CVD has been the leading cause of mortality in the USA for nearly a century( Reference Mozaffarian, Benjamin and Go 1 ). Although numerous risk factors are implicated, epidemiological evidence supports that the magnitude of postprandial hyperglycaemia (PPH) is an independent predictor of CVD-related mortality( 2 ). The mechanisms by which PPH induce CVD are under investigation( Reference Jacome-Sosa, Parks and Bruno 3 , Reference Mah and Bruno 4 ). PPH transiently impairs vascular endothelial function (VEF) in an oxidative stress-dependent manner that limits the bioavailability of nitric oxide (NO∙)( Reference Mah, Noh and Ballard 5 ), an endothelial-derived vasodilator that regulates vascular homoeostasis( Reference Liu and Huang 6 ). Dietary modification is a leading strategy to limit PPH-mediated impairments in VEF either by attenuating PPH and/or limiting downstream oxidative stress responses that induce vascular injury.
PPH following an oral glucose challenge induces oxidative stress as evidenced by increases in the lipid peroxidation biomarkers malondialdehyde( Reference Mah, Noh and Ballard 5 ) and F2-isoprostanes (F2-IsoPs)( Reference Sampson, Gopaul and Davies 7 ). F2-IsoPs are non-enzymatic oxidation products of arachidonic acid (AA)( Reference Sampson, Gopaul and Davies 7 , Reference Ceriello, Esposito and Piconi 8 ) and specific stereoisomers, such as 8-iso-PG F2α (8-iso-PGF2α ), are well associated with CVD( Reference Bauer, Ripperger and Frantz 9 ). F2-IsoPs contribute to CVD by increasing platelet aggregation and immune cell adhesion and inducing vasoconstriction( Reference Bauer, Ripperger and Frantz 9 ). Separate from this, PPH increases the formation of methylglyoxal (MGO), a precursor to advanced glycation end products that provokes inflammation and oxidative stress( Reference Giacco and Brownlee 10 ), which would be expected to contribute to impairments in VEF.
Brachial artery flow-mediated dilation (FMD) is a non-invasive ultrasound measurement of endothelial nitric oxide synthase (eNOS)-mediated, NO∙-dependent vasodilation that assesses VEF and CVD risk( Reference Matsuzawa, Kwon and Lennon 11 , Reference Harris, Nishiyama and Wray 12 ). In healthy men following an oral glucose challenge, PPH was positively correlated with lipid peroxidation and both PPH and lipid peroxidation were inversely correlated with FMD responses( Reference Mah, Noh and Ballard 5 , Reference Ballard, Mah and Guo 13 ). These data indicate that postprandial increases in blood glucose are associated with increases in oxidative stress and decreases in vascular function. Indeed, PPH-mediated oxidative stress is implicated in impairing VEF by reducing NO∙ bioavailability( Reference Mah, Noh and Ballard 5 ).
Findings from observational studies are equivocal regarding egg consumption in relation to CVD risk( Reference Djoussé and Gaziano 14 – Reference Rong, Chen and Zhu 17 ), with some suggesting neutral or even adverse effects on cardiovascular health. In contrast, controlled studies indicate favourable metabolic outcomes of egg consumption on PPH-mediated CVD risk( Reference Blesso, Andersen and Barona 18 – Reference Pelletier, Thouvenot and Belbraouet 22 ). Chronic consumption of eggs improves insulin sensitivity( Reference Blesso, Andersen and Barona 18 ), which would be expected to promote tissue glucose uptake. In healthy men, consumption of whole eggs and egg yolks, when added to a controlled meal (6 % protein, 49 % fat, 45 % carbohydrate) increased circulating cholecystokinin (CCK)( Reference Pelletier, Thouvenot and Belbraouet 22 ), a gastrointestinal hormone that functions to delay gastric empting. This potentially slowed glucose absorption to limit PPH( Reference Pelletier, Thouvenot and Belbraouet 22 ). Chronic ingestion of eggs also attenuates lipid peroxidation( Reference Blesso, Andersen and Barona 18 , Reference Garces-Rimon, Gonzalez and Uranga 23 ) and neither their acute nor chronic ingestion impairs VEF( Reference Katz, Evans and Nawaz 19 – Reference Njike, Faridi and Dutta 21 ). However, no postprandial studies have examined the different fractions of eggs on gut-level CCK responses to limit PPH-mediated oxidative stress leading to impairments in VEF. We hypothesised that co-ingestion of a glucose challenge with egg-based meals would protect against glucose-induced impairments in VEF consistent with a mechanism of delayed gastric emptying that limits PPH and downstream lipid peroxidation. To test this, prediabetic men completed a four-arm, randomised, cross-over trial in which they ingested isoenergetic meals of glucose alone or in combination with egg-based meals. We then assessed VEF and biomarkers of oxidative stress and cardiometabolic health during the 3-h postprandial period.
Methods
Participants
Men with prediabetes (n 20) were enrolled on the basis of age (25–50 years), BMI between 25 and 35 kg/m2, total cholesterol <6·2 mmol/l, and resting systolic and diastolic blood pressure of ≤140 mmHg and ≤90 mmHg, respectively. Middle-aged men were specifically enrolled to circumvent confounding factors of age and sex on FMD responses( Reference Harris, Nishiyama and Wray 12 ). Participants were required to have fasting blood glucose between 5·6 and 6·9 mmol/l according to established criteria for prediabetes( 24 ). They were also required to be non-vegetarian, non-smokers, non-users of dietary supplements or vasoactive medications (>1 month), participate in <7 h/week of aerobic exercise, be weight stable (±2 kg for 3 months), consume <3 alcoholic drinks/d, and self-reported to have no known history of CVD.
Chemicals and reagents
HPLC-grade solvents and the following chemicals were purchased from Fisher Scientific: acetic acid, formic acid, hexane, hydrochloric acid (HCl), methanol, sodium hydroxide (NaOH), acetonitrile, perchloric acid (PCA). 5-methylquinoxaline (5-MQ), o-phenylenediamine (OPD) and MGO were purchased from Sigma-Aldrich. Unlabelled (d0-) and octa-2H-labelled (d8-) AA were purchased from Cayman Chemical.
Study design
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board at The Ohio State University (2014H0307). Written informed consent was obtained from all subjects before enrolling (Fig. 1). This study was registered at ClinicalTrials.gov (NCT02364570). Participants completed a four-arm, randomised, cross-over trial in which they visited the study centre after an overnight fast (10–12 h) on four occasions separated by a washout period of at least 7 d. During each visit, participants ingested isoenergetic meals (about 1674 kJ (400 kcal); Table 1) of either glucose alone (GLU; 100 g in 296 ml water, Trutol®; Thermo Scientific), or glucose (75 g in 296 ml water) in combination with 1·5 whole eggs (EGG), seven egg whites (WHITE) or two egg yolks (YOLK). Eggs were purchased (Giant Eagle) and egg-based meals were scrambled without any additional ingredients and frozen based on mass into single-serve portions at the beginning of the study. Portioned meals were thawed overnight in a ceramic container and microwaved immediately before ingestion. All meals were consumed within 10 min. Each test meal provided participants with a typical energy content of breakfast for American men( 25 ). The amount of glucose provided in the egg-based meals (75 g) is equivalent to that of an oral glucose tolerance test, and has been demonstrated to induce PPH, oxidative stress, and impairments in VEF by our group( Reference Mah, Noh and Ballard 5 ). Blood samples were collected before test meal ingestion at baseline (t=0 min) of each trial and at 30 min intervals during the 3-h postprandial period. Brachial artery FMD, blood pressure, and heart rate were also measured at these time points. Participants’ blood pressure and heart rate were measured using an automated blood pressure monitor (Omron BP760; Omron Healthcare, Inc.).
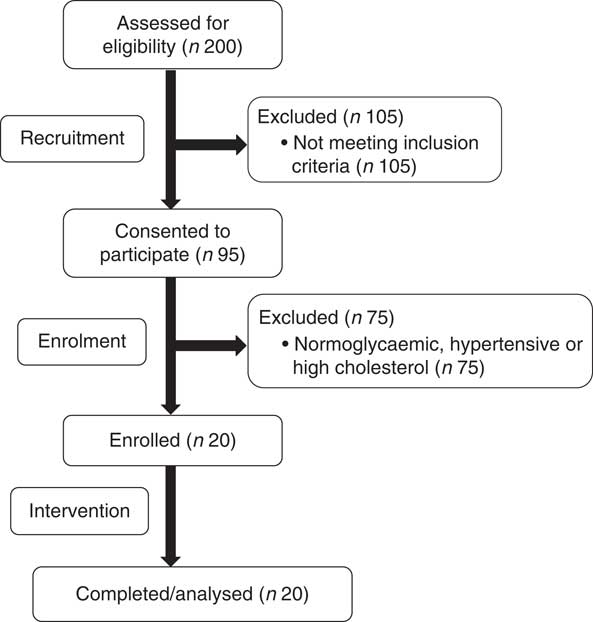
Fig. 1 Recruitment, enrolment, and intervention for prediabetic subjects who participated in the four-arm, randomised, cross-over trial.
Table 1 Energy, mass and macronutrient content of study test meals (Numbers and percentages of energy for each macronutrient within each meal)
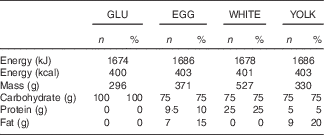
GLU, glucose; EGG, whole eggs; WHITE, egg whites; YOLK, egg yolks.
Sample handling
Venous blood samples were collected into evacuated tubes containing EDTA or sodium heparin from a flexible catheter inserted into the antecubital vein of the left arm. Plasma was obtained following centrifugation (4°C, 15 min, 1500 g ), aliquoted into cryovials, snap-frozen in liquid N2, and stored at −80°C until analysis.
Dietary modifications and analysis
Participants were instructed by a registered dietitian to consume identical euenergetic diets, free of major sources of eggs, for each 3-d period before each trial. They were also instructed to abstain from exercise, caffeine and alcohol for 48 h before each trial to prevent their confounding effects on FMD responses( Reference Harris, Nishiyama and Wray 12 ). Energy and nutrient intakes were assessed by 3-d food records before each trial and were analysed using Nutrition Data System for Research (University of Minnesota, NDSR 2015).
Ultrasound measures of vascular health
Carotid intima-media thickness (cIMT), an index of atherosclerosis risk, was assessed at baseline of each trial as described( Reference Stein, Korcarz and Hurst 26 ). In brief, a 13-MHz ultrasound transducer connected to a high-resolution T300 ultrasound system (Terason) was used to image the far wall of both the left and right common carotid arteries. Images were recorded for 10 s and analysed using edge-detection software (Carotid Analyzer for Research, Medical Imaging Applications). cIMT did not differ (P>0·05) among trials and mean values are reported.
FMD was assessed using high-frequency ultrasound as we described( Reference Mah, Noh and Ballard 5 , Reference Ballard, Mah and Guo 13 ). In brief, the brachial artery of the right arm was visualised by placing the transducer above the antecubital crease using the aforementioned ultrasound system. Pre-occlusion brachial artery diameter was recorded for 1 min, after which an automated blood pressure cuff (Hokanson E20; Hokanson, Inc.) placed immediately distal to the olecranon process of the right arm was rapidly inflated to perform lower arm occlusion (200 mmHg, 5 min). Post-occlusion vessel diameter recordings were initiated 1 min before cuff deflation and for 3 min thereafter. Pre- and post-occlusion images were analysed using edge-detection software (Medical Imaging Applications). Brachial artery FMD (%) was calculated as: (peak post-occlusion diameter (mm)−pre-occlusion diameter (mm))/pre-occlusion diameter (mm)×100. All FMD measurements were performed by the same technician and images were analysed in a blinded manner.
Clinical chemistries
Plasma glucose, total cholesterol, HDL-cholesterol, and TAG were measured according to the manufacturer’s instructions (Pointe Scientific) using a UV2600 spectrophotometer (Shimadzu). LDL-cholesterol was calculated according to the Friedwald equation( Reference Friedewald, Levy and Fredrickson 27 ). Plasma insulin (ALPCO) and CCK (LifeSpan Biosciences, Inc.) were measured by ELISA according to the manufacturers’ instructions using a Synergy H1 microplate reader (Biotek Instruments). The homoeostatic model assessment of insulin resistance (HOMA-IR) was calculated from plasma collected at baseline (t=0 min) of each invention day as follows: plasma insulin (μIU/ml)×plasma glucose (mmol/l)/22·5( Reference Matthews, Hosker and Rudenski 28 ). HOMA-IR did not differ (P>0·05) among trials and mean values are reported. Plasma F2-IsoPs were assessed by ELISA by measuring levels of 8-iso-PGF2α according to the manufacturer’s instructions (Enzo Life Sciences, Inc.).
Plasma arachidonic acid
To better define postprandial alterations in F2-IsoPs, plasma AA was measured as described( Reference Taylor, Bruno and Frei 29 ), with modifications to use an LCMS-2020 instrument (Shimadzu). In brief, 100 μl of plasma was mixed with 900 μl of water and 1 ml of 1 m NaOH in methanol before saponification (60°C, 30 min). The saponified sample was acidified to pH 2·85–3·0 using 600 μl of 3 M-HCl and 15 μl d8-AA (50 μm final concentration) was added as internal standard. The mixture was extracted with hexane and centrifuged (2500 g , 10 min, 25°C) and supernatant was dried under N2 gas before resolubilisation with methanol containing 0·1 % formic acid. Samples were then injected onto the liquid chromatography-MS system equipped with an autosampler maintained at 4°C (SIL-20AC), a degassing unit (DGU-20A5), a column oven set to 30°C (CTO-20A) and two LC-30AD pumps. Instrument control was performed using Shimadzu LabSolution (version 5.7). Samples were separated on a Kinetex C18 column (100×2·1 mm, 2·6 μm; Phenomenex) using an isocratic flow rate of 0·25 ml/min and mobile phase consisting of methanol containing 0·05 % acetic acid. Nebulising and drying gases were supplied at 1·5 and 15 litres/m, and block and desolvation line temperatures were 450 and 300°C, respectively. Detection was performed using single-ion monitoring following electrospray ionisation performed in negative mode. Endogenous AA (d0-AA) and d8-AA (internal standard) were quantified at a mass:charge ratio (m/z) of 303 and 310, respectively.
Plasma methylglyoxal
Plasma MGO was measured using HPLC-UV as described( Reference Masterjohn, Mah and Guo 30 ), with minor modifications. In brief, MGO standard or 500 μl of EDTA plasma was mixed with 100 μl 2·8 m PCA and 30 μl 212·68 mm OPD, respectively; 4 μl 5-MQ (11·5 nm final concentration) was added as an internal standard. Following incubation (25°C in the dark for 24 h) in a shaking water bath to derivatise MGO to 2-methylquinoxaline (2-MQ), samples were centrifuged (10 min, 15 000 g , 4°C) and the supernatant filtered (0·22 μm) before injecting onto a Waters Alliance 2965 HPLC system. 2-MQ and 5-MQ were separated at 1·0 ml/min on a Nov-Pak C18 column (150×3·9 mm, 4 μm; Waters) using a binary gradient of mobile phase A (20 % acetonitrile in water) and mobile phase B (100 % methanol) as follows: 0–12 min, 0 % B, 12–14 min, 80 % B, 14–16 min, 100 % B, and 16–20 min, 0 % B. 2-MQ and 5-MQ were detected at 315 nm and quantified using peak area relative to internal standard based on a standard curve prepared in parallel.
Statistical methods
Sample size was determined using data from previous work examining postprandial changes in FMD responses following an oral glucose challenge( Reference Mah, Noh and Ballard 5 ). Our power calculation indicated a minimum of nine subjects were needed to reject the null hypothesis with 90 % power (P<0·05). Data are expressed as means with their standard errors and were analysed using GraphPad Prism (version 7). Data reported are change from baseline (Δ) to better visualise between-treatment effects during the postprandial period. Time, treatment and time×treatment interaction effects for postprandial responses for FMD and plasma biomarkers were evaluated using two-way repeated-measures (RM) ANOVA with Bonferroni’s correction to evaluate pairwise differences. AUC during the 180 min postprandial period (AUC0–180 min) was calculated using the trapezoidal rule. Between-trial effects on dietary intakes, postprandial AUC0–180 min, and baseline values were evaluated using one-way RM ANOVA with Bonferroni’s posttest. Correlation coefficients (r) were calculated using multiple linear regression while controlling for within-subject RM( Reference Bland and Altman 31 ). P<0·05 was considered statistically significant for all analyses.
Results
Participants and dietary intakes
All enrolled participants (n 20) completed the study in its entirety without any adverse effects (Fig. 1). Participants had fasting plasma glucose indicative of prediabetes (Table 2). On average, participants were considered obese on the basis of BMI, although five of the twenty participants had a BMI indicative of being overweight. They were also normolipidaemic, normotensive and not at increased risk for CVD based on established cIMT criteria( Reference Stein, Korcarz and Hurst 26 ). According to HOMA-IR, values were indicative of insulin resistance based on established criteria( Reference Gayoso-Diz, Otero-González and Rodriguez-Alvarez 32 ). Participants’ energy and nutrient intakes did not differ among trials with the exception that α-tocopherol intakes were lower during GLU and WHITE compared with EGG and YOLK (online Supplementary Table S1). However, α-tocopherol intakes were substantially lower than dietary recommendations regardless of trial, consistent with 92 % of American men failing to meet dietary recommendations( Reference Maras, Bermudez and Qiao 33 ).
Table 2 Participant characteristics (Mean values with their standard errors; n 20)
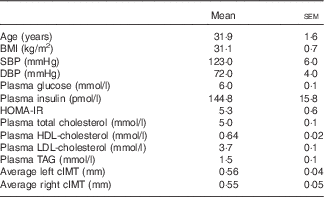
SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homoeostatic model assessment of insulin resistance; cIMT, carotid intima-media thickness.
Brachial artery flow-mediated dilation
Fasting FMD responses were not different (P>0·05) among trials (Table 3). Postprandial changes in FMD (ΔFMD, %) from baseline (i.e. t=0 min) showed main effects due to time, treatment, and time×treatment interaction effects (P<0·0001; Fig. 2). FMD responses decreased relative to baseline at 30–60 min in GLU and 30–180 min in YOLK, but only at 30 min in EGG and WHITE. Compared with GLU and YOLK, decreases in FMD were attenuated at 30–60 min in EGG and WHITE, but YOLK was not different from GLU. FMD responses were lower in YOLK compared with WHITE at 90–180 min and EGG at 150–180 min. This was corroborated by AUC0–180 min which was similarly higher in EGG and WHITE compared with GLU (P<0·0001; Fig. 2). Although FMD responses were lower in YOLK at 90–180 min compared with GLU, this was only significant at 150 min and did not result in a significant difference in AUC0–180min. Blood pressure and heart rate did not differ among treatments at baseline or postprandially (P>0·05; data not shown). Pre-occlusion and maximal post-occlusion diameter and shear rate AUC did not differ among treatments (P>0·05; Supplementary Table S2). These data suggest that co-ingestion of glucose with whole egg and egg white meals, but not yolks, protects against postprandial impairments in VEF.
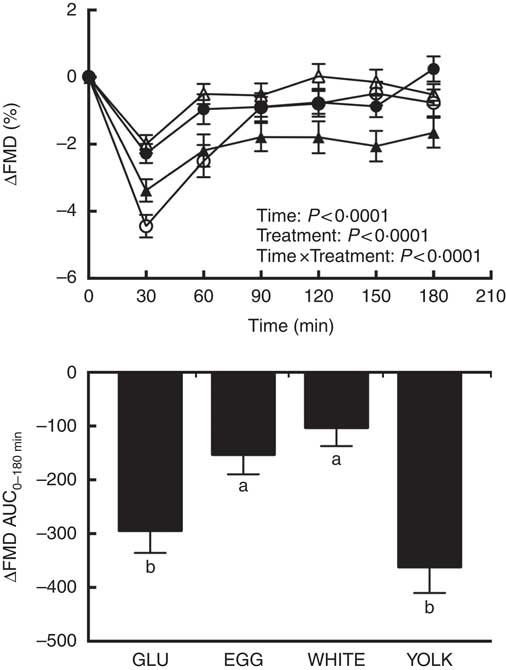
Fig. 2 Postprandial flow-mediated dilation (FMD) responses and AUC0–180 min following ingestion of glucose in the absence or presence of egg-based meals by prediabetic men. Postprandial responses were analysed using two-way repeated-measures (RM) ANOVA with Bonferroni’s post hoc test. AUC0–180 min was calculated using the trapezoidal rule and analysed using one-way RM ANOVA with Bonferroni’s post hoc test. Values are means (n 20), with their standard errors. ![]() , Glucose (GLU);
, Glucose (GLU); ![]() , whole eggs (EGG);
, whole eggs (EGG); ![]() , egg whites (WHITE);
, egg whites (WHITE); ![]() , egg yolk (YOLK). a,b Mean values with unlike letters are significantly different (P<0·05).
, egg yolk (YOLK). a,b Mean values with unlike letters are significantly different (P<0·05).
Table 3 Baseline values for flow-mediated dilation (FMD) and plasma biomarkers from each intervention arm (Mean values with their standard errors; n 20)
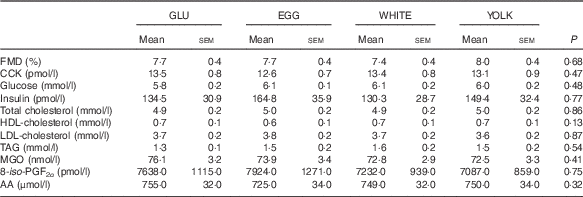
GLU, glucose; EGG, whole eggs; WHITE, egg whites; YOLK, egg yolks; CCK, cholecystokinin; MGO, methylglyoxal; 8-iso-PGF2α , 8-isoprostaglandin-F2α ; AA, arachidonic acid.
Plasma cholecystokinin, glucose, and insulin
Fasting concentrations of plasma glucose, insulin, and CCK were not different among study visits (Table 3). Postprandial changes in CCK (ΔCCK) showed treatment effects only. At 30 min and 120 min in WHITE and 90–180 min in EGG, CCK levels were greater compared with GLU (P<0·05; Fig. 3(A)). Egg-mediated changes in CCK were corroborated by analysis of AUC0–180 min (P<0·01; Fig. 3(A)) which was similarly higher in EGG and WHITE compared with GLU, whereas YOLK was not different from GLU or other egg-based meals.
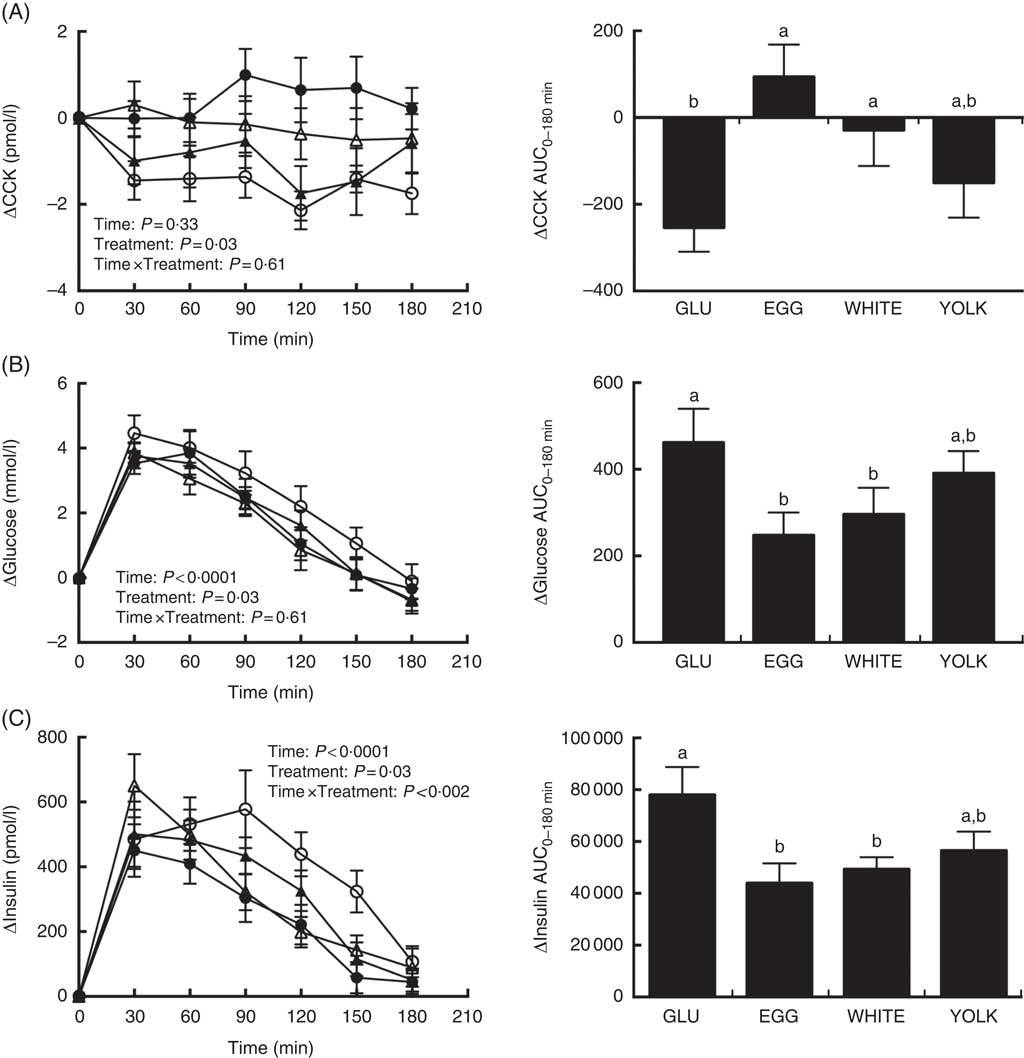
Fig. 3 Postprandial cholecystokinin (CCK) (A) glucose (B) and insulin (C) responses and AUC0–180min following ingestion of glucose in the absence or presence of egg-based meals by prediabetic men. Postprandial responses were analysed using two-way repeated-measures (RM) ANOVA with Bonferroni’s post hoc test. AUC0–180 min was calculated using the trapezoidal rule and analysed using one-way RM ANOVA with Bonferroni’s post hoc test. Values are means (n 20), with their standard errors. ![]() , Glucose (GLU);
, Glucose (GLU); ![]() , whole eggs (EGG);
, whole eggs (EGG); ![]() , egg whites (WHITE);
, egg whites (WHITE); ![]() , egg yolk (YOLK). a,b Mean values with unlike letters are significantly different (P<0·05).
, egg yolk (YOLK). a,b Mean values with unlike letters are significantly different (P<0·05).
Postprandial levels of ΔGlucose showed time and treatment effects (Fig. 3(B)). Specifically, plasma glucose increased at 30–120 min relative to baseline in GLU and YOLK but only at 30–90 min relative to baseline in EGG and WHITE. Furthermore, increases were significantly attenuated at 120 min in EGG and WHITE relative to GLU, whereas YOLK was not different from GLU. AUC0–180 min of ΔGlucose showed that EGG and WHITE were similarly lowered compared with GLU whereas YOLK was not different from GLU, EGG or WHITE.
ΔInsulin showed time, treatment and time×treatment interactive effects (Fig. 3(C)) such that insulin increased relative to baseline at the following times in each treatment: GLU (30–150 min), EGG (30–120 min), WHITE (30–90 min), YOLK (30–120 min). Compared with GLU, insulin increased to a lesser extent in EGG and WHITE at 90–150 min. In addition, insulin increased to a greater extent at 30 min in WHITE compared with EGG. AUC0–180 min of postprandial insulinaemia showed similarly lower levels in EGG and WHITE compared with GLU, whereas YOLK was not different from GLU or other egg-based meals. In addition, ΔFMDAUC and ΔInsulinAUC were negatively correlated (r −0·28, P<0·05), suggesting that lowering of insulinaemia is associated with improved VEF. Collectively, these data suggest that vasoprotection in EGG and WHITE is likely due to attenuating glycaemia and insulinaemia, which may be attributed to a CCK-mediated delay in gastric emptying.
Plasma lipids
Fasting concentrations of plasma TAG and total, HDL and LDL-cholesterol did not differ among study trials (Table 3) nor did their respective postprandial AUC0–180 min responses differ among treatments (Fig. 4). However, there were main effects due to time (Fig. 4(A)–(D)). Plasma TAG showed a significant main effect of time. However, this occurred without any statistically significant post hoc effects for time. Plasma total cholesterol decreased postprandially and failed to return to baseline levels by 180 min. Plasma LDL-cholesterol followed the same trend as total cholesterol. Plasma HDL-cholesterol decreased at 60–150 min relative to baseline before returning to baseline by 180 min.
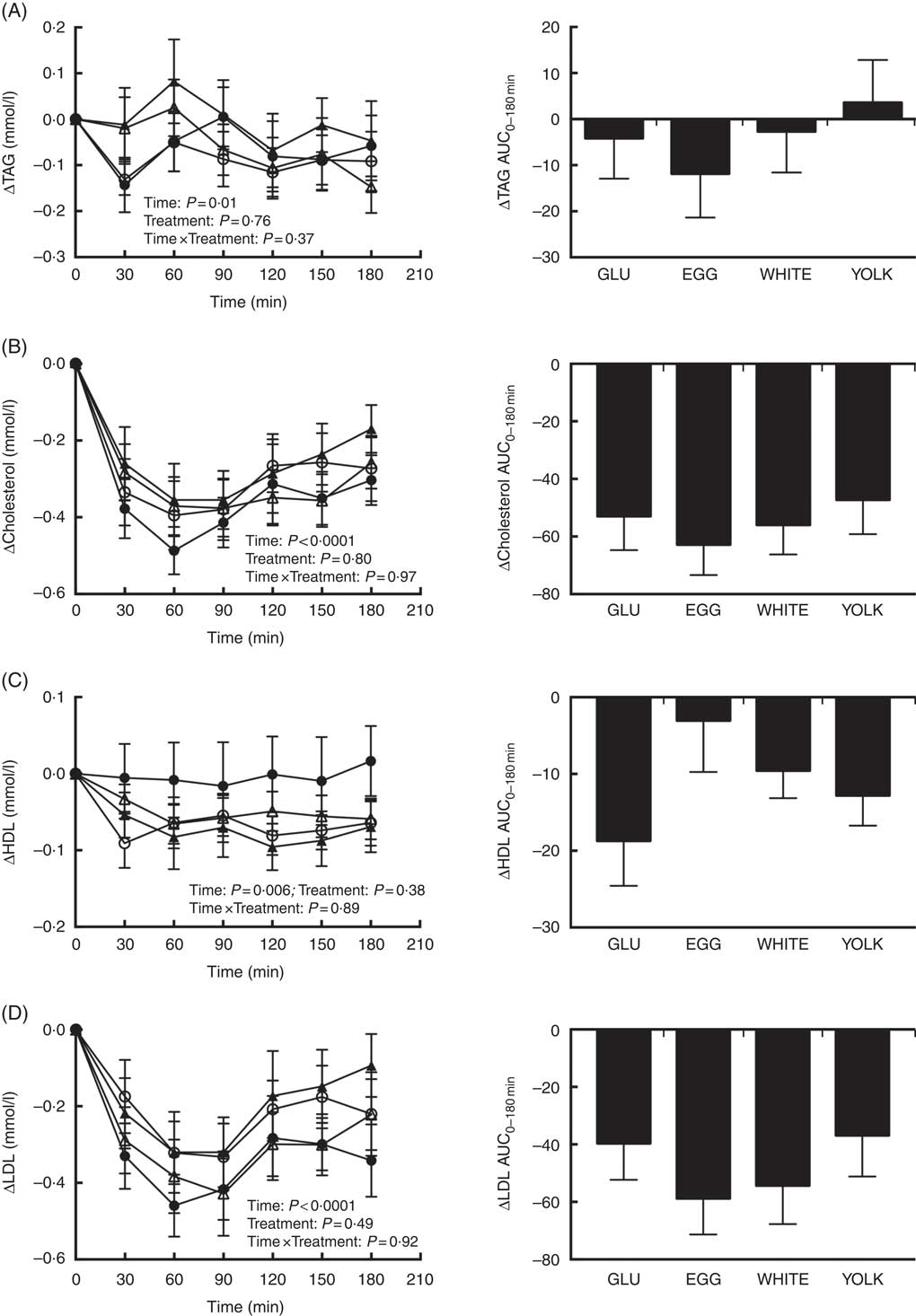
Fig. 4 Postprandial TAG (A), total cholesterol (B), HDL-cholesterol (C) and LDL-cholesterol (D) responses and AUC0–180 min following ingestion of glucose in the absence or presence of egg-based meals by prediabetic men. Postprandial responses were analysed using two-way repeated-measures (RM) ANOVA with Bonferroni’s post hoc test. AUC0-180 min was calculated using the trapezoidal rule and analysed using one-way RM ANOVA with Bonferroni’s post hoc test. Values are means (n 20), with their standard errors. ![]() , Glucose (GLU);
, Glucose (GLU); ![]() , whole eggs (EGG);
, whole eggs (EGG); ![]() , egg whites (WHITE);
, egg whites (WHITE); ![]() , egg yolk (YOLK). AUC of TAG, cholesterol, HDL and LDL did not differ among treatments (P>0·05).
, egg yolk (YOLK). AUC of TAG, cholesterol, HDL and LDL did not differ among treatments (P>0·05).
Plasma methylglyoxal
Fasting MGO concentrations did not differ among trials (Table 3). Plasma ΔMGO responses were unaffected by treatment, consistent with no difference in AUC0–180 min between treatments despite EGG and WHITE being 37–45 % lower than GLU (Fig. 5). However, MGO response did exhibit a statistically significant main effect for time. Specifically, plasma MGO levels were increased at 30–120 min postprandially relative to baseline before returning to levels no different from baseline by 150 min. MGO levels peaked at 30 min, which was greater (P<0·05) than levels at 90–180 min. Additionally, MGO levels were greater at 60 min relative to 90–180 min, at 90 min relative to 120–150 min and at 120 min relative to 150–180 min.
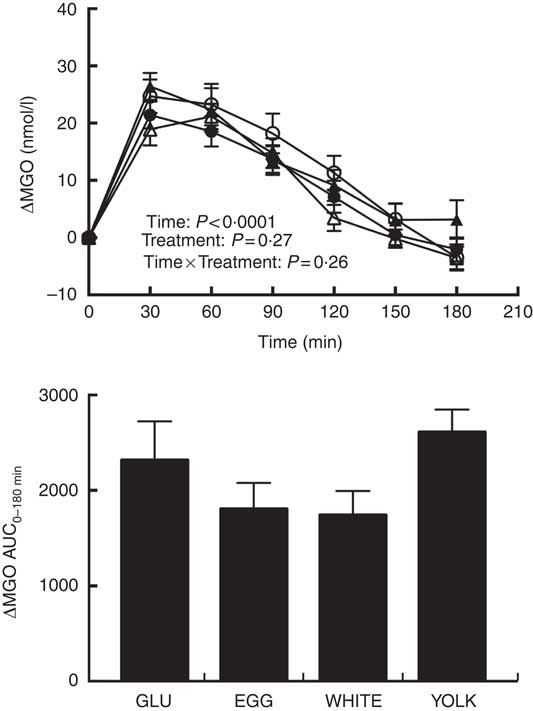
Fig. 5 Postprandial methylglyoxal (MGO) responses and AUC0–180 min following ingestion of glucose in the absence or presence of egg-based meals by prediabetic men. Postprandial responses were analysed using two-way repeated-measures (RM) ANOVA with Bonferroni’s post hoc test. AUC0–180 min was calculated using the trapezoidal rule and analysed using one-way RM ANOVA with Bonferroni’s post hoc test. Values are means (n 20), with their standard errors. ![]() , Glucose (GLU);
, Glucose (GLU); ![]() , whole eggs (EGG);
, whole eggs (EGG); ![]() , egg whites (WHITE);
, egg whites (WHITE); ![]() , egg yolk (YOLK). MGO AUC did not differ among treatments (P>0·05).
, egg yolk (YOLK). MGO AUC did not differ among treatments (P>0·05).
Plasma F2-isoprostanes and arachidonic acid
Baseline concentrations of 8-iso-PGF2α and AA did not differ among trials (Table 3). Main effects due to time and treatment were observed for postprandial changes in plasma 8-iso-PGF2α (Fig. 6(A)). Relative to baseline, Δ8-iso-PGF2α levels increased at 60–150 min in GLU, 120 min in EGG, 90–120 min in WHITE, and 30–150 min in YOLK. In addition, compared with GLU, increases were attenuated at 90–120 min in WHITE and 90–150 min in EGG, whereas YOLK did not differ from GLU at any time point. Although we observed treatment effects at the aforementioned time points postprandially, we did not observe any differences in AUC despite 39–54 % lower values in EGG and WHITE compared with GLU. Because F2-IsoPs are derived from AA, we calculated the ratio of 8-iso-PGF2α :AA as an indicator of the extent of lipid peroxidation. ΔAA decreased postprandially regardless of treatment (P<0·01), with levels significantly lower at 90–180 min compared with baseline (Fig. 6(B)). We therefore normalised changes in 8-iso-PGF2α to changes in AA (Fig. 6(C)). Data showed significant time, treatment and time×treatment interactive effects in Δ8-iso-PGF2α :ΔAA. Relative to baseline, the ratio of 8-iso-PGF2α :AA increased at 60–180 min in GLU, 90–120 min in EGG, 90–150 min in WHITE and 30–180 min in YOLK. In addition, 8-iso-PGF2α :AA was increased to a lesser extent at 90–180 min in EGG and WHITE compared with GLU, with YOLK not different from GLU. Likewise, AUC0–180 min was similarly lower in EGG and WHITE compared with GLU, whereas YOLK was not different from GLU and higher than EGG and WHITE. This finding suggests egg- and egg white-based meals protect against PPH-mediated impairments in VEF by attenuating lipid peroxidation.
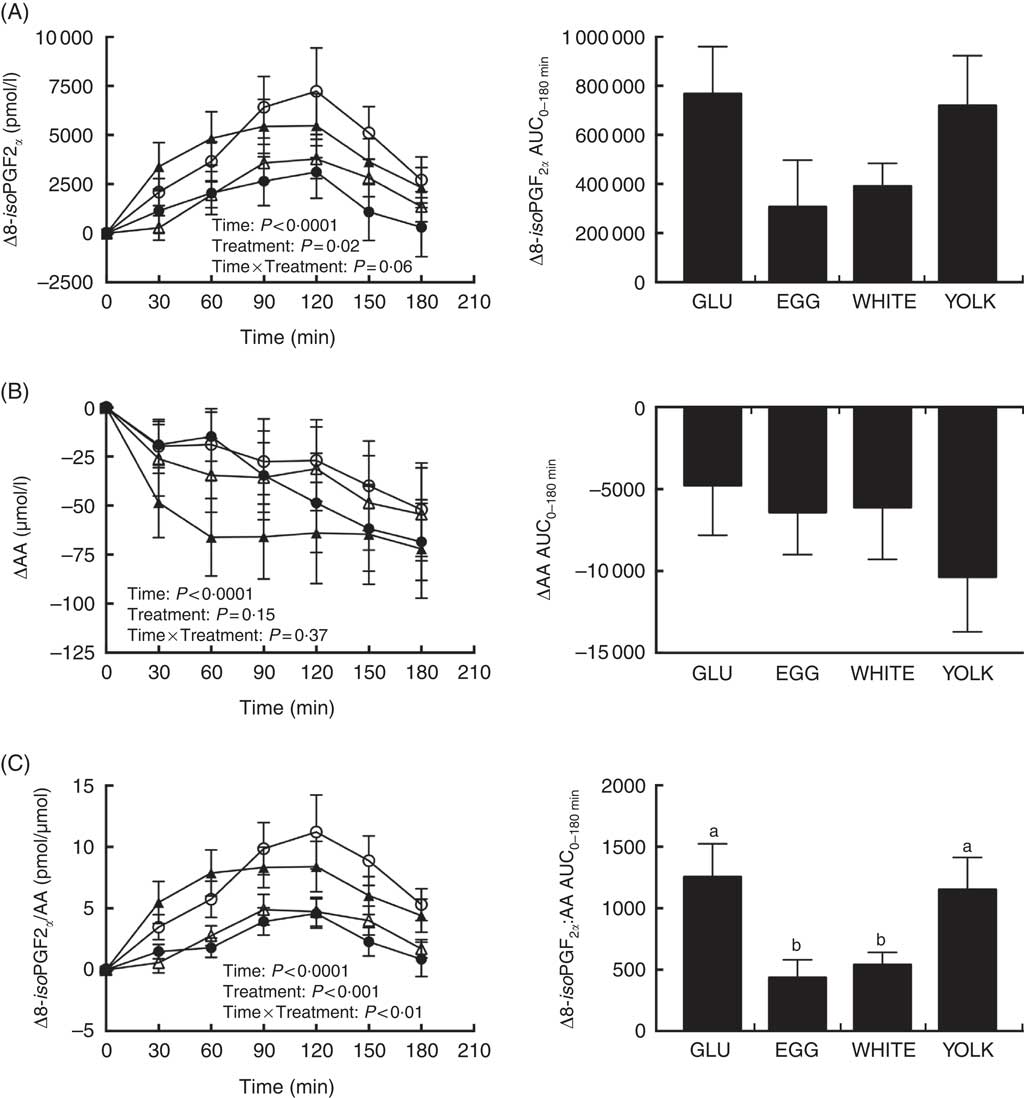
Fig. 6 Postprandial responses and AUC0–180 min for 8-isoprostaglandin F2α (8-iso-PGF2α) (A), arachidonic acid (AA) (B), and the ratio of 8-iso-PGF2α:AA (C) following ingestion of glucose in the absence or presence of egg-based meals by prediabetic men. Postprandial responses were analysed using two-way repeated-measures (RM) ANOVA with Bonferroni’s post hoc test. AUC0–180 min was calculated using the trapezoidal rule and analysed using one-way RM ANOVA with Bonferroni’s post hoc test. Values are means (n 20), with their standard errors. ![]() , Glucose (GLU);
, Glucose (GLU); ![]() , whole eggs (EGG);
, whole eggs (EGG); ![]() , egg whites (WHITE);
, egg whites (WHITE); ![]() , egg yolk (YOLK). a,b Mean values with unlike letter are significantly different (P<0·05).
, egg yolk (YOLK). a,b Mean values with unlike letter are significantly different (P<0·05).
Discussion
This study demonstrates that co-ingestion of whole eggs with glucose protects against PPH-mediated impairments in VEF by limiting lipid peroxidation independent of changes in MGO or circulating lipids. This is potentially mediated by the egg white fraction, based on whole egg and egg white meals similarly attenuating glycaemia and lipid peroxidation. Vasoprotective activities of whole eggs and egg whites occurred in association with gut-level improvements in CCK, which would be expected to limit PPH-mediated lipid peroxidation by delaying gastric emptying. Thus, simply reducing the glucose content in egg-based meals did not confer vasoprotection, but rather replacing carbohydrate with either whole eggs or egg whites limits postprandial impairments in VEF otherwise induced by glucose ingestion.
Test meals were formulated to be isoenergetic rather than equal in carbohydrate. This approach was undertaken specifically to rule out confounding effects of varying energy intakes on postprandial responses. This is consistent with others showing that carbohydrate quantity in test meals that varied in energy content differentially affected postprandial glycaemia( Reference Wolever and Bolognesi 34 ). In addition, the use of egg-based test meals served as a modifiable food matrix to investigate the influence of the macronutrient ratio on postprandial responses. Findings show an inter-related role between the intestinal hormone CCK, PPH, and downstream lipid peroxidation, suggesting that whole egg and egg white-based meals mediate vasoprotection along the gut-vessel axis. Data show that co-ingestion of glucose with whole eggs and egg whites, but not egg yolks, attenuates postprandial decreases in FMD otherwise lowered by glucose ingestion alone. Reducing glucose quantity did not improve FMD responses, but rather the macronutrient being substituted in lieu of glucose played an important role. Indeed, meals containing the egg white fraction attenuated postprandial impairments in VEF as assessed by FMD, suggesting that egg-fraction composition differences in meals regulate VEF. Future studies are warranted to examine dose–response effects of egg whites and yolks in relation to whole eggs on postprandial vascular health.
Bioactive peptides resulting from enzymatic hydrolysis of egg white and yolk proteins exhibit blood pressure-lowering activities in spontaneously hypertensive rats (SHR)( Reference Miguel, Lopez-Fandino and Ramos 35 , Reference Yoshii, Tachi and Ohba 36 ). However, feeding egg white hydrolysates in SHR compared with egg yolk hydrolysates resulted in a greater lowering of blood pressure( Reference Jahandideh, Majumder and Chakrabarti 37 ). Contrary to those findings, blood pressure was unaffected by egg treatments in our study, and likely reflects participants’ normotensive status. Nonetheless, mesenteric arteries of SHR treated with whole egg hydrolysates( Reference Jahandideh, Majumder and Chakrabarti 37 ) or the egg white-derived peptide ovokinin( Reference Matoba, Usui and Fujita 38 ) exhibited greater NO∙-dependent vasorelaxation consistent with our findings of egg-mediated improvements in FMD responses that reflect NO∙-dependent vasodilation( Reference Harris, Nishiyama and Wray 12 ).
In hyperlipidaemic adults, 6 weeks daily consumption of 0·5 cups egg substitute (99 % egg whites), but not an equal amount of protein from whole eggs, improved fasting FMD responses( Reference Njike, Faridi and Dutta 21 ). To the contrary, we show improvements in FMD following consumption of either whole eggs or egg whites. Differences are likely due to study duration in which our postprandial study had strict dietary control. We also acknowledge that differences in protein quantity may also explain vasoprotection by WHITE (25 g) and EGG (9·5 g) compared with YOLK (5 g). No postprandial studies have examined the dose-dependent effect of egg protein on glycaemia and FMD. However, postprandial studies demonstrate that glycaemic responses were dose-dependently attenuated by increasing amounts of whey protein isolate that was co-ingested with glucose( Reference Paterson, Smart and Lopez 39 ). Thus, future studies are needed to determine differential and dose-dependent effects of egg white and egg yolk proteins with a carbohydrate challenge on postprandial glycaemia and FMD.
Gastric emptying helps to regulate postprandial glycaemia, indicating a role of gut physiology on postprandial VEF. CCK functions to delay gastric emptying and its secretion is greatest in response to dietary protein( Reference Brennan, Luscombe-Marsh and Seimon 40 ). In agreement, we showed that CCK was greatest in response to meals containing whole eggs and egg whites. This is consistent with others showing greater CCK following the co-ingestion of whole eggs with a standardised mixed-meal compared with the mixed meal alone( Reference Pelletier, Thouvenot and Belbraouet 22 ). This may be attributed to whole egg hydrolysates increasing CCK in murine endocrine cells( Reference Geraedts, Troost and Fischer 41 ) and egg white peptides inhibiting intestinal proteases( Reference Kovacs-Nolan, Phillips and Mine 42 ) that would otherwise digest intraluminal CCK releasing factors leading to lower levels of CCK( Reference Liddle 43 ). Contrary to these findings, we showed greater CCK levels following consumption of the mixed-meal with egg whites but not egg yolks. These differences may reflect the higher energy content of test meals and/or varying quantity of egg components (i.e. two whites v. two yolks) used in earlier studies( Reference Pelletier, Thouvenot and Belbraouet 22 ). Differences in participant health status may also account for observed changes in that we studied prediabetic, obese adults rather than healthy adults( Reference Pelletier, Thouvenot and Belbraouet 22 ). Indeed, obese mice fed a high-fat diet had lower small intestinal CCK expression( Reference Duca, Swartz and Sakar 44 ). Obese adults also exhibit greater CCK following a high-protein meal compared with a high-fat meal( Reference Brennan, Luscombe-Marsh and Seimon 40 ). Although increasing the solid mass content of a solid/liquid meal delays gastric emptying( Reference Collins, Horowitz and Maddox 45 ), which would be expected to be reflected by greater levels of CCK, we did not observe any increases in CCK based on increasing solid food mass in egg-based meals. Future studies are needed to examine egg white-derived bioactive peptides on other gut hormones (e.g. PYY, GLP-1) that also regulate gastric emptying.
Whole egg and egg white meals attenuated postprandial glycaemia compared with glucose ingestion alone, which is expected due to decreased carbohydrate quantity. However, glycaemic responses following ingestion of the egg yolk meal was not different compared with glucose ingestion alone, demonstrating that simply reducing carbohydrate content did not lead to an attenuation of glycaemia. Whole eggs as part of a standard breakfast limited postprandial glycaemia in healthy adults( Reference Pelletier, Thouvenot and Belbraouet 22 ). Chronic ingestion of 2 eggs/d as part of a high-protein diet for 12 weeks also improved 2-h blood glucose following a glucose challenge in adults with the metabolic syndrome (MetS)( Reference Blesso, Andersen and Barona 18 ). Inclusion of protein with a glucose challenge also attenuated postprandial glycaemia, likely by delaying gastric emptying, as protein is a potent stimuli for gastric regulatory hormones( Reference Karamanlis, Chaikomin and Doran 46 ). We observed that peak insulin concentrations (30 min) were greater in WHITE compared with EGG, which may have aided in glucose uptake. Whole egg and egg white, but not egg yolk meals, attenuated postprandial insulinaemia compared with glucose ingestion alone. Insulin binding to its receptor results in phosphorylation of its substrate (IRS-1). This leads to the activation of protein kinase B (Akt), which phosphorylates eNOS at Ser1177 and increases its activity to stimulate NO∙ production( Reference Muniyappa and Sowers 47 ). However, despite increased insulin concentrations during GLU compared with EGG and WHITE, we observed that postprandial FMD responses declined. This may be due to oxidative stress impairing insulin signalling( Reference Muniyappa and Sowers 47 ), but future studies are needed to determine any protective effect of egg whites and yolks on oxidative-stress mediated impairments in insulin signalling.
Glucose induces oxidative stress through increased formation of mitochondrial superoxide, accumulation of proinflammatory mediators and advanced glycation end products, and activity of enzymes (e.g. protein kinase C, NADPH oxidase) that contribute to the generation of reactive oxygen species (ROS)( Reference Jacome-Sosa, Parks and Bruno 3 , Reference Mah and Bruno 4 , Reference Giacco and Brownlee 10 ). Regardless of source, glucose-induced increases in ROS decrease NO∙ bioavailability thereby leading to vascular dysfunction( Reference Mah and Bruno 4 , Reference Cai and Harrison 48 ). PPH has been shown to increase MGO( Reference Masterjohn, Mah and Guo 30 ). MGO also increases mitochondrial ROS generation( Reference Brouwers, Niessen and Haenen 49 ), and increases in MGO following glucose ingestion impairs VEF as assessed by FMD in dogs( Reference Adolphe, Drew and Huang 50 ). However, in this study, improvements in VEF were independent of changes in MGO as responses did not differ between test meals. In addition, improvements in VEF occurred independent of changes in plasma lipids. Thus, we measured F2-IsoPs/AA to examine PPH-mediated lipid peroxidation on VEF( Reference Bauer, Ripperger and Frantz 9 ). We provide novel evidence that co-ingestion of glucose with whole eggs or egg whites attenuated increases in F2-IsoPs/AA. This suggests that the vasoprotective activities of whole eggs and eggs whites are due to limiting glycaemia and downstream lipid peroxidation, consistent with evidence that oxidative stress is positively correlated with the magnitude of PPH and that PPH and lipid peroxidation are inversely related with FMD( Reference Mah, Noh and Ballard 5 , Reference Ballard, Mah and Guo 13 ). In diabetic adults, F2-IsoPs increased following a glucose challenge( Reference Sampson, Gopaul and Davies 7 ) and both whole eggs and a yolk-free substitute lowered plasma oxidised LDL in the MetS adults( Reference Blesso, Andersen and Barona 18 ). Although hydrolysates from egg whites and yolks exhibit free-radical scavenging activity( Reference Davalos, Miguel and Bartolome 51 , Reference Nimalaratne, Lopes-Lutz and Schieber 52 ), we show that egg yolk meals failed to attenuate lipid peroxidation, suggesting a more prominent role of bioactive constituents of egg whites to improve VEF.
Separate from serving as a marker of oxidative stress, F2-IsoPs provoke CVD by modulating platelet activation, vascular inflammation, inducing vasoconstriction and vascular smooth muscle cell proliferation, inhibiting angiogenesis, and dysregulating cardiac ion channels( Reference Bauer, Ripperger and Frantz 9 ). Furthermore, AA can be enzymatically (i.e. cyclooxygenase, lipoxygenase) oxidised to prostaglandins, thromboxanes, and leukotrienes, which exert similar action as F2-IsoPs( Reference Needleman, Turk and Jakschik 53 ). The physiologic roles of F2-IsoPs and AA metabolites to negatively affect VEF occur separate from directly reducing NO∙ bioavailability (i.e. NO∙-independent). Thus, more research is needed to examine NO∙-dependent and -independent pathways on VEF in response to egg-based meals. Prior postprandial studies in adults with the MetS suggest no sex-specific effects( Reference Ballard, Mah and Guo 13 ), therefore women were not included, but future studies should examine the effects of sex hormones on responses to egg-based meals.
In conclusion, this study shows that replacing a portion of an oral glucose challenge with whole eggs or egg whites, but not yolks, protects against postprandial decreases in VEF by attenuating glycaemia and oxidative stress. Co-ingestion of carbohydrate-based meals with whole eggs or egg whites may serve as an effective dietary approach to mitigate PPH-mediated impairments in VEF that otherwise increases CVD risk. However, future studies are needed to examine the vasoprotective benefits of eggs as part of more complex mixed meals. Nonetheless, in the absence of validated dietary strategies, the accumulation of acute insults mediated by PPH at the vascular endothelium that contribute to CVD risk will remain problematic. This is of public health importance as half of adult Americans have prediabetes or diabetes( Reference Menke, Casagrande and Geiss 54 ), and humans spend the majority of their day in the postprandial state.
Acknowledgements
The authors thank Kevin Schill for their assistance with J. D. M. in coordinating the study and analysis of vascular data.
The present study was funded by the American Egg Board/Egg Nutrition Center. American Egg Board/Egg Nutrition Center had no role in the design, analysis, or writing of this article.
The authors contributions are as follows: R. S. B., J. S. V. and E. M. were responsible for the study design; A. N. L. analysed participant dietary records. J. D. M., C. C., J. L., E. J. R. and K. D. B. were responsible for collecting and analysing data; and J. D. M. and R. S. B. wrote the initial draft of the manuscript and all authors contributed to the editing and review of this manuscript. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517003610












