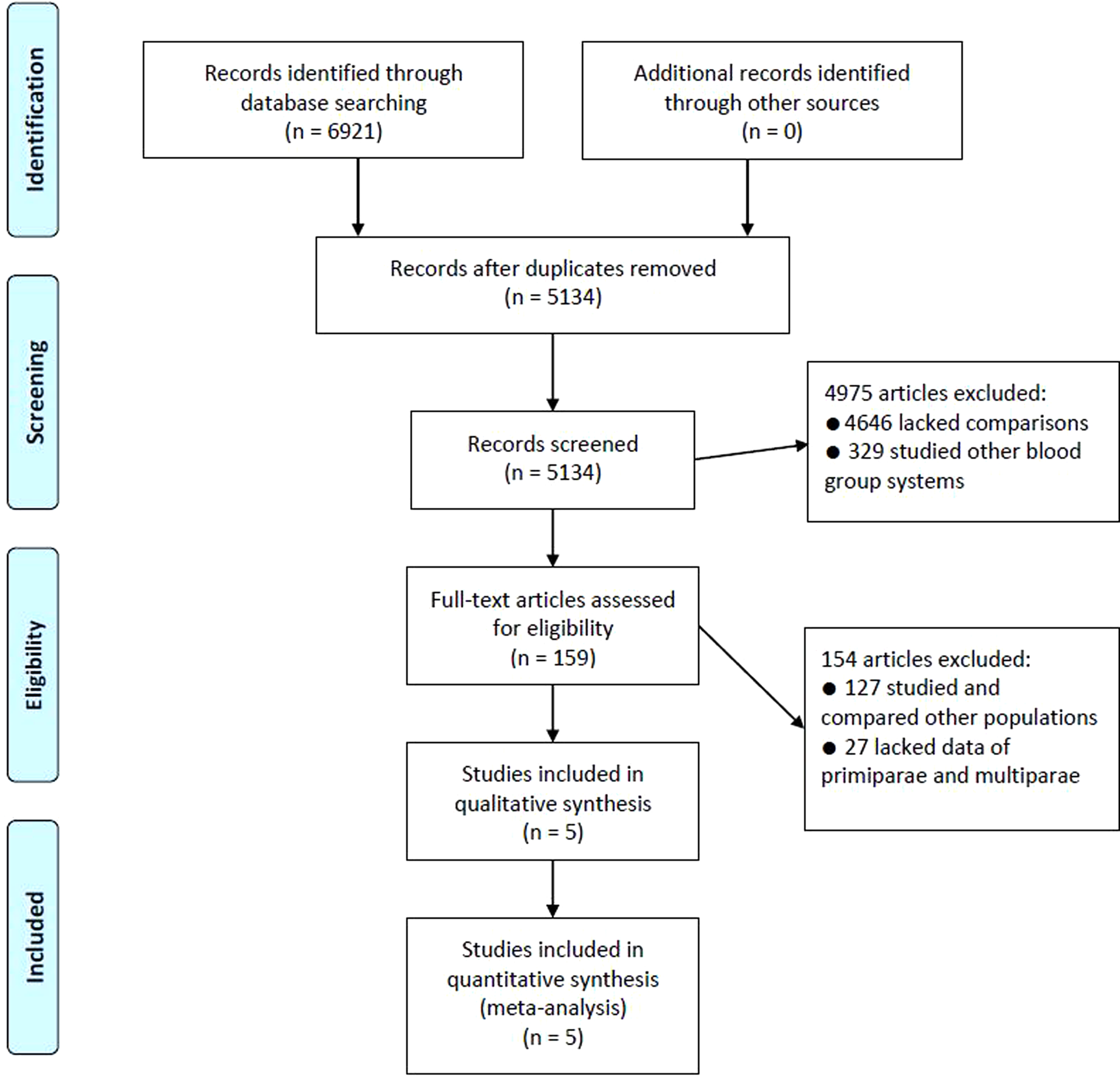
Fig. 1. Flow-chart of study selection.
Studies characteristics
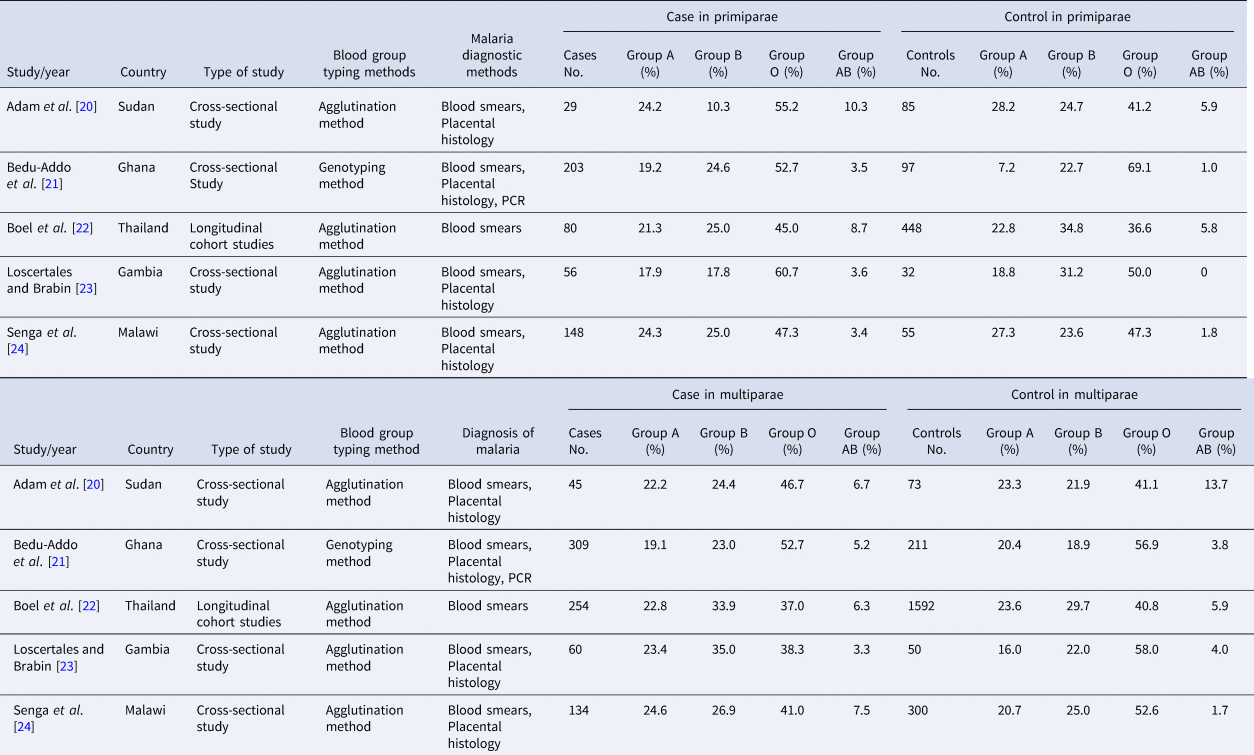
As it was shown in Table 1, five studies satisfied inclusion criteria. The publication date of them ranged from 2006 to 2014. All studies were conducted in malaria-endemic countries, four of them were cross-sectional studies while another was a longitudinal cohort study. The blood group typing method was the agglutination method in four studies and genotyping method in another study. A blood smear was a diagnostic method of malaria in all studies, while placental histology in four of them and PCR in one of them were used.
Table 1. Characteristics of studies included in the meta-analysis
Quantitative synthesis
Five studies were included in the meta-analysis and all of them were conducted in hospitals of malaria-endemic regions. The risk of malaria was not statistically different between primiparae with blood group A and non-A (OR = 1.12, 95% CI was 0.69 to 1.81, P = 0.65) (Fig. 2a), blood group B and non-B (OR = 0.76, 95% CI was 0.52 to 1.12, P = 0.16) (Fig. 2b), blood group O and non-O (OR = 1.08, 95% CI was 0.66 to 1.77, P = 0.75) (Fig. 2c), blood group AB and non-AB (OR = 1.82, 95% CI was 0.94 to 3.52, P = 0.07) (Fig. 2d).

Fig. 2. Forest plot for meta-analysis of malaria risk in primiparae with different blood group. (a) Forest plot of primiparae with blood group A versus non-A. (b) Forest plot of primiparae with blood group B versus non-B. (c) Forest plot of primiparae with blood group O versus non-O. (d) Forest plot of primiparae with blood group AB versus non-AB.
In multiparae with different blood group, the risk of malaria was comparable between blood group A and non-A (OR = 1.02, 95% CI was 0.83 to 1.27, P = 0.82) (Fig. 3a), blood group AB and non-AB (OR = 1.31, 95% CI was 0.67 to 2.55, P = 0.43) (Fig. 3b). But the risk in blood group B was significantly higher than that in non-B group (OR = 1.23, 95% CI was 1.01 to 1.50, P = 0.04) (Fig. 3c). In contrast, multiparae with blood group O had a significantly reduced risk of malaria than non-O group (OR = 0.78, 95% CI was 0.63 to 0.97, P = 0.03) (Fig. 3d).

Fig. 3. Forest plot for meta-analysis of malaria risk in multiparae with different blood group. (a) Forest plot of multiparae with blood group A versus non-A. (b) Forest plot of multiparae with blood group AB versus non-AB. (c) Forest plot of multiparae with blood group B versus non-B. (d) Forest plot of multiparae with blood group O versus non-O.
Discussion
The present study systematically reviewed relevant knowledge about the ABO blood group and malaria infection in pregnant women, simultaneously reassessed the risk of malaria in primiparae and multiparae with different blood group. Comparable risk of malaria was shown in primiparae with different ABO blood group, as well as multiparae with blood group A and non-A, blood group AB and non-AB. However, the meta-analysis showed significantly higher risk in multiparae with blood group B than non-B group, but the significantly lower risk in multiparae with blood group O than non-O group (P < 0.05). This suggested a protective role of blood group O but a harmful role of blood group B against malaria infection in multiparae.
Although the advancement of preventive and therapeutic methods, malaria continues to bring about a devastating impact on people's health and livelihoods [Reference Gunda and Chimbari25]. Gestational malaria is one manifestation of malaria infection. It signifies the presence of parasitaemia in the placenta, cord or peripheral blood [Reference Maestre and Carmona-Fonseca4]. Compared with non-pregnant counterparts, the pregnant is more susceptible to malaria and has a higher risk of severe infection [Reference Lagerberg2, Reference Maestre and Carmona-Fonseca4, Reference Okoko, Enwere and Ota26]. It is estimated that more than 125 million pregnant women are at risk of malaria annually [Reference Cates27].
The pathogenesis of malaria is intricate and the ABO blood group may play a part in it. Malaria is endemic in many countries of Africa, South America and Asia [Reference Garcia28]. The distribution of the ABO blood group is also inhomogeneous in populations of different regions. For example, the O allele has a higher frequency in sub-Saharan Africa, but the B antigen is more common in Asians [Reference Loscertales11]. These differences may interact with the prevalence of some infectious diseases including malaria [Reference Abegaz29]. It has been reported that the risk of severe malaria increased in human with blood group B but decreased in those with blood group O [Reference Degarege16, Reference Panda30]. The underlying mechanism of variable susceptibility of malaria in human with different ABO blood group has not been clearly clarified. Shared ABO antigens with Plasmodium, impairment of merozoite penetration in red blood cells, rosetting and cytoadherence are thought to be essential factors in the selected invasion of Plasmodium [Reference Loscertales11]. The protective role of blood group O has intricate mechanisms. Individuals with blood group O may be selectively advantaged from the co-existence of anti-A and anti-B antibodies shared by microorganisms [Reference Saitou and Yamamoto31]. Diversity of surface glycan molecules in red blood cells and ligands such as merozoite surface protein 1, apical membrane antigen 1 and erythrocyte-binding antigen in Plasmodium may also lead to different host susceptibility [Reference Loscertales11, Reference Paul32, Reference Nasr33]. Reduced resetting is potentially another mechanism involved in it [Reference Loscertales11, Reference Rowe34, Reference Vigan-Womas35].
Antibodies against malaria vary with the intensity of transmission and their level may increase over successive pregnancies [Reference Beeson and Duffy36]. The protective immunity against malaria may be weakened in the absence of exposure for a few years [Reference McClure37]. It may also diminish in women during the first pregnancy. Primigravida is actually more susceptible to maternal and placental infection for the lack of immunity to placenta-specific cytoadherence proteins [Reference Maestre and Carmona-Fonseca4, Reference Okoko, Enwere and Ota26, Reference Panda30]. The repeated exposure of particular parasites phenotypes in subsequent pregnancies may increase the protection. The human placenta can provide the parasite with a particular immune environment and induce antigen switch of a different antigen variant (VAR2CSA) in case of Plasmodium falciparum infection. This contributes to the clonal selection observed in placental malarial parasites [Reference Maestre and Carmona-Fonseca4, Reference Gamain38, Reference Beeson and Brown39]. Therefore, the parity specific risk of malaria in pregnant women with different ABO blood group in this meta-analysis is reasonable.
The present study has some limitations. Firstly, the high value of I2 was shown in two groups of the meta-analysis. This suggested heterogeneity of included studies. Different malaria diagnostic and blood group typing methods were potential factors resulting in the heterogeneity. Species of plasmodium studied, malaria prevalence, local socio-economic patterns, life habits of residents were possibly different between included studies. These factors may also lead to the heterogeneity. Secondly, all included studies were conducted in malaria-endemic regions. Data of susceptibility in pregnant women of less endemic regions was absent in reported studies. Thirdly, only five studies satisfied the inclusion criteria and were included in the meta-analysis. More eligible studies are needed to confirm the results of the present study.
Conclusions
The present study showed that the risk of malaria may increase in multiparae with blood group B, but a decrease in those with blood group O. Primiparae with different ABO blood group may have comparable risk. More preventive and interventional methods should therefore be recommended in multiparae with blood group B to reduce the incidence of malaria in this population. However, considering the heterogeneity and a limited number of included studies, more rigorous studies with high quality and large sample size are needed to provide a high level of evidence to confirm these results.
Financial support
This work was supported by the Research Projects Fund of Southwest Medical University (No. 2020ZRQNB001), and the Project Fund of Hospital Affiliated to Southwest Medical University (No. 20091).
Conflict of interest
We declare that we have no competing interests.
Data availability statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.







