1. Introduction
The type species of the helicoprionid (Eugeneodontiformes, Chondrichthyes) genus Campyloprion, Campyloprion annectans, was described by Eastman (Reference Eastman1902) from an incompletely preserved symphyseal tooth whorl presumed to be from the Pennsylvanian or lower Permian of North America. The exact age and locality of this type specimen is unknown. Due to the fragile nature of the tooth whorls, their arched fragments of various length are rarely found.
In the 20 years after the first description, Karpinsky (Reference Karpinsky1924a) recorded the presence of a short lingual fragment of a tooth whorl from the Gzhelian deposits of the Moscow Region and assigned it to a separate species of the genus Helicoprion Karpinsky, Reference Karpinsky1899, Helicoprion ivanovi. Later, Obruchev (Reference Obruchev and Obruchev1964) transferred this species to the genus Campyloprion Eastman, Reference Eastman1902 and figured, but did not provide a description of, a much larger specimen consisting of two detached whorl parts from the same locality.
Little data have been obtained since then, with all newer records being confined to the Pennsylvanian of the Midcontinent and its western boundaries in North America, as well as to central Russia (Itano & Lucas Reference Itano and Lucas2018). Before this publication, the type species C. annectans Eastman, Reference Eastman1902 had been thought to be characteristic of North America while all Russian specimens had been assigned to Campyloprion ivanovi (Karpinsky Reference Karpinsky1924a). The holotype of the latter species PIN 1655/132, as well as a more complete specimen PIN 1655/1, originate from the Kosherovo Formation, Dobryatinian Substage, or Horizon in the Russian stratigraphic tradition (Gzhelian, Pennsylvanian) exposed in a quarry by the Rusavkino village in the Moscow Region (Fig 1a, b).
Figure 1 Schematic map of Karpinskiprion ivanovi comb. nov. localities in Russia: (a) geographical position of the Rusavkino type locality in the Moscow region; and (b) position of the Perevozinka locality in the Volgograd Region of Russia.
Apart from these two, there are three other poorly known or undescribed specimens from Russia. A short fragment of a very large tooth whorl recently found in the collections (PIN 1655/653) was figured by Itano & Lucas (Reference Itano and Lucas2018). Petukhov et al. (Reference Petukhov, Petrov, Pakhomov, Lebedev and Ivanov2011) reported a find of a Campyloprion sp. whorl fragment in the Kasimovian deposits (Upper Pennsylvanian) in the vicinity of the town of Zhirnovsk (Volgograd Region), this one being the earliest record in Russia. One more, yet undescribed, tooth whorl fragment from the Lower Gzhelian deposits comes from the Shchelkovo quarry in the Moscow Region. These last two specimens are deposited in the private Universe History Museum (Moscow Region, Dedovsk) (Petukhov et al. Reference Petukhov, Petrov, Pakhomov, Lebedev and Ivanov2011).
The fragmented nature of previously known materials resulted in attention being focused on the projecting parts of the tooth crowns, viz. their shape, proportions and serration along the cutting edges. However, Itano & Lucas (Reference Itano and Lucas2018) suggested that whorl growth followed a logarithmic spiral and that the angle between a radius and a tangent to the spiral α may be used as a taxonomic criterion.
By analogy with the better-known tooth whorl of Helicoprion, the whorl in Campyloprion was previously deduced to be of spiral form and consist of more than one coil (Zangerl Reference Zangerl and Schultze1981; Ginter et al. Reference Ginter, Hampe, Duffin and Schultze2010; Naugolnykh Reference Naugolnykh2017; Itano & Lucas Reference Itano and Lucas2018) and was, by analogy, thought to belong to the lower jaw. Due to the fragmentary nature of the available material, the extent of the spiral and whether or not it comprised more than a full volution was unknown. Zangerl (Reference Zangerl and Schultze1981) and Ginter et al. (Reference Ginter, Hampe, Duffin and Schultze2010) both state that the teeth ‘do not form a tooth spiral’. Presumably they meant by this assertion that the whorl comprised less than one full volution, possibly much less. In the absence of gross whorl data, Itano & Lucas (Reference Itano and Lucas2018) suggested that crown morphology characters be used and proposed that new, more complete, materials and further study might result in re-evaluation of the taxonomy of these materials.
In 2019, a new, most complete, specimen was discovered in the Volgograd Region of Russia by one of the current authors (A.V.I.). As discussed further in the Results section, our attempts to assign the new specimen to either of the two known species is hindered by absence of necessary features characterising the type species (Itano & Lucas Reference Itano and Lucas2018). Given that the holotype of C. annectans lacks details of the crowns and so is not diagnostic at the species level, and also is of unknown provenance, we have to regard it a nomen dubium. There is no possibility to select a neotype as specimens attributed earlier to C. annectans are insufficiently well-preserved, as required by the International Code of Zoological Nomenclature (1999, Art. 75.5). Since this species was explicitly designated as the type species of the genus Campyloprion by Eastman, this requires suppression of the genus also.
For this reason, we establish here a new genus Karpinskiprion Lebedev et Itano gen. nov., based upon the only species, K. ivanovi (Karpinsky Reference Karpinsky1924a). Whether there is only one Russian species or two is uncertain because of the insufficient amount of material, but it seems possible that there is only one species, showing variability in ontogeny and among individuals.
Apart from this systematic revision, we also describe the oldest, juvenile section of the whorl preserved in a new specimen and unknown before, as well as the youngest parts of previously described specimens, which illustrate the way new crowns had been added, and some morphological features unnoticed earlier. The new specimen allows us to trace changes of tooth formation during life of the same individual, what we name here organogenetic (to stress difference from ontogenetic, relating to the organism as a whole) changes.
2. Geographical and geological settings
In the summer of 2019, one of the field crews led by one of the current authors (A.V.I.) of the scientific and educational expedition ‘Navigating Universities Flotilla’ of the Natural History Museum of the Gagarin Saratov State Technical University, in collaboration with the Lomonosov Moscow State University, Borissiak Palaeontological Institute of the RAS, State Land Management University, and Higher School of Economics, supported by the Youth Club of the Russian Geographical Society, discovered an isolated limestone slab in an abandoned local quarry. This slab included a large fragment of a tooth whorl. In September 2019 the section was described and sampled bed by bed. During this work, another fragment of the same whorl specimen was uncovered in bedrock (Ivanov et al. Reference Ivanov, Lebedev, Novikov, Romanova and Yashkov2020). Preparation of the new specimen revealed the internal volutions, clearly demonstrating the coiled nature of this fossil for the first time. These new specimens provide an opportunity to revisit assumptions made previously.
The tooth whorl was found in a quarry in the upstream of the left tributary of the Perevozinka River, in its turn the left tributary of the Medveditsa River 15 km to the east south-east of the town of Zhirnovsk and 6 km to the south-east from the abandoned Perevozinka (formerly Neubalzer) village (50.925015 N, 44.989927 E; Volgograd Region, Russia).
The quarry exposes the Upper Pennsylvanian (Upper Kasimovian–Lower Gzhelian) deposits in the axial part of Don-Medveditsa tectonic dislocations located in the south-east of the East European Platform (EEP), not far from the margin of the Peri-Caspian Depression.
Two lithological units are clearly expressed in the quarry. The lower unit is mostly composed of limestones: crinoid–fusulinid rudstones, wackestones, mainly as slump breccias showing crinoid fragments and rare shells of fusulinid foraminifers. The upper unit unconformably overlies the lower one with erosional contact and consists of dolomites and dolomitised limestones. Disconformity is also stressed by the presence of marls with limestone gravel gradually turning into clays up the section. Dolomites are cavernous – these caverns are often formed as a result of dissolution of fusulinid and gastropod shells and crinoid ossicles; there are also imprints of fenestellid bryozoans and brachiopod valves found. The middle part of this layer includes numerous chert concretions. The whorl was found in the lower part of this layer.
The section has never been studied before. According to the geological map composed by Saltykov (Reference Saltykov2009, p. 78, text- fig. 4.1) these deposits may belong to the Kurakino or Parubino Formations, belonging to the middle and upper parts of the Kasimovian Stage.
Biostratigraphic analysis of the section is based upon fusulinid foraminifers and conodont elements. The fusulinid assemblage of the lower unit is characteristic of the upper part of the Khamovnikian or lower Dorogomilovian regional substages (written communication by T. Isakova, Geological Institute of the Russian Academy of Sciences, 2022). This suggests that the age of the lower-most bed relates to a boundary interval between the Khamovnikian and Dorogomilovian of the EEP (Alekseev et al. Reference Alekseev, Nikolaeva, Goreva, Donova, Kossovaya, Kulagina, Kucheva, Kurilenko, Kutygin, Popeko, Stepanova, Lucas, Schneider, Wang and Nikolaeva2022). Very rare conodont elements identified as Streptognathodus cf. firmus Kozitskaya found in the lower unit suggest a somewhat younger age.
The lower part of the upper unit yielded no conodonts; however, up section their assemblages are abundant: Idiognathodus simulator Ellison, Idiognathodus auritus Chernykh,. Idiognathodus sinistrum Chernykh, and more rarely Streptognathodus pawhuskaensis (Harris & Hollingsworth), as well as a single specimen of Gondolella bella Stauffer & Plummer. The sample taken immediately from the slab containing the tooth whorl shows the same assemblage.
This conodont assemblage is typical of the base of the I. simulator Zone. The first appearance of its index species is recommended to be regarded as the base of the global Gzhelian Stage of the Upper Pennsylvanian (Heckel et al. Reference Heckel, Alekseev, Barrick, Boardman, Goreva, Isakova, Nemyrovska, Ueno, Villa and Work2008; Villa et al. Reference Villa, Alekseev, Barrick, Boardman, Djenchuraeva, Fohrer, Forke, Goreva, Heckel, Isakova, Kossovaya, Lambert, Martinez-Chacon, Mendez, Nemyrovska, Remizova, Samankassou, Sanchez de Posada, Ueno, Wahlman and Work2009). Thus, the deposition of this layer was coeval with the Kosherovo Formation of the Rusavkino and Gzhel sections in central Russia (Alekseev et al. Reference Alekseev, Goreva, Isakova, Kossovaya, Lazarev, Davydov, Alekseev and Goreva2009), which also yielded Campyloprion specimens described by earlier authors (Karpinsky Reference Karpinsky1924a; Obruchev Reference Obruchev and Obruchev1964; Itano & Lucas Reference Itano and Lucas2018).
3. Materials and methods
For this study we use the previously described holotype of Helicoprion (Campyloprion) ivanovi Karpinsky, Reference Karpinsky1924a, PIN 1655/132, as well as specimens figured by Obruchev (Reference Obruchev and Obruchev1964) (PIN 1655/1) and Itano & Lucas (Reference Itano and Lucas2018) (PIN 1655/1 and 1655/653) coming from the same locality. The holotype had been collected by A.P. Ivanov in 1907. The collectors of PIN 1655/1 and 1655/653 are unknown.
A new specimen PIN 1655/656 was found in two larger fragments preserving the younger part of the tooth whorl separated by a crack from the rest, representing the older (juvenile) part of the whorl (Fig. 2). Some small pieces had been collected separately, later glued together and fitted into their place in the first fragment. There is no direct contact between the two larger parts of the whorl, but the configuration of the matrix blocks presented reasonably well-preserved contact surfaces making possible the assembly of two parts. Externally, the base of the outer coil fragment is damaged. Projecting parts of a few crowns (cutting blades) are destroyed, mostly during life or before burial. The youngest (latest formed) part of the whorl is missing, as well as a section of an inner volution. Four isolated fragments of cartilage are preserved on the exposed surface of the whorl, most likely being displaced from their original position. It is proposed that these fragments belong to the same individual as the whorl, otherwise their concentration and affinity is difficult to explain.

Figure 2 Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a), an incomplete symphyseal tooth whorl, specimen PIN 1655/656. A local quarry at the left tributary of the Perevozinka River, 6 km to the south-east from the abandoned Perevozinka (formerly Neubalzer) village, Volgograd Region, Russia; Lower Gzhelian, Upper Pennsylvanian. Embedding matrix removed from the photograph to highlight the whorl. Black counter arrows show an area from which cartilage samples had been taken for thin sections. cp = cartilage patch. Scale bar = 4 cm.
Superficially the specimen is mostly well-preserved, although the base and crowns are covered by a network of thin cracks and, dentine in the smaller fragment is damaged along the main break. Locally the vascularised dentine of the whorl base is more fragile than that in the other areas due to spotty dolomitisation of surrounding rock. Finally, due to this process, the whorl is very slightly deformed so that it does not fit a plane and slightly undulates. This is especially noticeable along the inner margins of the base.
The holotype of Karpinskiprion (Campyloprion) ivanovi (Karpinsky Reference Karpinsky1924a) PIN 1655/132 (Fig. 3) and specimen PIN 1655/653 (Fig. 4) are short fragments of the outer volutions of the tooth whorls from their lingual-most parts. Specimen PIN 1655/1 consists of two much larger parts missing a contact – one of those also belongs to this whorl region. Some of the cutting blades are broken off (Fig. 5).
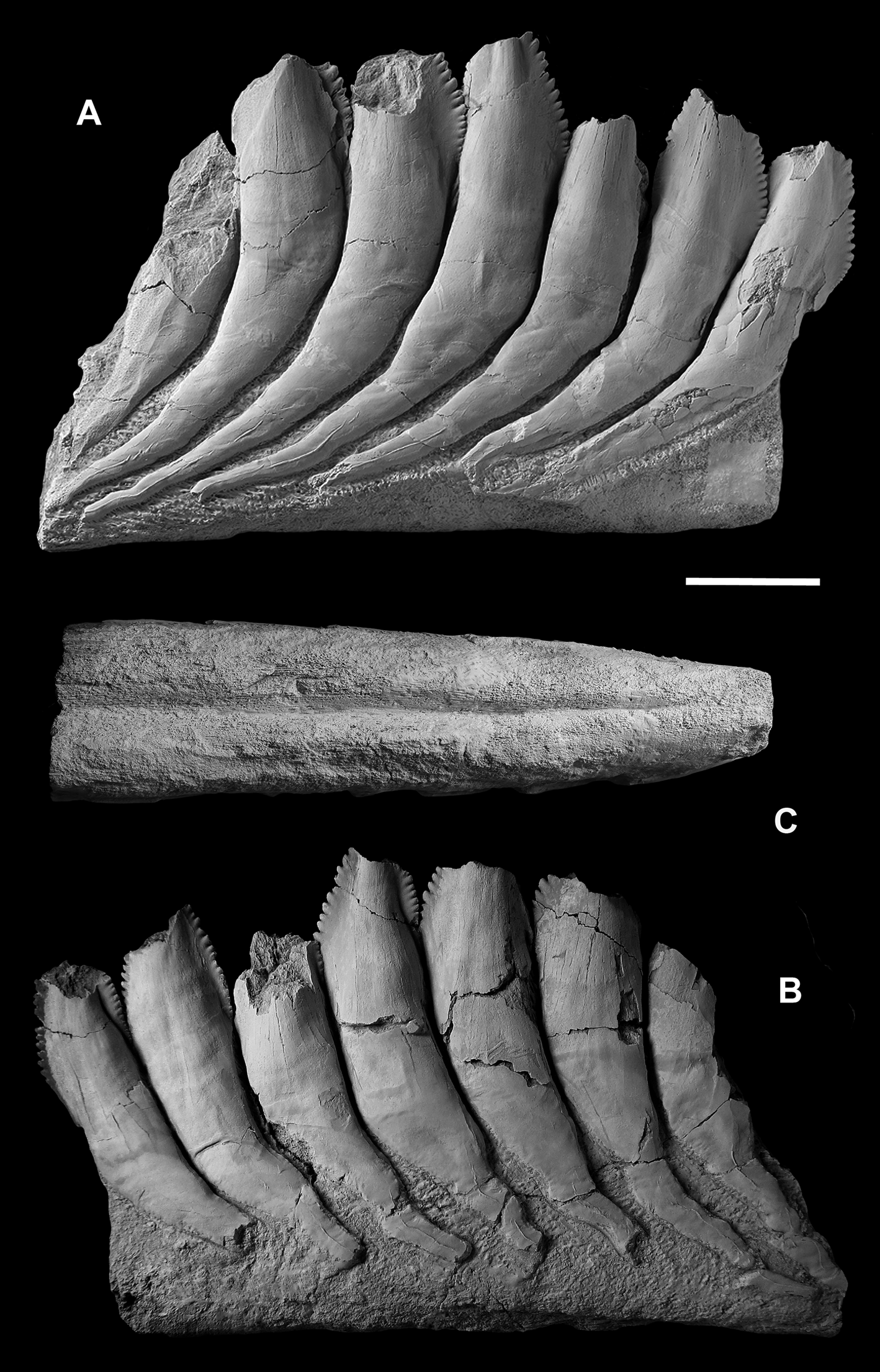
Figure 3 Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a), the lingual-most section of the tooth whorl, holotype specimen PIN 1655/132: (a) right view; (b) left view; and (c) basal view. A quarry by the Rusavkino village, Balashikha District, Moscow Region; Kosherovo Formation, Dobryatinian Substage, Gzhelian, Pennsylvanian. Scale bar = 2 cm.
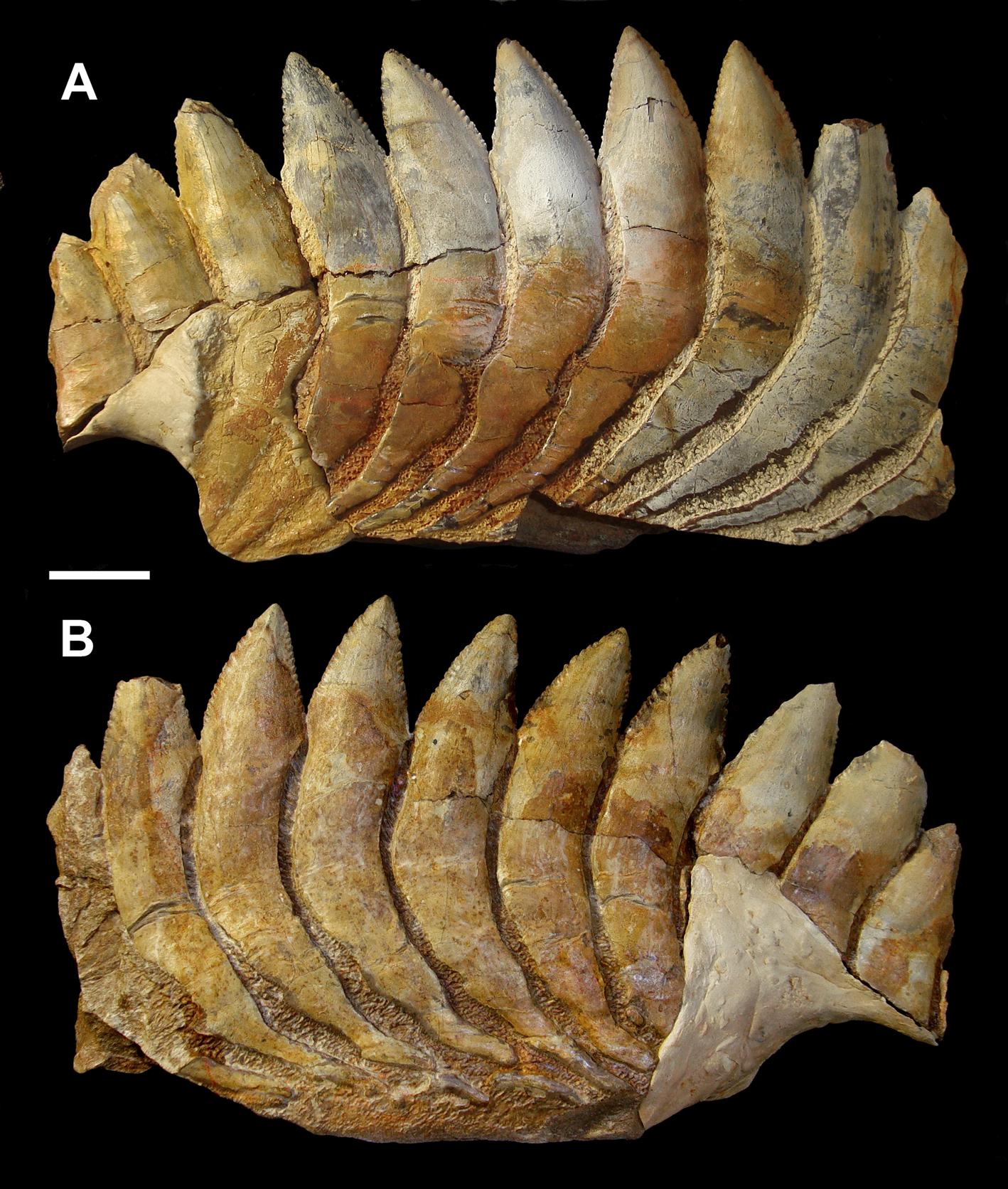
Figure 4 Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a), a part of the tooth whorl, specimen PIN 1655/653: (a) left view; and (b) right view. A quarry by the Rusavkino village, Balashikha District, Moscow Region; Kosherovo Formation, Dobryatinian Substage, Gzhelian, Pennsylvanian. Scale bar = 2 cm.
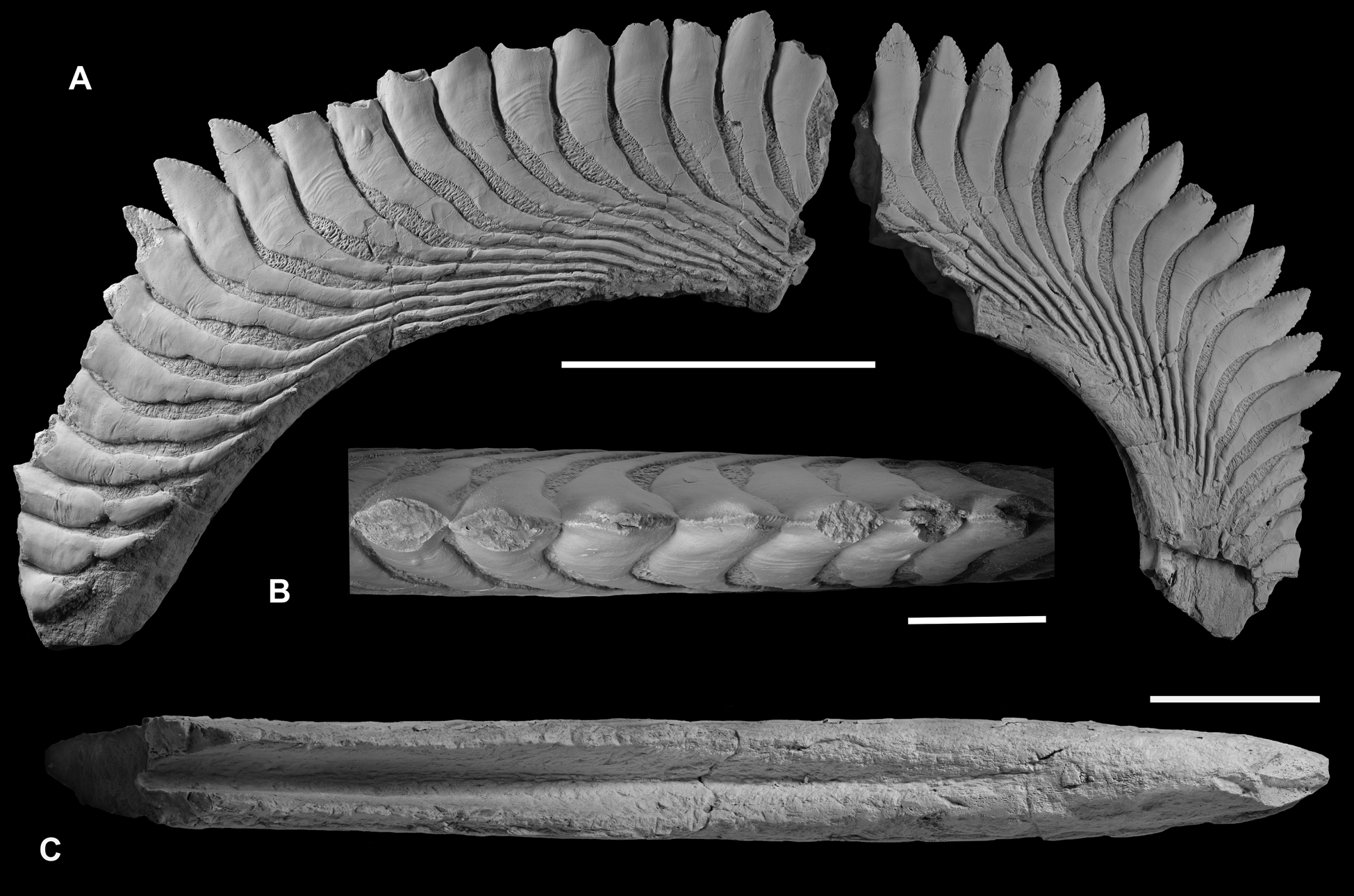
Figure 5 Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a), specimen PIN 1655/1: (a) quarry by the Rusavkino village, Balashikha District, Moscow Region; Kosherovo Formation, Dobryatinian Substage, Gzhelian, Pennsylvanian. (a) fragments of an outer coil of a single tooth whorl, right view; (b) a part of the fragment at the left, apical view; and (c) same, basal view. Scale bars: (a) = 10 cm; and (b) and (c) = 5 cm.
The holotype specimen and PIN 1655/1 had been prepared manually by chiselling the embedding dolomite in the early 1920s and 1950s. Specimen PIN 1655/656 was cleaned of matrix mechanically by various electric instruments, then manually by chisels of various diameters. Specimen PIN 1655/653 was originally prepared manually; later three labialmost crowns missing bases had been attached to the whorl by means of plastic adhesive and the whole specimen was treated by a 10% solution of acetic acid. Thin sections were prepared manually by standard techniques, specimens being immersed in epoxy resin.
Microtomography was carried out on the Neoscan NEOSCAN 80 in the A.A. Borissiak Palaeontological Institute of the Russian Academy of Science (PIN), Moscow, Russia, software Version 2.2.4 at source voltage 110 kV, source current 146 μA, camera exposure 533 ms, filter Cu 1.0 mm, image pixel size 35.132805 μm and rotation step 0.200°. Reconstruction was made by NeoScan software.
Most of the specimens had been coated with a sublimate of ammonium chloride before photography.
Specimens figured in this paper are deposited in the A.A. Borissiak Palaeontological Institute of the Russian Academy of Science (PIN), Moscow, Russia, in the collection 1655; F.N. Chernyshev Central Scientific Research Geological Prospecting Museum (TsNIGR Museum), St. Petersburg, Russia, in the collections 1865 and 1670; National Museum of Natural History (USNM), Washington, DC, USA, in the collections USNM PAL; and Museum of Paleontology (UCMP), University of California, Berkeley, USA.
4. Results
Itano & Lucas (Reference Itano and Lucas2018) described a number of problems regarding the species composition of the genus Campyloprion Eastman, Reference Eastman1902. The most important one these authors faced was a low comparability of the holotypes between the type species and C. ivanovi (Karpinsky Reference Karpinsky1924a). In the former no crowns are completely preserved while in the latter the whorl fragment is too short to measure the spiral angle. For this reason, the authors included the spiral angle 60° characteristic of C. annectans as a generic, rather than species character in the diagnosis. Based on a computer fit to the apices of the smaller of the two pieces of PIN 1655/1, the spiral angle in the C. ivanovi specimen PIN 1655/1 was found to equal 67.4°. In principle it is possible to determine the spiral angle of a logarithmic spiral even from a specimen preserving a small fraction of a volution and even to determine whether the spiral is of logarithmic form or of another form (Aldridge Reference Aldridge2020). In practice, such a determination is difficult to make on an imperfect specimen comprising only a small fraction of a volution. The new specimen PIN 1655/1 comprises approximately 1.75 volutions, enabling a more precise determination of its spiral form (see Discussion).
Usage of these criteria resulted in the suggestion that some North American specimens are more closely related to the Russian species. Specimen NMMNH P-68551 from the Tinajas Member, Atrasado Formation, Late Missourian (= Late Kasimovian), Socorro County, New Mexico, USA was identified as Campyloprion cf. C. ivanovi (Karpinsky Reference Karpinsky1924a). On the contrary, comparison of the type species holotype to that of C. ivanovi (Karpinsky Reference Karpinsky1924a) revealed significant distinctions in height/width ratios of the tooth cutting blades, as well as the extent of imbrication between the neighbouring crowns. The assignment of PIN 1655/1 to C. ivanovi was questioned because in this specimen the crown height/width (h/w) values vary from approximately 1.05 to 1.15, versus approximately 1.5 in the holotype PIN 1655/132, and a serration depth/serration separation (sd/ss) value of approximately 0.24, versus approximately 0.6 in the holotype specimen. Itano & Lucas (Reference Itano and Lucas2018) noted that in its h/w value PIN 1655/1 is close to that in C. annectans (approximately 1.0). Estimated imbrication of adjacent crowns in PIN 1655/1 is less pronounced than that in the holotype specimen of the same species. However, a more precise comparison is hampered by incompleteness of the holotype and only known specimen of C. annectans.
Thus, Itano & Lucas (Reference Itano and Lucas2018) suggested that the discovery of more complete remains definitely referable to C. ivanovi might change the systematic status of PIN 1655/1. They suggested that C. annectans and C. ivanovi would remain distinct species, but the latter would have to be assigned to another genus, or that more than two species of Campyloprion are represented in the assemblage of known Pennsylvanian specimens. An alternative option was that all Russian specimens represent variation within a single species, or that C. ivanovi is a junior synonym of C. annectans.
Karpinsky (Reference Karpinsky1924a) in his description of Helicoprion ivanovi suggested some characters which distinguish it from ‘other Helicoprion species (p. 369)’ (at that time). He listed imbricated contacts of the neighbouring crowns, prolongation of the serrated margins to imbricated sections of the crown margins, presence of a longitudinal keel on spurs, different cross-section shape, convex labial and almost straight lingual margins of the crowns, oblique apical direction of marginal serration, absence or presence of secondary incisions on the denticles, and depth and shape in section of the basal furrow. An important character overlooked by Karpinsky but noted by Obruchev (Reference Obruchev1953, Reference Obruchev and Obruchev1964) is relative length of spurs. In Helicoprion these lateral projections of the crowns are much shorter, so that the line drawn through the mid-length of the projecting part of the crown crosses basally one or two spurs of the adjoining crowns, while in Campyloprion from three to six.
The new specimen described below provides a good opportunity to evaluate the significance of these and newly found characters and to present interpretations regarding organogenetic growth and function of the whorl.
Apart from revising the genus Karpinskiprion (Campyloprion), we suggest refining of the composition of the family Helicoprionidae Karpinsky, 1911 and erect a new family Helicampodontidae Itano et Lebedev fam. nov. In it, we place some genera previously placed in the Edestidae Jaekel, Reference Jaekel1899 and the Helicoprionidae.
5. Systematic palaeontology
Class Chondrichthyes Huxley, Reference Huxley1880
Subclass Euchondrocephali Lund & Grogan, Reference Lund and Grogan1997
Order Eugeneodontiformes Zangerl, Reference Zangerl and Schultze1981
Family Helicampodontidae Itano et Lebedev fam. nov.
Type genus Helicampodus Branson, Reference Branson1935
Family composition: Apart from the type genus, Parahelicampodus Nielsen, Reference Nielsen1952; Sinohelicoprion Liu & Chang, Reference Liu and Chang1963; Hunanohelicoprion Liu, Reference Liu1994.
Diagnosis: Known from arcuate symphyseal tooth whorls, presumed to belong to the lower jaw. Whether the complete tooth whorls are spiraliform and whether older teeth were shed is unknown. Crowns triangular, laterally compressed, edges serrated. Tooth spurs project lingually, taper basally. Longitudinal groove along the basal surface of the fused tooth bases.
Remarks: Justification for inclusion of the genera Helicampodus, Parahelicampodus, Sinohelicoprion and Hunanohelicoprion in the new family Helicampodontidae is based on their similarities with one another and their differences from the type genera of the Helicoprionidae and the Edestidae.
Helicampodus has had an uncertain family classification. It was placed in the Helicoprionidae by Obruchev (Reference Obruchev and Obruchev1964) and in the Edestidae by Zangerl (Reference Zangerl and Schultze1981) and Ginter et al. (Reference Ginter, Hampe, Duffin and Schultze2010). According to Zangerl (Reference Zangerl and Schultze1981) and Ginter et al. (Reference Ginter, Hampe, Duffin and Schultze2010), the Edestidae are distinguished by having crown spurs of symphyseal teeth that point lingually, while those of the Helicoprionidae (=Agassizodontidae) point labially. Application of this diagnosis to Helicampodus is complicated by disagreement as to the orientation of the tooth whorls. Branson (Reference Branson1935) believed that the tooth whorl of Helicampodus kokeni Branson, Reference Branson1935, the type species of the genus, was oriented with the crown spurs pointing labially, as in Helicoprion, despite evidence to the contrary. In particular, he ignored the evidence of crown size (large crowns are likely to be more recently formed) and tooth wear (older teeth are likely to be more worn). Helicampodus egloni Obruchev, Reference Obruchev, Ruzhentsev and Sarycheva1965, is known from a much more complete tooth whorl than is H. kokeni. Tooth wear is not apparent, but the fact that the crown spurs project lingually rather than labially indicates a gradual shift in the size of the crowns with position on the whorl and the assumption that younger teeth are larger. Accepting the evidence that the crown spurs point lingually in Helicampodus, Zangerl (Reference Zangerl and Schultze1981) and Ginter et al. (Reference Ginter, Hampe, Duffin and Schultze2010) placed that genus in the Edestidae. However, Helicampodus teeth differ in their basal structure from those of Edestus Leidy, Reference Leidy1856, the type genus of the Edestidae, in having a concave rather than convex basal surface of the fused tooth bases (Branson, Reference Branson1935; text- fig. 1c). In this respect, Helicampodus resembles Helicoprion. In both Helicampodus and Helicoprion, the tooth bases are tightly fused. In Edestus, the bases are not completely fused, and mature teeth are shed. Given the strong differences in morphology between Helicampodus and the type genera of both the Helicoprionidae and the Edestidae, we consider it justified to erect a new family. Sinohelicoprion and Hunanohelicoprion are quite similar to Helicampodus but are provisionally retained as distinct genera. In the type species of both genera, Sinohelicoprion changhsingensis Liu & Wang, 1963 and Hunanohelicoprion xiandongensis Liu, Reference Liu1994, respectively, the basal surfaces are concave, as in Helicampodus. However, the crown spurs of both species are described as pointing labially rather than lingually. This assertion is likely based on an assumed homology with Helicoprion, as the short sections of tooth whorl that comprise the holotypes are so short that it would be difficult to observe a change in tooth size with position. The situation with regard to the orientation of the tooth whorl of Sinohelicoprion has been clarified by new computerised tomography-scanning observations of a specimen referred to Sinohelicoprion sp. by Tapanila et al. (Reference Tapanila, Pruitt, Wilga and Pradel2020). This specimen, USNM 235393, from the Permian of Wyoming, United States, clearly shows an increase in tooth size with position that indicates that the crown spurs are directed lingually (Tapanila et al. Reference Tapanila, Pruitt, Wilga and Pradel2020; text- fig. 11). Clear evidence of the orientation of the tooth whorl of Hunanohelicoprion is lacking, since the known tooth whorls are so incomplete, but the similarity of the teeth to those of both Sinohelicoprion and Helicampodus indicates a close relationship to those genera. Parahelicampodus is known only from a fragment of a tooth whorl comprising parts of a few teeth, so its orientation is ambiguous. However, the similarity of the teeth to those of Helicampodus indicates a close relationship to that genus. The Edestidae are then restricted to Edestus, Lestrodus Obruchev, Reference Obruchev1953 and Edestodus Obruchev, Reference Obruchev1953. Edestus and Edestodus are sometimes synonymised (e.g., Zangerl Reference Zangerl and Schultze1981; Ginter et al. Reference Ginter, Hampe, Duffin and Schultze2010), but this is unjustified, given that they can easily be distinguished.
Family Helicoprionidae Karpinsky, 1911
Family composition: Apart from the type genus, Agassizodus St. John & Worthen, Reference St. John and Worthen1875; Parahelicoprion Karpinsky, Reference Karpinsky1924b; Sarcoprion Nielsen, Reference Nielsen1952; Toxoprion Hay, Reference Hay1909; Shaktauites Tchuvashov, Reference Tchuvashov2001; and Karpinskiprion Lebedev et Itano gen. nov.
Diagnosis (emended after Obruchev Reference Obruchev and Obruchev1964; Zangerl Reference Zangerl and Schultze1981; Lebedev Reference Lebedev2009): Known from arcuate or spiraliform symphyseal tooth whorls, belonging to the lower jaw. Crowns laterally compressed, tooth spurs project labially. Lateral teeth, when present, organised into numerous series. Longitudinal groove along the basal surface of the fused tooth bases.
Remarks: Zangerl (Reference Zangerl and Schultze1981) erected the Agassizodontidae to include genera related to Helicoprion Karpinsky, Reference Karpinsky1899. He noted partial correspondence of the Agassizodontidae to Helicoprionidae but did not explain the reasons for erecting the new family name. His opinion was followed by Ginter et al. (Reference Ginter, Hampe, Duffin and Schultze2010) and Tapanila et al. (Reference Tapanila, Pruitt, Wilga and Pradel2020). However, Helicoprionidae Karpinsky, 1911 has priority over the Agassizodontidae (Lebedev Reference Lebedev2009).
Genus Karpinskiprion Lebedev et Itano, gen. nov.
Campyloprion: Eastman Reference Eastman1902, p. 151; Obruchev Reference Obruchev and Obruchev1964, p. 253; Zangerl Reference Zangerl and Schultze1981, p. 86; Ginter et al. Reference Ginter, Hampe, Duffin and Schultze2010, p. 125; and Itano & Lucas Reference Itano and Lucas2018, p. 408.
Helicoprion: Karpinsky Reference Karpinsky1924a, p. 369; and Khabakov Reference Khabakov and Gorsky1939, p. 150.
Type species: Helicoprion ivanovi Karpinsky, 1924.
Generic composition: Only type species
Diagnosis: Helicoprionids characterised by presence of tooth whorls in the upper and lower jaws. Number of crowns per volution increases with whorl growth. Approximately 12–17 crowns are present within a 90° sector of a tooth whorl. Marginal crown denticles oriented obliquely apically. Denticle margin plain, secondary incisions absent. Radial line drawn through the cutting blade axis crosses from three to six spurs of the next crowns in the outer (adult) volution. Longitudinal keels run along the spurs.
Karpinskiprion ivanovi (Karpinsky Reference Karpinsky1924a), comb. nov.
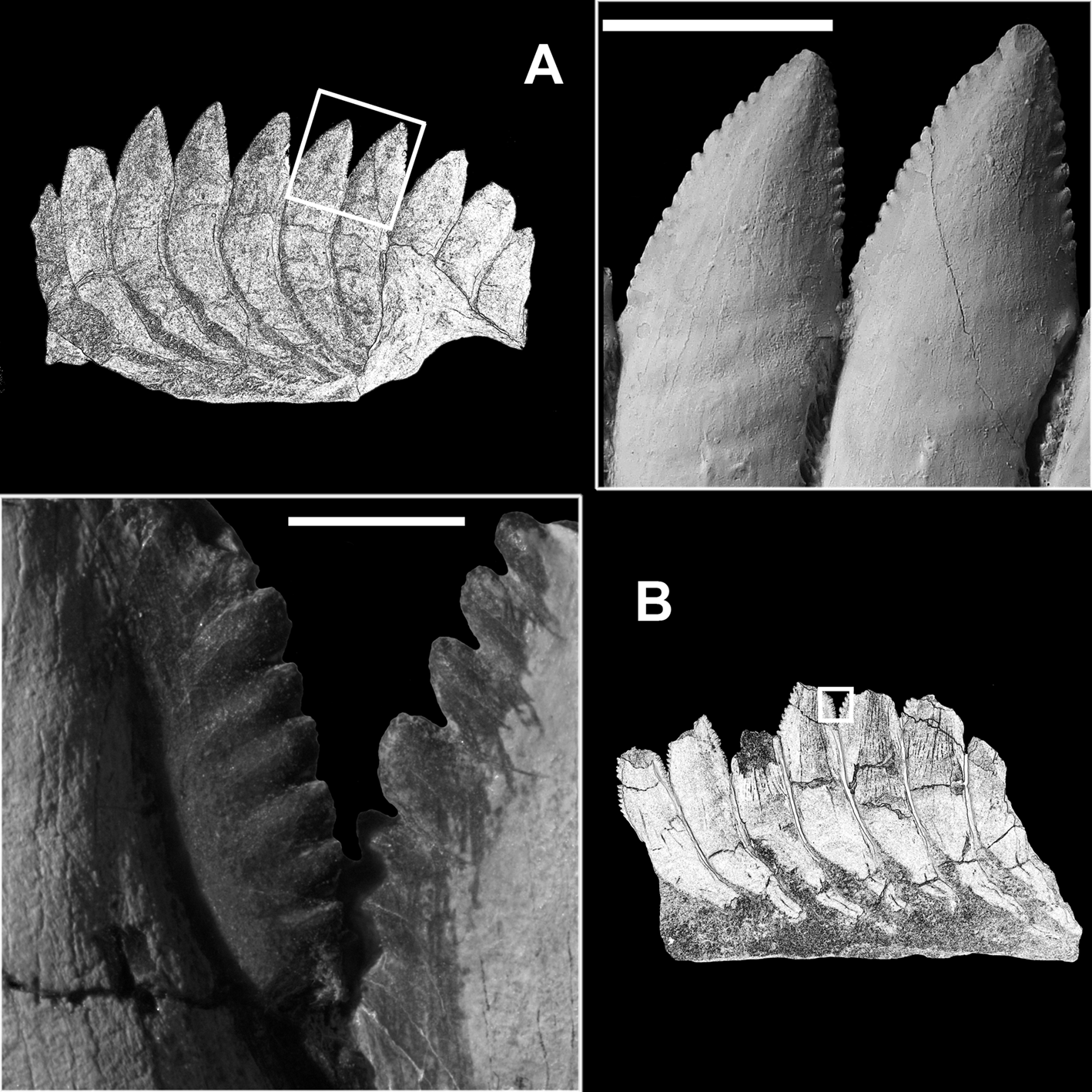
Figure 6 Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a): (a) two neighbouring cutting blades of the crowns of the specimen PIN 1655/653, showing serrated margins and the basal contact; and (b) type of serration on the preserved cutting blades in the holotype specimen PIN 1655/132. Both specimens from a quarry by the Rusavkino village, Balashikha District, Moscow Region; Kosherovo Formation, Dobryatinian Substage, Gzhelian, Pennsylvanian. White boxes on the reduced specimen images show the position of the enlarged area. Scale bars: (a) = 2 cm; and (b) = 3 mm.
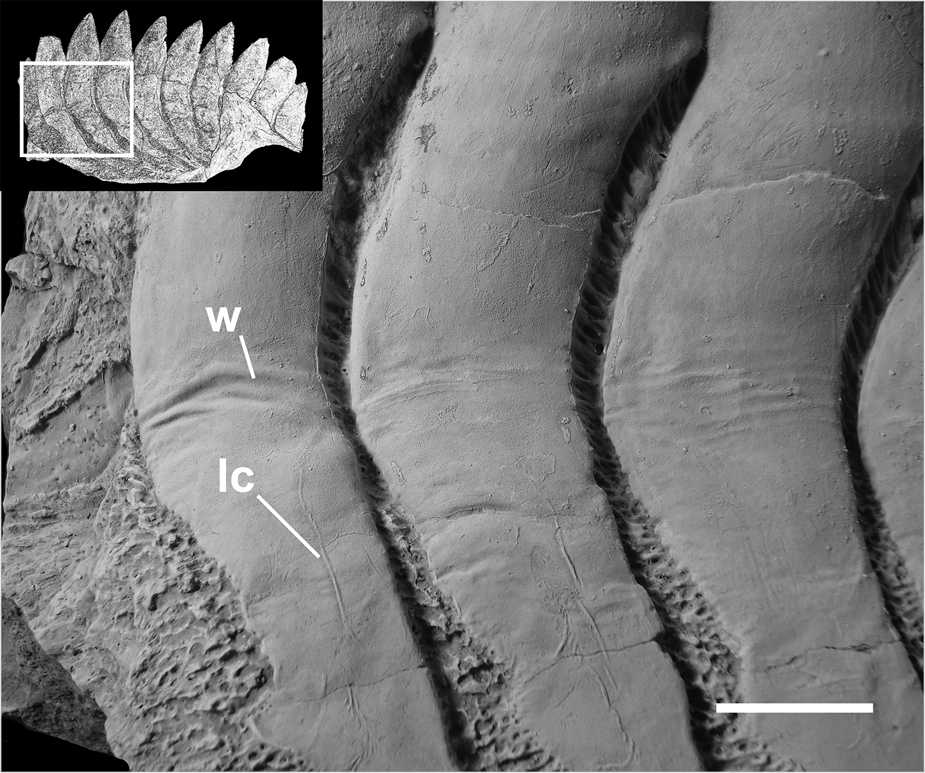
Figure 7 A fragment of a symphyseal tooth whorl of Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a), specimen PIN 1655/653, showing wrinkled dentine and lateral crests on the spurs. A quarry by the Rusavkino village, Balashikha District, Moscow Region; Kosherovo Formation, Dobryatinian Substage, Gzhelian, Pennsylvanian. White box on the reduced specimen image shows the position of the enlarged area. lc = lateral crest; and w = wrinkled surface of crown dentine. Scale bar = 2 cm.
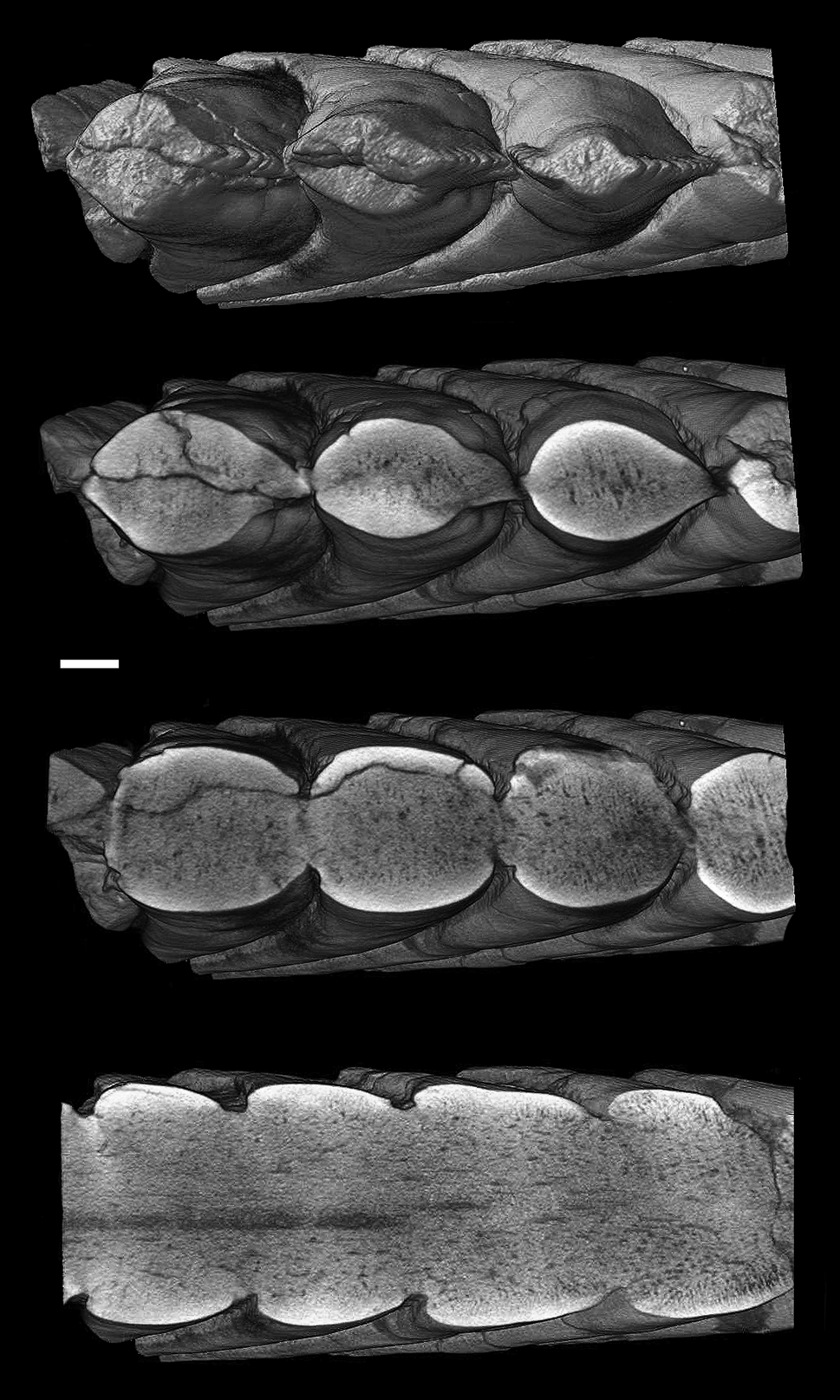
Figure 8 Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a), virtual serial sections of the holotype specimen PIN 1655/132. Scale bar = 1 cm.

Figure 9 Organogenetic transformation of helicoprionid tooth crowns: (a) Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a); and (b) Helicoprion bessonowi Karpinsky, Reference Karpinsky1899. (a) Growth from left to the right, (b) growth from right to the left.

Figure 10 Ornamental elements on the spurs in Karpinskiprion ivanovi comb. nov. (Karpinsky, Reference Karpinsky1924a): (a) holotype specimen PIN 1655/132, left view; and (b) specimen PIN 1655/653, left view. A quarry by the Rusavkino village, Balashikha District, Moscow Region; Kosherovo Formation, Dobryatinian Substage, Gzhelian, Pennsylvanian. White box on the reduced specimen image shows the position of the enlarged area. lc = lateral crests; and lt = lateral tubercles. Scale bars: (a) = 1 cm; and (b) = 2.5 mm.
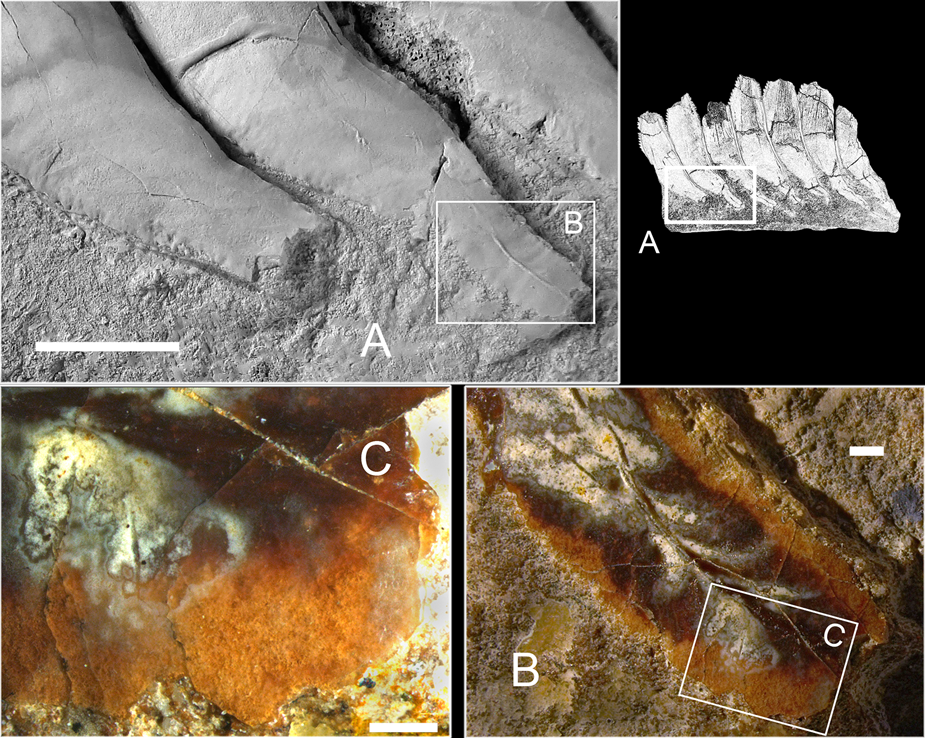
Figure 11 Spur formation in Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a): (a) holotype specimen PIN 1655/132, right view; and (b) and (c), the enlarged areas of (a). A quarry by the Rusavkino village, Balashikha District, Moscow Region; Kosherovo Formation, Dobryatinian Substage, Gzhelian, Pennsylvanian. White boxes show the positions of the enlarged areas. Scale bars: (a) = 1 cm; (b) = 750 mkm; and (c) = 500 mkm.

Figure 12 Spur malformation in Karpinskiprion gen. nov. (a)–(g) Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a); (h) Karpinskiprion sp. (a) holotype specimen PIN 1655/132, right view; (b)–(e) specimen PIN 1655/1; (c) is an enlarged area of (b), right view; (e) is an enlarged area of (d), left view; (f) is an enlarged area of specimen PIN 1655/653; (g) is an enlarged area of specimen PIN 1655/656; (a–f) a quarry by the Rusavkino village, Balashikha District, Moscow Region, Russia; Kosherovo Formation, Dobryatinian Substage, Gzhelian, Pennsylvanian; (g) local quarry at the left tributary of the Perevozinka River, 6 km to the south-east from the abandoned Perevozinka (formerly Neubalzer) village, Volgograd Region, Russia; Lower Gzhelian, Upper Pennsylvanian; and (h) specimen USNM 443547, locality 68 ATR-153, Middle Fork, Kivalina River, Alaska, USA; Pennsylvanian. Scale bars: (a, b, e, f, g) = 1 cm; (c) = 5 mm; (d) = 2 mm; and (h) = 5 cm.
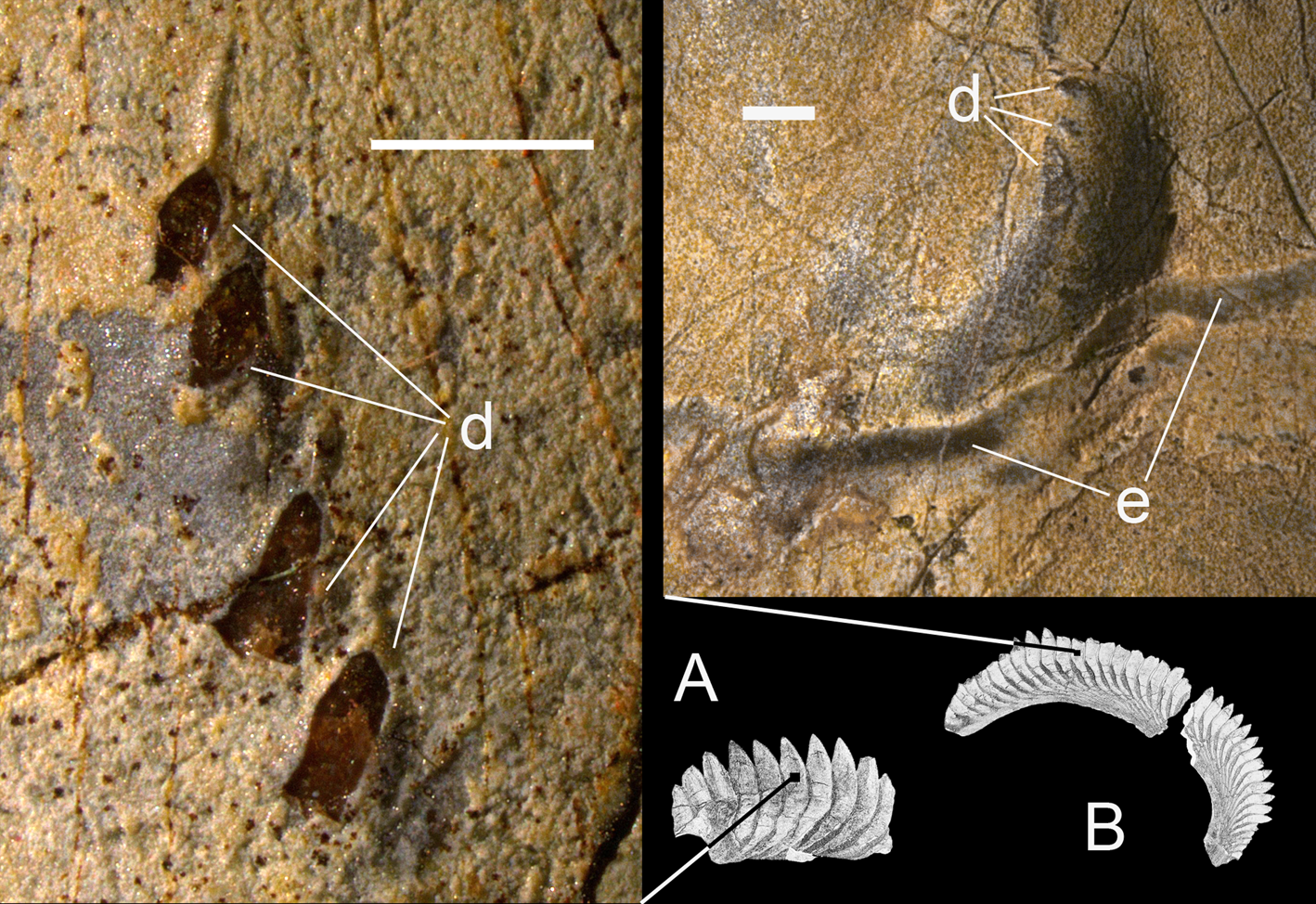
Figure 13 Denticles arranged into rows in Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a): (a) specimen PIN 1655/653; and (b) PIN 1655/1. In (a) the denticle apices are broken off exposing compact dentine. d = denticles; and e = a band of preserved enameloid. Scale bars: (a) = 2 mm; and (b) = 1 mm.

Figure 14 Areas presenting latest growth stages in Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a) (a, b); and Helicoprion bessonowi Karpinsky, Reference Karpinsky1899 (c, d). p = lingual process of the base. Scale bars: (a) and (b) = 2 cm; and (c) and (d) = 1 cm.

Figure 15 Juvenile (oldest) part of the tooth whorl of Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a), specimen PIN 1655/656. Scale bar = 5 mm.
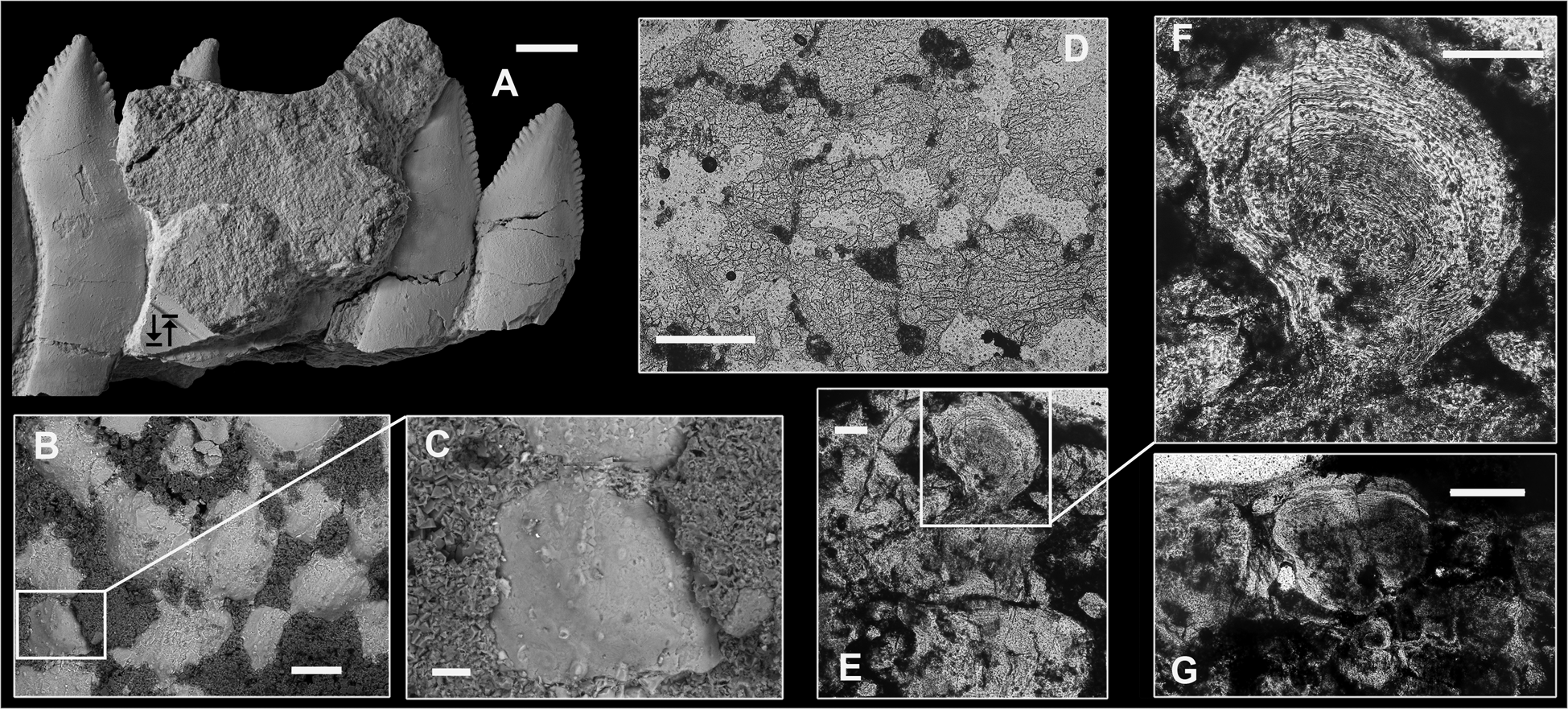
Figure 16 Calcified cartilage in Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a), specimen PIN 1655/656: (a) the largest cartilage fragment on the outer coil of the specimen PIN 1655/656. Black counter arrows show an area from which cartilage samples had been taken for thin sections; (b) scanning electron microscopy image of the cartilage surface; (c) enlargement of an area in (b); in (b and c) cubic dolomite crystals fill in depressions between tubercles; (d) horizontal section showing disposition and interconnection of globules. Visual reticulation results from minute cracks; (e) vertical section showing irregularly disposed globules and their interconnection; (f) enlargement of a globule in (e) showing concentric growth lines; and (g) three large globules separated by interconnecting tissue. Scale bars: (a) = 1 cm; (b) = 250 mkm; (c) = 50 mkm; (d) = 500 mkm; (e) and (f) = 100 mkm; and (g) = 200 mkm.

Figure 17 Calcified cartilage structure in Helicoprion bessonowi Karpinsky, Reference Karpinsky1899: (a) horizontal section showing a network of calcified cartilage units with radial inner structure; and (b) multilayered arrangement of cartilage. Redrawn from Karpinsky, Reference Karpinsky1899. Scale bars: (a) = 500 mkm; and (b) = 1 mm.

Figure 18 Drawing of the specimen PIN 1655/1 presenting wear facets (dark grey), dentine wrinkles (light grey) and preserved enameloid areas (black): (a) right view: and (b) left view. Scale bar = 5 cm.

Figure 19 Wear facets in the specimen PIN 1655/1: (a, b) right side view; and (c, d) left side view. Note parallel lifetime scratch marks. Scale bars: (a) = 5 mm; (b) = 2 mm; (c) = 10 mm; and (d) = 3 mm.
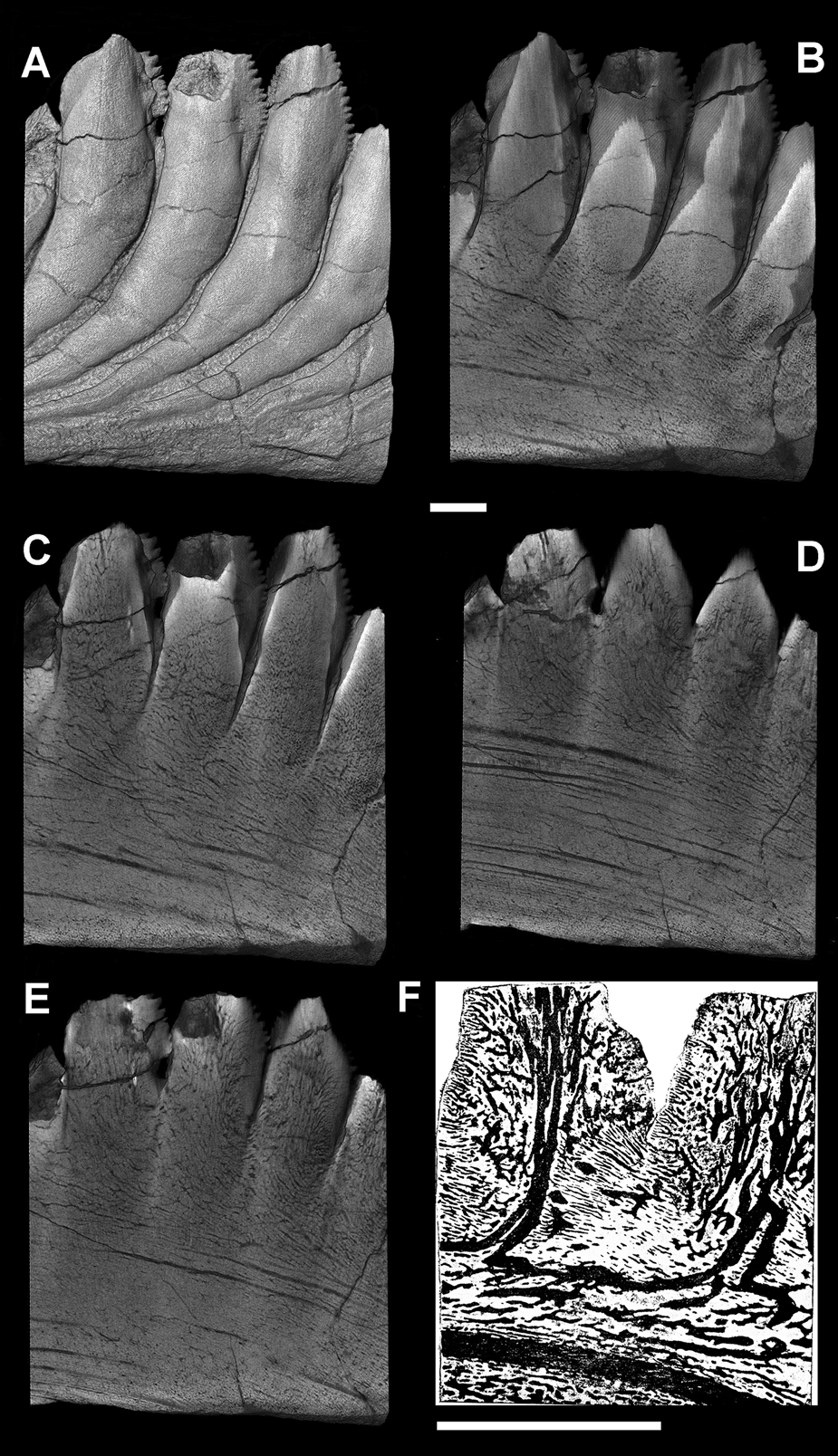
Figure 20 Internal structure of the tooth whorl in Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a), holotype specimen PIN 1655/132, as revealed by microtomography scanning (a–e), longitudinally, and (f) a longitudinal sagittal section of the apical part of the whorl in Helicoprion bessonowi Karpinsky, Reference Karpinsky1899. (f) redrawn from Karpinsky, Reference Karpinsky1899. Scale bars: (a–f) = 1 cm.

Figure 21 Internal structure of the tooth whorl in Karpinskiprion ivanovi comb. nov. (Karpinsky, Reference Karpinsky1924a), holotype specimen PIN 1655/132, as revealed by microtomography scanning (a), transversally, and (b) a transverse section of the whorl in Helicoprion bessonowi Karpinsky, Reference Karpinsky1899. (b) redrawn from Karpinsky, Reference Karpinsky1899. Scale bars: (a) and (b) = 5 mm.

Figure 22 Enameloid in the crowns of Karpinskiprion ivanovi comb. nov. (Karpinsky, Reference Karpinsky1924a) and weathering effects: (a) specimen PIN 1655/653; and (b, c) holotype specimen PIN 1655/132. de = denteon projections to the surface exposed by weathering; ep = preserved enameloid patches; and ev = enameloid preserved in the dentine valleys. Scale bars: (a) = 2.5 mm; (b) = 750 mkm; and (c) = 500 mkm.

Figure 23 Histological structure of the tooth crown of Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a). Transverse vertical thin sections prepared from a crown fragment of the specimen PIN 1655/1: (a)–(b) thin section PIN 1655/1c; and (c)–(f) thin section PIN 1655/1d. dt = dentine tubules; en = enameloid; and vc = vascular canals. Scale bars: (a) and (f) = 200 mkm, (b) = 50 mkm, and (c)–(e) = 100 mkm.

Figure 24 Coiling in Helicoprion Karpinsky, 1899 and Karpinskiprion gen. nov.: (a, c and d) present loose coiling pattern. (a) Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a), specimen PIN 1655/656; and (b, c) Helicoprion bessonowi Karpinsky, Reference Karpinsky1899, (b) holotype specimen F.N. Chernyshev Central Scientific Research Geological Prospecting Museum 1/1865; (c) specimen PIN 1769/3 (figured by Karpinsky, Reference Karpinsky1911); and (d) Helicoprion sp., specimen UCMP 140632, Nevada, USA, Antler Peak Fm., Permian. Scale bars: (a) and (c) = 3 cm; (b) = 2 cm; and (d) = 1 cm.

Figure 25 Karpinskiprion ivanovi comb. nov. (Karpinsky Reference Karpinsky1924a). Specimen PIN 1655/656, with an overlaid logarithmic spiral having spiral angle 75.75° (spiral curve). The arrow points to a region where the crowns are anomalously short. Not to scale.
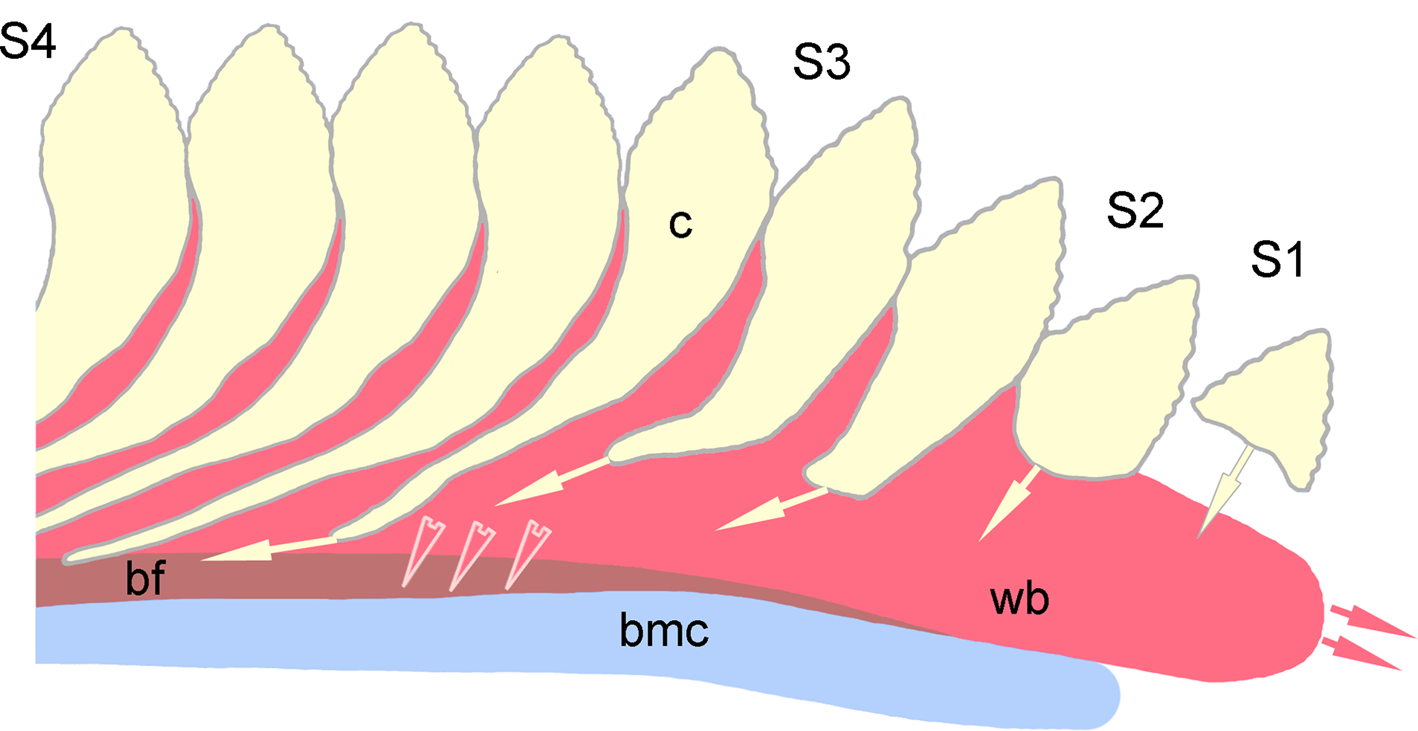
Figure 26 Schematic reconstruction of whorl growth in Karpinskiprion gen. nov. S1–S4 = four consecutive developmental stages. bf = whorl base flanges; bmc = basic mandibular cartilage; c = tooth crowns; and wb = whorl base. Not to scale.
Helicoprion ivanovi: Karpinsky Reference Karpinsky1924a, p. 369, text-figs 1, 2, 3a; Khabakov Reference Khabakov and Gorsky1939, p. 150, pl. 36, figs 2a, b; Obruchev Reference Obruchev1953, pl. 5, fig. 1; and Ivanova & Obruchev (In Ivanova) Reference Ivanova, Obruchev and Ivanova1958, p. 145, pl. 21, fig. 5.
Campyloprion ivanovi: Obruchev Reference Obruchev and Obruchev1964, pl. 3, fig. 2; Zangerl Reference Zangerl and Schultze1981, p. 86, text- fig. 98f; Ginter et al. Reference Ginter, Hampe, Duffin and Schultze2010, p. 125, text- fig. 120a; and Itano & Lucas Reference Itano and Lucas2018, p. 411, text-figs. 12–14.
Holotype: PIN 1655/132, a lingual part of the outer volution of a symphyseal tooth whorl; from a local quarry by the village of Rusavkino, Balashikha District, Moscow Region, Russia; Kosherovo Formation, Dobryatinian Regional Substage, Lower Gzhelian, Upper Pennsylvanian.
Diagnosis: As for the genus.
Referred materials: Specimens PIN 1655/1, two fragments of the same tooth whorl, and a very large tooth whorl specimen PIN 1655/653 from the type locality.
Description: The new specimen PIN 1655/656 (Fig. 2) consists of two almost complete volutions. The inner volution demonstrates the oldest, juvenile crowns. There are three small patches and one large patch of mineralised cartilage which are scattered irregularly at the surface of the whorl, superimposed over the tooth crowns or located close to those. The maximum whorl diameter is 45 cm.
It is not possible to count the exact number of tooth crowns in a whorl because of the missing parts of the outer (second) coil, but this value may be estimated. In the first (inner) coil there are 42 crowns, thus, the insertion angle (sensu Tapanila & Pruitt Reference Tapanila and Pruitt2013) is 8.57°. In the preserved parts this number may be calculated approximately by counting the number of crowns in a 90° sector (making 16 or 17 × 4 = 64–68), in total 106–110 crowns in two coils, without taking into account the missing lingual section. Thus, the number of crowns per volution increased during whorl growth. This may be due to deviation from a logarithmic spiral growth type. For comparison, in Helicoprion, in which the whorl follows this growth pattern, there are approximately 40 teeth (depending on the species and the specimen) in each volution (Tapanila & Pruitt Reference Tapanila and Pruitt2013) and may reach 43 in Helicoprion bessonowi (Karpinsky Reference Karpinsky1899). As an exclusion, in Helicoprion davisii (Woodward, 1886), there are only 34–38 crowns per volution (Teichert Reference Teichert1940).
In contrast to Helicoprion, in which the tooth crown axis is mostly straight, then turns labially while following the spur at an obtuse angle, in Karpinskiprion the crown axis forms a gentle arc (Karpinsky Reference Karpinsky1924a). There are 10–18 denticles, set on both the lingual and the labial cutting margins, in the outer volution of the Karpinskiprion whorl (Fig 6a, b). Locally, the lateral surface of the crown forms wrinkled growth irregularities most likely resulting from uneven deposition of dentine during growth. Variously expressed wrinkles are best seen in the lingual quadrant of the outer volution, especially well expressed in the holotype PIN 1655/132, and specimens PIN 1655/1 and PIN 1655/653 (Figs 3–5, 7). Superficially, the neighbouring crowns are in contact only at the base of serrated margins (Fig 6a, b). Basally from this level, individual crowns fuse internally, seen in section only (Fig. 8, online Supplementary video 1 available at https://doi.org/10.1017/S1755691022000251). The cutting blade width ranges from 2 mm in the smallest tooth in the juvenile volution to 20.5 mm in the largest tooth in the outer volution; and height ranges from 1.1 mm to 22.4 correspondingly (measurements after Itano & Lucas Reference Itano and Lucas2018) (Fig. 9).
In the last preserved cutting blade, the length of its labial margin is 23.4 mm; it bears 18 denticles, with 7.7 denticles per 10 mm. The length of the lingual cutting blade margin is 25.1 mm; it bears 17 denticles, with 6.6 denticles per 10 mm. In the cutting blade number 10 there are nine denticles on the 3.0 mm long lingual margin, making 30 denticles per 10 mm.
Imbrication, an overlap of serrated margins of the adjoining crowns, was previously regarded to be an important taxonomic character (for example, Karpinsky Reference Karpinsky1924a; Itano & Lucas Reference Itano and Lucas2018), mostly because this feature is well expressed in the holotype specimen of Karpinskiprion (Figs 6b, 8). However, neither in PIN 1655/1, nor in PIN 1655/653 and PIN 1655/656 is this feature observed at all, or is only present to a smallest degree (Fig. 6a). For this reason, we suggest that this feature be regarded as individual variation. In fact, imbrication is the only character not following the bilateral symmetry characteristic of the whorl, and it is present in only one Russian specimen, the holotype. Imbrication is also present in the specimen of Karpinskiprion cf. Karpinskiprion ivanovi from New Mexico, NMMNH P-68551 (Itano & Lucas Reference Itano and Lucas2018, text- fig. 15B).
Lateral projections of the tooth crowns (spurs) are very long, the radial line drawn through the projecting part of the crown crosses basally from three to six spurs of the adjoining younger crowns, except in the juvenile and the youngest sections, in which the spurs are much shorter (Fig. 9). Spurs in the outer volution bear minute longitudinal median ridges running almost all the midline length of the spur (Fig. 10a). These ridges may occasionally be interrupted and/or give off short branches. In PIN 1655/653 there is an example of small denticles present on the spur surface in the place normally occupied by a ridge (Fig. 10b).
Generally, the spurs are semi-rounded in section. However, in the holotype PIN 1655/132 the spur tips from the left side seem to be unfinished, showing the external surfaces of circumvascular structures (Fig. 10a). On the right side of the same specimen, the spur of the lingual-most segment is not formed at all, and two or three previous ones are low, and demonstrate very thin, uneven margins (Fig 11a–c). The coating enameloid is almost transparent (Fig 11b, c). Opaque areas in the middle and arranged in a bifurcating manner seem to be recently formed bunches of dentine canals.
Apart from incompletely developed spurs observed on both sides of the holotype specimen (Figs 3, 11a) and from the right side of the specimen PIN 1655/1 (Fig. 5a), several cases of spur malformation are recorded. These may be divided into three groups: (a) growth interruption, both temporary and final; (b) growth distortion; and (c) fusion. We regard all of these to be a result of dental lamina dysfunction caused by some mechanical obstacle preventing growth or some other unknown cause. Notably, no malformations of the cutting blades are known, suggesting absence of such obstacles.
Growth interruptions are recorded in completely formed spurs 6 and 7 from the right side of the holotype specimen. In this case the distal part of spur 7 is connected to the rest of the crown only by a narrow neck, while the distal part of spur 6 is separated completely, also being displaced with respect to the rest of the crown (Fig. 12a). On the right side of the specimen PIN 1655/1, two newly formed spurs are also constricted (Fig 12b, c). From the left side, growth was interrupted, but soon after released in two adjoining spurs, although their growth became reoriented. A fine example of growth distortion and simultaneous fusion is presented in the same specimen, in which the last five newly formed spurs from the left side are affected (Fig 12d, e). In this case, the spur of the crown 4 intervened in the growth of the previous crown 5 hindering its development. At the same time, the spur of the crown 2 fused at the same place, and both became connected to a very large common spur. This blocked growth of crown 3 which produced almost no spur. Finally, an almost normally, but strongly displaced, spur 1 follows very closely the spur of the crown 2. All of these spurs are unusually closely spaced.
Unilateral constriction is seen on the left side of the specimen PIN 1655/653 (Fig. 12f), and at its right side two distal parts of the spurs are separated completely. In the specimen PIN 1655/656, one of the spurs became ‘jammed’ between the neighbouring ones and stopped its growth without reaching the base margin (Fig. 12g).
The most extreme example is observed on the isolated whorl fragment of Karpinskiprion sp., USNM PAL 443547, from north-west Alaska figured by Tapanila et al. (Reference Tapanila, Pruitt, Wilga and Pradel2020) (Fig. 12h). Fusion of the basal parts of the crowns and of the spurs of several of the more labial (right side) teeth creates a continuous band of tissue including at least seven teeth. Basal to the continuous band, the spurs separate and continue basally and labiad. The partially preserved most-lingual tooth crown and its spur is nearly separated from the next more labial crown and spur, with only a narrow region of fusion. An anomaly in this specimen is that the youngest (last formed) crowns are narrower than some of the older ones, which is the opposite of what would be expected if tooth size increased continually with age. Perhaps the last formed teeth represent a gerontic stage or result from lifetime injury.
In another well-known member of the same family, Helicoprion, no spur malformations are observed, in contrast to Karpinskiprion, where malformations occur in all known specimens. This might signify the difference in gene regulation of tooth crown formation between these two genera.
Some cutting blades in the specimens PIN 1655/653 and PIN 1655/1 demonstrate more or less strongly expressed denticles on the lateral sides of the crowns (Fig 13a, b). These denticles are arranged into short radial rows located approximately in the middle between the line drawn through the basal contact of the adjoining cutting blades and the level of the wrinkled dentine.
On all organogenetic stages, the surface between the crowns, including spurs, is ornamented by a network of rough anastomosing ridges enclosing pits penetrated by vascular pores. The whorl base is coarsely striated all along its length (Figs 2, 3, 5) – its depth (width) comprises approximately a quarter of the whole whorl depth. However, in the lingual-most section of the whorl seen in all available specimens, multiple spur tips reach or almost reach the whorl margin, probably resulting from incomplete formation of the whorl base. The basal groove is proportionally deep all along the whorl, although it becomes progressively shallower in the lingual-most section, finally fading; here the whorl base becomes convex (Figs 3c, 5c).
In the holotype specimen PIN 1655/132 and in the specimen PIN 1655/1, the lingual-most parts of the whorl are preserved (Figs 3, 5, 12b, d). Karpinsky (Reference Karpinsky1924a) mentioned that in the holotype there was a blunt thick process destroyed during preparation; this broken surface was subsequently polished (Fig. 14a). The process is reconstructed by a dashed line in his specimen drawing (Karpinsky Reference Karpinsky1924a: text-fig. A, labelled P) (Fig. 14b). In the same figure he also marked the depth of the basal groove, noting its tapering towards the mouth, though he ascribed this to local abrasion. The shape and position of the lingual projection is very much alike in its shape and proportional size to those seen in the Helicoprion bessonowi holotype TsNIGR museum 1/1865 and specimen TsNIGR museum 1/1670 (Fig 14c, d), although in the former the projection is only very lightly phosphatised. The homologous section of the whorl of the specimen PIN 1655/1, from its right side, demonstrates a very short lingual crown base with no spur, and in the next two crown spurs labiad are still very short (Fig. 12b). From the left side, the spurs are much longer but malformed, leaving basally a large free area which ought to be occupied by spurs at the later stage (Fig. 12d).
Juvenile stage: The juvenile, toothless section of the tooth arch is not preserved, being either unphosphatised or dissolved diagenetically (Fig. 15). The smallest tooth crowns are diamond-shape, their main lobe being approximately twice as deep as wide; in the crowns added later the crown height increased in respect to its width. In the first preserved cutting blade the labial edge bears two denticles basally and an undulating margin apically; the lingual edge is undulating; the apex is inclined lingually. In the second cutting blade labially there are two larger denticles basally and three apically; lingually there is one basal denticle and the rest of the edge is undulating. The same pattern is observed of the third cutting blade. In the fourth lobe there are six denticles on the labial margin, two large denticles basally and three small denticles apically. The number of denticles reaches 10 on each side, starting from element number 10. Its width is 2.9 mm, height 3.2 mm, labial margin length is 3.2 mm and lingual margin length is 3.0 mm. The labial margin bears six denticles; its basal section is smooth and on the lingual margin there are nine denticles. The first spurs are formed starting from element number 2; in the smallest elements they almost reach the margin of the whorl base. In the elements number 2 to number 9 (where distinguishable), spurs are short and directed labially at an obtuse angle being connected to the cutting blade by a neck. The radial line drawn through the lobe axis crosses only one, two or a maximum of three spurs of the next crowns, in contrast to 3–6 in the adult (outer) volution. Within the first (oldest) whorl the spurs are directed at an acute angle to the base margin, rather than subparallel to it, as in the second volution. Crowns of elements number 6 and number 8 were broken during life.
Cartilage fragments: As mentioned before, there are three small patches and one large patch of calcified cartilage preserved in association with the tooth whorl in PIN 1655/656 (Figs 2, 16a). These might be attributed to the same individual, becoming stuck to the whorl, because the chances that these four pieces belonged to another fish are very small (but see below). Whether these patches belonged to cranial or postcranial structures is unclear. The external surface of these cartilage patches is macroscopically granulated (Fig. 16a); under the scanning electron microscope the cartilage surface is composed of unevenly placed and irregularly shaped tubercles separated by pits of various shape and size infilled by surrounding matrix (Fig 16b, c). Thin sections reveal a network of calcified elements interconnected by anastomoses. In horizontal section (Fig. 16d) variously sized cartilage units mostly arranged in irregular rows are interconnected to each other by bridges or contact immediately. In the vertical sections (Fig 16e–g) these units do not seem to be arranged in any orderly manner. The space between them is filled with darker matter randomly interrupted by bridges. Separate elements demonstrate a globular, multi-layered, concentric structure; no traces of tesselation are discernible.
The histological structure of the cartilage in a close relative, H. bessonowi described by Karpinsky (Reference Karpinsky1899), differs strongly from the architecture described above. In this fish the cartilage demonstrates a radial structure of the cartilaginous units and their multilayered arrangement (Fig. 17). This may indicate that Karpinskiprion and Helicoprion are not as closely related as previously thought, or that the cartilage patches on the specimen PIN 1655/656 are allochthonous.
Wear traces: Two of the four available Karpinskiprion ivanovi specimens, PIN 1655/1 and PIN 1655/656, demonstrate a feature previously undescribed in any eugeneodontiform chondrichthyan, that is, wear traces on the lateral surfaces of the tooth crowns and not on the crown apices (Figs 12g, 18). In the latter specimen, both sides bear numerous facets of various sizes and shapes mostly limited from their sides by their crown spur margins. Areas bearing these facets start from crown 10 (counted from the latest formed crown) from the right side (Fig. 18a) and crown 11 from the left (Fig. 18b). The faceted area runs as a wide band along the whorl approximately limited in its outer extent by a line connecting points of transition of the crown bases into spurs. This position of the band corresponds to the point where whorl thickness increases towards the base. The width of the band affected by wear increases in the lingual direction from both sides of the whorl, and the band itself gently shifts basally.
The best-preserved facets show parallel, radially directed scratches (Fig. 19). In PIN 1655/656 similar facets are less well-expressed, although present from the left side, exposed by preparation. In this specimen there are only three facets observed approximately in the middle of the preserved part of the outer volution (Fig. 12g). The holotype specimen and PIN 1655/653 do not show any wear facets, seemingly because these samples are short, lingual most parts of the whorl in which these structures had not been formed yet. We suggest that these wear facets result from interaction of the antagonistic dental structures. At the same time, cutting blade apices do not show significant wear in any specimen.
Internal macrostructure: A section of the holotype specimen was analysed by microtomography, which revealed that the base is uniformly penetrated by subparallel, sometimes anastomosing, canals of variable diameter, running along the specimen length (Figs 20a–e, 21a, online Supplementary videos 2, 3). The largest of these passes approximately at the base of the apical third of the whorl depth and is possibly homologous to that of the main blood supply canal described by Karpinsky (Reference Karpinsky1899) in H. bessonowi (Figs 20f, 21b) and by Teichert (Reference Teichert1940) in H. davisii (Woodward, 1886), but is proportionally much thinner. All vascular canals extending basally from this largest canal are smaller in diameter. Crownward, within the bases of the cutting blades, vascular canals become smaller in diameter, change their predominant orientation to oblique linguo-coronal and richly branch. Within the cutting blade, canal bunches are mostly sub-vertical in the central part of the crown, with offshoots towards the crown surface.
Histological structure: Teeth of eugeneodontiform chondrichthyans are thought to be devoid of an enameloid layer with only a compact form of dentine composing the outer layer of the crown. Duffin (Reference Duffin2016) reported the presence of an outer compact layer composed of single crystallites in several eugeneodontiform genera, but his studies did not include Campyloprion. As noted by Karpinsky (Reference Karpinsky1924a), most of the whorl including both bases and crowns is composed of trabecular dentine (vasodentine in his terminology). In all Karpinskiprion specimens examined here the external surface of the tooth crowns is composed of a murky, opaque matter, most likely resulting from weathering, although enamel and enameloid are usually regarded to be the most resistant skeletal tissues. However, three better-preserved locations, seen in all specimens, present exceptions, in which some of the material superficially resembling enameloid is still well-preserved (Fig. 18). The best-expressed locations are spur surfaces, which are completely or partially coated with a rather thick semi-transparent tissue, which occasionally forms longitudinal ridges and tubercles described above (Figs 10, 11). Another common location is valleys formed between the wrinkles in the middle of the crowns (Figs 12b, d, e, 22a). The third presents very rare cases of preservation of minute enameloid patches on the lateral surface of the crowns in the holotype specimen PIN 1655/132 (Fig. 22b) and in PIN 1655/1 (Fig. 13d). These elongated subparallel sheets had probably been preserved after diagenetic dissolution of the rest of the superficial material. Their transparent matter rests upon earthy and soft, opaque matter, possibly changed diagenetically, which in its turn overlies crown dentin composing its outer layers. In the holotype specimen, in the areas in which the surface of the trabecular dentin is affected by weathering to even greater extent, the terminal projections of denteons become exposed (Fig. 22c).
Karpinskiprion (Campyloprion) tooth structure had never been studied before in thin sections. However, more than 120 years ago a profound histological study had been performed by Karpinsky (Reference Karpinsky1899), who provided a thorough description of dental tissues in H. bessonowi, the closest relative of Karpinskiprion. Karpinsky stated that the Helicoprion whorl is totally composed of ‘vasodentine’ (trabecular dentine). Its ‘spongy’ variety constitutes the base, being penetrated by thick and numerous vascular canals, most of which run in parallel to the margins of this structure. The walls of these canals are formed by circumvascular dentine, the lamellar structure of which is visible in polarised light. Closer to the main vascular canal, trabecular dentine changes its structure from spongy to ‘fibrous’. The crown surface is coated by a thin layer of dentine penetrated by branching tubules of ‘tubular vasodentine’, which are directed normally to the crown surface and even enter the tissue composing the serration denticles of the cutting margin. The crown surface is sheathed by a thin layer of enameloid composed of ‘transverse fibres’ seen only at crossed nicols.
Apart from PIN 1655/656, all specimens used in this study are figured, and thus could not be used for thin sections. However, a small detached fragment of the crown from the broken side of PIN 1655/1 was available. The sample comes from the middle part of the crown lobe and bears a wrinkled surface with some enameloid tissue preserved in its valley. Two vertical transverse sections, PIN 1655/1c and PIN 1655/1d, were prepared from this sample, both showing an area of the middle part of the crown adjoining the superficial wrinkled area.
In its general features, the histological structure seen in K. ivanovi is analogous to that described by Karpinsky (Reference Karpinsky1899) in Helicoprion. Due to specific preservation affecting the enameloid layer (see above), this tissue is found only locally, as seen in the presented sections (PIN 1655/1c and PIN 1655/1d, Fig. 23). In the former section the boundary between the dentine and enameloid (en) is wavy, but the external surface of the latter is almost straight, resulting in an alternating thickness of this layer (Fig 23a, b). The superficial layer of dentine is penetrated by numerous branching tubules (dt). In PIN 1655/1d the wavy nature of the boundary is less expressed and the enameloid layer mostly preserves its thickness throughout the specimen (Fig 23c–e). As usual, large vascular canals (vc) are running within a mass of trabecular dentine penetrated by minute branches of dentine canals, but no concentric circumvascular dentine is present possibly suggesting that after the crown had been formed, no deposition within the tubes took place, maybe due to rapid crown formation.
Presence of enameloid contrasts with the opinion presented by Duffin (Reference Duffin2016) on the absence of an enameloid layer in Eugeneodontiformes. However, no helicoprionids had been included in his research.
6. Discussion
6.1. Tooth whorl and enclosing cartilage
As suggested by Karpinsky (Reference Karpinsky1911, Reference Karpinsky1915) the whorl in Helicoprion might be enclosed in the jaw cartilage, but his first earlier graphic reconstruction did not show this. Obruchev (Reference Obruchev1953) reconstructed the whorl in Helicoprion with symphyseal cartilage filling the space between the coils. His reconstruction was based upon the presence of cartilage patches remaining there (Obruchev Reference Obruchev1953; Lebedev Reference Lebedev2009, text-fig. 6b). Bendix-Almgreen (Reference Bendix-Almgreen1966), on the basis of uniquely preserved materials from the Phosphoria Formation (Idaho), stated that the whorls in Helicoprion rested upon an unpaired basic mandibular cartilage and became enclosed in a case formed by Meckel's cartilages, with this enclosure of the whorl currently generally accepted (for example, Bendix-Almgreen Reference Bendix-Almgreen1966; Ginter et al. Reference Ginter, Hampe, Duffin and Schultze2010; Tapanila et al. Reference Tapanila, Pruitt, Pradel, Wilga, Ramsay, Schlader and Didier2013; Ramsay et al. Reference Ramsay, Wilga, Tapanila, Pruitt, Pradel, Schlader and Didier2015).
In general features, the symphyseal tooth whorl growth mode in Karpinskiprion does not seem to differ much from that described in another member of the same family, Helicoprion (Lebedev Reference Lebedev2009; Tapanila & Pruitt Reference Tapanila and Pruitt2013). In fact, only two major characters related to growth mode are distinct, that is the tight versus loose coiling mode and spur formation.
It is most parsimonious to suggest that in Karpinskiprion the jaw structure approximately followed the same pattern. The difference between these two genera is coil packing. Volutions in Karpinskiprion are widely separated (Fig. 24a). In the Helicoprion holotype (Fig. 24b) and many other specimens the volutions are set so close to each other that the distance between the crown apices of the preceding coil approximately equals the height of the projecting part of the crown. However, there are some exceptions. In specimen PIN 1769/3 (in which unfortunately only one complete crown is preserved, figured by Karpinsky, Reference Karpinsky1911) originating from the same type locality as all other specimens, this distance between coils is 2.5–3 times larger than the crown height (Fig. 24c). Another example of the same deviation is presented by the specimen UCMP 140632 from Nevada, United States, deposited in the Museum of Paleontology in the University of California (Hanger & Strong Reference Hanger and Strong1998). This specimen is incompletely preserved, but there is a single preserved tooth crown on the internal coil, the height of which is also approximately three times smaller than the distance between this coil and the outer one (Fig. 24d). We suggest that these loosely coiled specimens might represent small-crowned morphs of H. bessonowi and Helicoprion nevadaensis (or Helicoprion sierraensis, these species may be synonymous), respectively. We also speculate here that small crown size might correlate with increased depth of the basic mandibular cartilage. However, the biological importance of loose coiling in Karpinskiprion remains an open question.
6.2. Spiral shapes of tooth whorls of Karpinskiprion and other helicoprionids
Itano & Lucas (Reference Itano and Lucas2018) proposed the use of the spiral angle of the logarithmic spiral of the tooth whorl as a taxonomic criterion for certain helicoprionids, but this is only useful if tooth whorl shape is in fact well-described by these spirals. It is well-established that the tooth whorl of Helicoprion is well-described by a logarithmic spiral with spiral angle of 81°–83° (Tapanila & Pruitt Reference Tapanila and Pruitt2013). There are, however, deviations from a logarithmic spiral for the innermost and outermost parts of the tooth whorl (see, e.g., Itano & Lucas Reference Itano and Lucas2018, text-fig. 5). Itano & Lucas (Reference Itano and Lucas2018) showed also that the tooth whorl of Shaktauites is well-described by a logarithmic spiral with spiral angle equal to 76°. In both cases, more than one volution of the spiral was preserved. This aids in determination of the spiral angle. For all previously known specimens of Karpinskiprion, determination of the spiral angle was made difficult by imperfect preservation, particularly the missing apices of the crowns, and the fact that none of the available specimens preserved more than a small fraction of a volution. Estimates for the spiral angle of Karpinskiprion ranged from 58° to 67°. Moreover, it was not known a priori that the tooth whorls were in fact well-described by logarithmic spirals.
Analysis of PIN 1655/656, the only specimen of Karpinskiprion known to preserve more than one volution of the tooth whorl, reveals that the tooth whorl of Karpinsiprion is only well-described by a logarithmic spiral for about the innermost 1.5 volutions. Figure 25 is a photograph of PIN 1655/656 with an overlay (red) of a logarithmic spiral having a spiral angle of 75.75°. While there is good agreement of the red curve with a curve passing through the crown apices over most of the inner (labial) portion, there are three anomalies:
(1) The innermost (most labial) portion of the tooth whorl deviates from the spiral curve.
(2) The outermost (most lingual) portion of the tooth whorl deviates from the red curve.
(3) Over a relatively small interval, indicated by the red arrow, the crowns are anomalously short.
Two small tooth whorls, USNM PAL330003 and PAL330004, from the Jacksboro Limestone Member of the Graham Formation, early Gzhelian Stage, near Jacksboro, Texas, United States, were found to be well-described by logarithmic spirals with a spiral angle of about 58° (Itano & Lucas Reference Itano and Lucas2018, text-figs. 6, 16). They were tentatively identified as Campyloprion sp. and were thought perhaps to represent the innermost parts of Campyloprion (Karpinskiprion) tooth whorls. However, they do not match well the innermost portion of PIN 1655/656, so they likely do not belong to Karpinskiprion. Tapanila et al. (Reference Tapanila, Pruitt, Wilga and Pradel2020) identified these two specimens as Toxoprion sp. The holotype and only known specimen of Toxoprion lecontei (Dean Reference Dean1898), the only species of Toxoprion, is believed to be from the Carbon Ridge Formation, Lower Permian (Cisuralian), near Eureka, Nevada, United States (Itano & Lucas Reference Itano and Lucas2018). That specimen is too poorly preserved to be able to assign the two specimens from Texas to the same genus. The Texas specimens cannot be assigned to any other genus with any degree of certainty. Hence, they are presently identified only as Helicoprionidae incertae sedis.
6.3. Tooth whorl growth
The lingual-most parts of the whorl bear data on its growth mode, making possible the reconstruction of the whorl formation sequence (Fig. 26). Tracing the whorl structure from this area towards its labial part makes it possible to follow the order in which this structure had been formed. The lingual-most structure includes a terminal process of the base mentioned by Karpinsky (Reference Karpinsky1924a) in the holotype specimen and also seen, although imperfectly preserved, in the specimen PIN 1655/1. The whorl formation process may be conditionally subdivided into stages:
Stage 1 (oldest). The terminal process formed as a knob, consisting of trabecular dentine and penetrated by longitudinal canals, and is suggested to be formed first in organogeny. At approximately the same time we hypothesise separate formation of the youngest cutting blade of the crown, never found separately in the fossil record. At this stage the basal side of the base is convex, suggesting no or little contact with the supporting basic mandibular cartilage.
Stage 2. Labiad, the cutting blades fused to the previously formed base with formation of short basal crown processes, the spurs. The base grows uniformly in the basal direction and is still convex.
Stage 3. The spurs continue growing labio-basally along the surface of the base. Base flanges start embracing the basic mandibular cartilage, forming the basal groove. Growing flanges give more space for growth of spur tips.
Stage 4. Completely formed spurs parallel to each other may almost reach the margin of the base flange. Spurs form a grater, secondarily participating in food processing at an adult stage as suggested by wear facets.
At stages 1–2 absence of a basal groove may suggest the lingual position of this whorl section in respect to the symphysis; the origin of this groove might document the start of contact with the basic mandibular cartilage, that is, to the symphyseal area.
Notably, the spur growth rate changes organogenetically. In the inner (juvenile) volution short spurs are only directed at an angle to the margin of the base flange, as in Helicoprion; during the animal's growth the spurs elongate and become arranged almost parallel to this margin, forming a comb-like pattern. We suggest that during life, space between spurs was intensely supplied with blood and was occupied by the dental lamina that made possible continuous spur growth resulting in ongoing elongation, after the basic part of the crown had been formed. This suggestion may be supported by structure of the lingual-most spurs in the best-preserved holotype specimen showing unfinished growth and described above. On its right side the spur of the lingual-most segment is not formed yet, and two or three previous ones are very short, their margins are uneven, very thin and almost transparent. In comparison to earlier formed spurs, the latest ones should also have been finalised, but were not because of the animal's death.
6.4. Tooth whorl position and function
Unlike in Helicoprion, the position of the symphyseal tooth whorl and its counteraction against the structures in the opposite jaws in Karpinskiprion are uncertain. In Helicoprion, the position of the symphyseal tooth whorl is proven to be in the lower jaw by the presence of cranial cartilages (Tapanila et al. Reference Tapanila, Pruitt, Pradel, Wilga, Ramsay, Schlader and Didier2013). The close similarity of the PIN 1655/656 specimen of Karpinskiprion to a tooth whorl of Helicoprion indicates that it might also be located in the lower jaw. While the nature of the dentition of the upper jaw of Karpinskiprion is not known directly from fossil evidence, certain inferences can be made on the basis of the wear observed on the symphyseal tooth whorls. The shape and position of the faceted areas on the lateral faces of the tooth crowns resulting from interaction with the antagonistic dental structures suggests that the last 10 tooth crowns either did not participate in this pseudo-occlusion, being still located too deep in the mouth, or that growth rate was so fast that abraded crowns became shifted outwards, out of the reach of the contact area to the antagonist dentition, very soon after the development of the facet. The most striking consequence of the presence of these wear facets is their placement on both sides of the whorl. Wear is not observed on any of the crown apices.
It is useful to compare and contrast the tooth wear observed in Karpinskiprion to that observed in Edestodus (Itano Reference Itano2015) and Edestus (Itano Reference Itano2018). These latter two closely related genera are known to possess symphyseal tooth whorls of similar size in both jaws. No wear was observed on the lateral faces of the crowns, but significant wear on the crown apices was observed. This wear was interpreted as being due to abrasion against the surface of rough-skinned prey, since the convex curvature of the tooth whorls would largely prevent occlusion between the teeth of opposing jaws. (An alternative interpretation is proposed by Tapanila et al. (Reference Tapanila, Pruitt, Wilga and Pradel2020), in which the opposing tooth whorls act against each other, like the blades of a reciprocating saw (see, e.g., Zangerl & Jeremiah Reference Zangerl and Jeremiah2004, p. 16), and the wear on the crown apices might be due to tooth-on-tooth contact.)
Sarcoprion edax Nielsen, Reference Nielsen1952 from the Permian of Greenland, known from articulated cranial material, possesses a gently arched lower symphyseal tooth whorl and an almost straight upper symphyseal tooth whorl (Nielsen Reference Nielsen1952). The teeth are worn on both lateral faces and on the crown apices. Wear at the apices is interpreted as the result of contact with prey. Wear on the lateral faces, however, resulted from contact between the teeth of the lower and upper symphyseal tooth whorls. In order to account for the wear on both lateral faces, it is necessary to posit a scissor-like action, with the positions of the lower and upper tooth whorls alternating from side to side with respect to each other (Nielsen Reference Nielsen1952, p. 32).
Helicoprion is not known to possess any large teeth in the upper jaw, although the possibility of the presence of a pavement of small Campodus-like teeth in the palatoquadrate has been noted (Lebedev Reference Lebedev2009). Lebedev (Reference Lebedev2009) noted radially directed scratches on the lateral faces of the symphyseal teeth, likely due to interaction with prey, but no wear facets such as those in Karpinskiprion were observed.
There would appear to be three possible configurations of the upper dentition in Karpinskiprion that could result in the observed wear to both lateral faces on teeth of the lower tooth whorl: (a) presence of an upper symphyseal tooth whorl, with the two opposing tooth whorls acting as scissor blades, moving alternately from side to side, as in Sarcoprion; (b) two closely spaced parasymphyseal tooth whorls in the upper jaw, with the lower tooth whorl fitting between them; or (c) a pavement dentition on the palatoquadrates.
The only available character making possible speculations on the presence of paired whorls in the upper jaw is the strongly expressed unilateral imbrication in the holotype specimen PIN 1655/132, not observed to the same extent in any other known specimen. This deviation from bilateral symmetry may suggest that this specimen is the only one of the available specimens representing one element of a paired whorl.
6.5. Feeding
The feeding mode may be reconstructed by analysis of morphology and wear traces, making it possible to interpret what type of food might be used as prey and how food processing might have occurred.
One of the most indicative morphological features for reconstructing the mode of food processing in vertebrates is serration of tooth crown margins. Serrated teeth are known in many extant and fossil carnivorous tetrapods and fishes, including recent sharks (for example, Berkovitz & Shellis Reference Berkovitz and Shellis2016; Moyer & Bemis Reference Moyer and Bemis2017). Despite general morphological similarities, serration is variable and is adapted for specific food objects (for example, Frazzetta Reference Frazzetta1988). Comparison of serration observed in the closely related helicoprionid genera Helicoprion and Karpinskiprion reveals some distinctions. For example, in Helicoprion crenulating cusps may be absent or are wider than deep and bear one or two vertical incisions (Lebedev Reference Lebedev2009), that differs from long and unincised denticles in Karpinskiprion (Fig. 6). While studying differences in tooth serration, Frazzetta (Reference Frazzetta1988) concluded that size of serrations and distance between their apices should correlate with the microrelief of the ‘substrate’, that is of the prey skin surface. Thus, observed types of serration in Karpinskiprion and Helicoprion should suggest different objects of prey taken.
Relatively good preservation of serrations and rarely broken denticles during life suggest that Karpinskiprion food objects were not protected by any type of rigid integument. Thus, we suggest that their diet included soft parts of large cephalopods, as already proposed for Helicoprion (Lebedev Reference Lebedev2009; Tapanila et al. Reference Tapanila, Pruitt, Pradel, Wilga, Ramsay, Schlader and Didier2013, 2018; Ramsay et al. Reference Ramsay, Wilga, Tapanila, Pruitt, Pradel, Schlader and Didier2015), but soft-skinned fish should be also regarded as a possible object of prey. The similarity of its triangle-shaped cutting blades with serrated margins to those observed in Helicoprion and Edestus suggests a grasping–slicing function of its dental system.
If tooth size growth in organogeny more or less corresponds to the total increase in fish size, we may evaluate the growth rate of Karpinskiprion during life. If the smallest cutting blade at the juvenile stage is 2 mm wide and the largest reaches 20.5 mm, and height of the same elements increased from 1.1 mm to 22.4 mm, that is a dramatic 10–20 times increase in size. Shape of the tooth crowns, absolute enlargement of the amount of marginal denticles in parallel with the reduction of denticle number per 1 cm and absolute enlargement of the denticle size make us suggest a significant change of diet during the life cycle of the fish. At the same time, the huge size of the adult fishes required prey of comparable size.
In contrast to the Permian Helicoprion community, there are almost no ammonoids in the Moscow Gzhelian basin. Although nautiloids recorded there may attain large size, they are rare. This also makes us think that large fishes with naked skin might constitute a significant part of Karpinskiprion's diet.
6.6. Palaeozoogeography
During the Middle and Late Pennsylvanian, seaway connections were very limited due to frequent sea level fluctuations under the influence of periodic glaciations in Gondwana. The origin of the conodont Idiognathodus simulator and an assemblage of other conodont species during the earliest Gzhelian might result from a short but very massive transgression after a strong eustatic sea level fall. This transgression is recorded in the Midcontinent of North America (Heckel et al. Reference Heckel, Alekseev, Barrick, Boardman, Goreva, Isakova, Nemyrovska, Ueno, Villa and Work2008), Moscow Basin (Alekseev et al. Reference Alekseev, Goreva, Isakova, Kossovaya, Lazarev, Davydov, Alekseev and Goreva2009), Tatarstan (Sungatullina Reference Sungatullina2008), on the Samara Bend (Ermakova et al. Reference Ermakova, Reimers, Alekseev and Zhamoida2012) and South Urals (Chernykh Reference Chernykh2012), Donets Basin (Kozitskaya et al. Reference Kozitskaya, Kosenko, Lipnyagov and Nemirovskaya1978) as well as in South China (Qi et al. Reference Qi, Barrick, Hogancamp, Chen, Hu, Wang and Wang2020).
The Central Russian shallow-water epicratonic sea occupying the eastern part of the EEP during the I. simulator sea level rise was at least several tens of metres deep (Gondolella conodont biofacies) that made possible intense faunistic migrations between the Moscow Basin and the Volga region of a distance of 700–800 km. However, the faunistic exchange between the Moscow Basin and the North American Midcontinent was much more limited, thus the similarity of even the pelagic conodont assemblages was not high (Barrick et al. Reference Barrick, Alekseev and Nemyrovska2000). The only possible seaway connection between these two basins might follow the Barents Sea, Spitsbergen, Sverdrup Basin in the Canadian Arctic Archipelago and further along the western margin of the North American continent. Despite these limitations, occurrence of Karpinskiprion in the Midcontinent and EEP in the approximately synchronous deposits also supports our assumption on the faunistic connections between these two basins despite their distant position. We suggest that these large cruising fishes were capable of long migrations; however, it remains unclear why the members of this genus had not been spread more widely, on a global scale, in a manner demonstrated by another helicoprionid, the early Permian Helicoprion (for its distribution see, e.g., Lebedev (Reference Lebedev2009)).
7. Conclusions
Restudy of the American and Russian species previously belonging to the genus Campyloprion revealed that the holotype of its type species C. annectans is not diagnostic at the species level and is also of unknown provenance. For this reason, we regard it as a nomen dubium. Selection of a neotype is not possible as specimens attributed earlier to C. annectans are insufficiently well preserved. Since this species was explicitly designated as the type species of the genus Campyloprion by Eastman, this requires suppression of the genus also. For this reason, a new genus Karpinskiprion Lebedev et Itano gen. nov., including the only species, K. ivanovi (Karpinsky Reference Karpinsky1924a) is established.
The composition of the family Helicoprionidae Karpinsky, 1911 is revised and a new family Helicampodontidae Itano et Lebedev fam. nov. is erected. The genus Helicampodus Branson, Reference Branson1935 is removed from the Edestidae and Hunanohelicoprion Liu, Reference Liu1994 from the Helicoprionidae to a new family Helicampodontidae, to include, apart from the type genus, Sinohelicoprion Liu & Chang, Reference Liu and Chang1963, Hunanohelicoprion Liu, Reference Liu1994 and Parahelicampodus Nielsen, Reference Nielsen1952.
A newly discovered specimen from Russia belonging to this genus is an almost complete tooth whorl; it demonstrates the coiled nature of these dental complexes comparable to those in Helicoprion. The type of the spiral in Karpinskiprion gen. nov., earlier presumed to follow the logarithmic pattern, is shown now to deviate from it, demonstrating an exceptional coiling type.
The organogenetic development of the tooth whorl in Karpinskiprion gen. nov. shows not only an increase of absolute size of tooth crowns but changes in the cutting blade shape, number of marginal denticles and relative growth of spur length. These changes suggest gradual changes in diet of the fish during its ageing. The organogenetic growth rate of Karpinskiprion gen. nov. tooth whorl suggests a 10–20 times size increase in size of the whole fish.
Growth stages of the lingual-most section of the whorl are reconstructed. The terminal process of the base growing first received bases of tooth crowns formed separately, after that the lateral crown projections, the spurs, start producing long sub-parallel processes along the base margin. During a short period, spurs elongated and became arranged almost parallel to this margin forming a comb-like pattern. The originally convex basal side of the base initially embraced the basic mandibular cartilage and formed lateral flanges housing a deep groove at the basal side of the whorl enclosing it. In general features, the growth process was similar to that in the type genus of the family, Helicoprion.
Two of four K. ivanovi specimens demonstrate almost symmetrical wear traces on both sides of the whorl without wear of the crown apices. We suggest that basal wear facets result from interaction of the antagonistic dental structures during mouth closure. Presence of these structures implies that either the mandibular joint made possible mouth closure with lateral movement, resulting in alternating left–right–left positioning of dental structures with respect to each other, or that the dental element in the antagonist jaw was paired (including the possibility of a bilaterally symmetrical dental pavement in the palatoquadrates). Facets show parallel radially directed lifetime scratches. Spurs bearing wear traces form a grater, secondarily participating in food processing at an adult stage.
Food objects of Karpinskiprion gen. nov. are presumed not to have been protected by any type of rigid integument. We suggest that the diet of these fishes included soft parts of shelled cephalopods and soft-skinned fish. Triangle-shaped cutting blades with serrated margins suggest a grasping–slicing function of its dental system.
The symphyseal tooth whorls of Karpinskiprion gen. nov. are found within a short stratigraphic interval, the Middle Kasimovian–Lower Gzhelian of the Upper Pennsylvanian, also being limited geographically. Occurrence of Karpinskiprion in North America and EEP in approximately synchronous deposits demonstrates the relatively open faunistic connections between these two basins. We suggest that these large cruising fishes were capable of long migrations. The possible seaway connection between these two basins might follow the direction through the Barents Sea, Spitsbergen, Sverdrup Basin in the Canadian Arctic Archipelago and further along the western margin of the North American continent.
8. Supplementary material
Supplementary material is available online at https://doi.org/10.1017/S1755691022000251.
9. Acknowledgements
Material from Perevozinka locality had been collected by the scientific and educational expedition ‘Navigating Universities Flotilla’. Fieldwork assistance from Eugenia G. Romanova and Ivan A. Yashkov (Museum of Geology, Oil and Gas, Khanty-Mansiysk, Russia) is gratefully acknowledged. Christian Klug (Institut für Paläontologie und Paläontologisches Museum, University of Zürich, Zürich, Switzerland) and an anonymous referee supported us with their first positive response to our study and provided useful comments and corrections that helped to improve manuscript quality. We are grateful to Pat Holroyd (University of California Museum of Paleontology, UCMP) for access to the aberrant Nevada specimen of Helicoprion UCMP 140632 and David Bohaska (National Museum of Natural History, USNM) for information regarding the specimen USNM PAL 443547. An inset map of the position of the Rusavkino locality in the Moscow Region of Russia was kindly provided by Yuri V. Yashunsky (Geological Institute of the Russian Academy of Sciences (RAS), Moscow, Russia). The authors acknowledge kind assistance from Roman A. Rakitov (Palaeontological Institute, Russian Academy of Sciences (PIN RAS)), who performed the scanning electron microscope studies and microtomography. Macrophotography was carried out by Sergey V. Bagirov (PIN RAS).
10. Financial support
This study was funded by the Russian Foundation for Basic Research and the Royal Society of London, project number 21–54–10003. A.V. Ivanov's research was supported by the government mandate of the Museum of Earth Sciences in the Moscow State University ААААА-А16-116042010089-2 ‘Biosphere functions of ecosystems, their components and rational use of natural resources’ and AAAA-A16-116042710030-7 ‘Museology and education by museum means in Earth Sciences’; within the framework of the government mandate of the Institute of Geography of the Russian Academy of Sciences AAAA-A19-119021990093-8 (FMGE-2019-0007) ‘Assessment of physical-geographical, hydrological and biotic changes in the environment and their consequences for creating the foundations for sustainable nature management’.
11. Competing interests declaration
None.





























