The management of patients with major depression who have failed to respond to antidepressant medication is a common clinical problem in general and old age psychiatry. Since I last reviewed this topic in APT, over 5 years ago (Reference CowenCowen, 1998), there have been a number of promising developments in the pharmacological management of treatment-resistant depression, but patient and practitioner are still faced with many difficulties when trying to select effective therapeutic approaches (Box 1).
Box 1 Developments in the pharmacological management of treatment-resistant depression
-
• Patients presenting to psychiatrists have usually already received at least two adequate trials of antidepressant medication without response
-
• More antidepressant medications are now available
-
• Treatment focus is on achieving clinical remission
-
• The evidence base of treatment remains poor
-
• Treatment algorithms and guidelines have been developed
Definition
The definition of treatment-resistant depression is somewhat arbitrary, but in pharmacological terms there is the presumption that the depressive syndrome has not responded to a trial of at least one antidepressant medication. It has been estimated that about 20–30% of patients with major depression fail to respond to treatment with a single antidepressant drug given in adequate dosage for an appropriate period. About half such patients will respond when switched to another antidepressant medication (see Reference FavaFava, 2000). It is therefore helpful to identify different degrees of treatment resistance depending on the nature of the interventions that have been unsuccessfully deployed (Fig. 1).
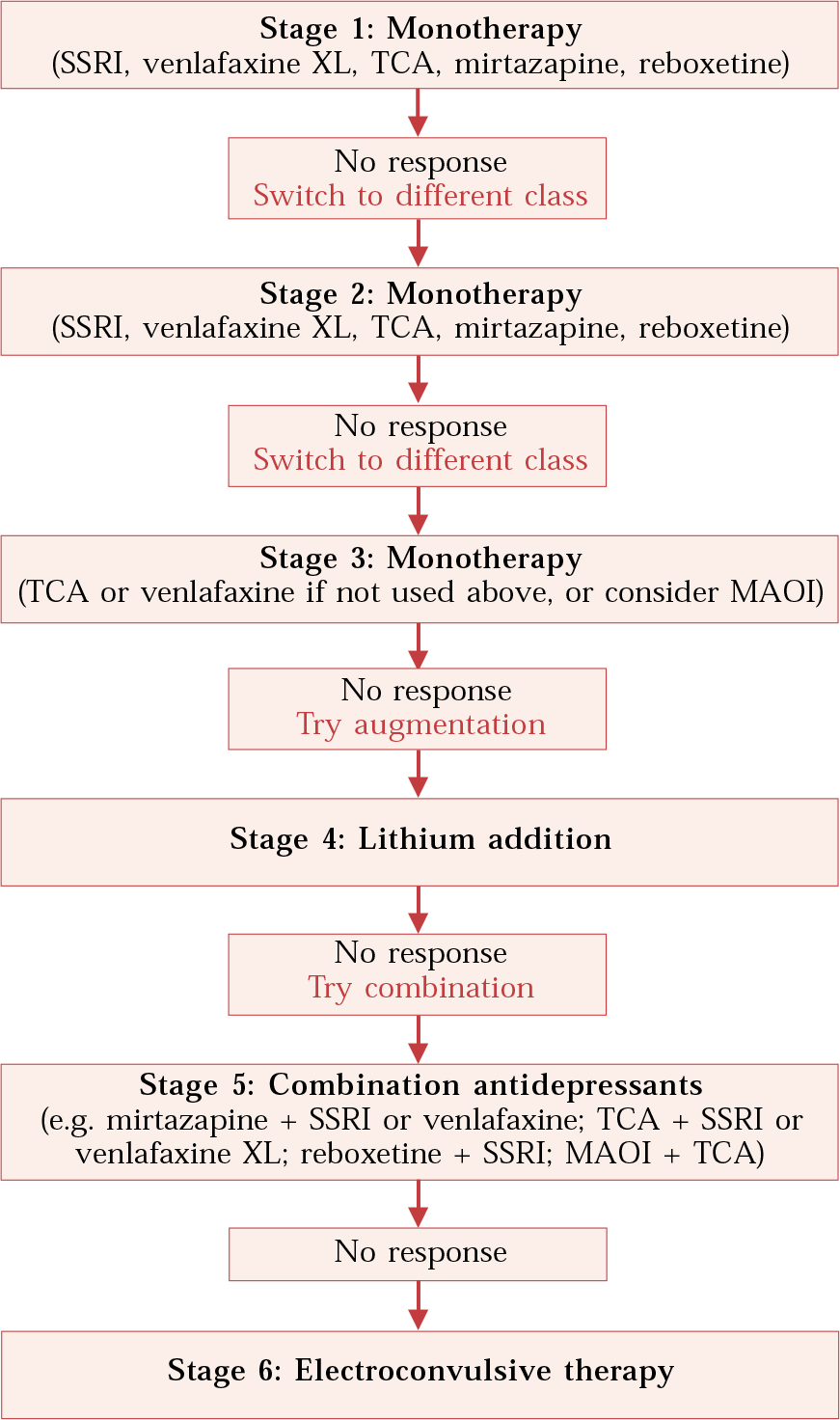
Fig. 1 Medication algorithm for treating depression at increasing levels of resistance. (Adapted from Reference Trivedi, Rush and CrismonTrivedi et al, 2004.)
Assessment
Patients with depression are often referred to psychiatrists after a considerable period of treatment with different pharmacological and psychological approaches. It is important to confirm the diagnosis of major depression and exclude general medical disorders. Psychiatric comorbidity such as substance misuse and personality disorder can worsen the outcome of depressive disorder. Other factors associated with lack of recovery from major depression include more severe depressive states, chronic social difficulties and continuing life events (see Reference Anderson, Nutt and DeakinAnderson et al, 2000).
It is important to enquire about the possibility of manic or hypomanic episodes, whether or not these appear to have been precipitated by antidepressant medication. Hypomanic symptoms can be difficult to detect from a clinical history, but even episodes of brief duration can have implications for treatment response (see below). Bipolar II disorder is not uncommon in patients who present with treatment-refractory depression, and, from the point of view of medication response, depressed individuals with a premorbid ‘hyperthymic’ personality (unusually energetic, cheerful and motivated) may resemble patients with a bipolar II disorder (Reference Hurowitz and LiebowitzHurowitz & Liebowitz, 1993).
The assessment should attempt to determine how long the depression has been present, its nature and course, and the treatments that have been used. Often, patients will have improved to some extent and this may influence whether or not major changes in pharmacological management are needed. It is also helpful to gain an idea of age at onset of the first depressive episode and the usual premorbid level of functioning. It is useful to develop with the patient a model for understanding the origin of the depression and possible maintaining factors under the usual headings of genetic risk (family history), temperament, childhood experiences, levels of social support and more recent stresses and losses. It is important to interview an informant, particularly if that person is a carer and therefore sharing the burdens imposed by the depressive illness.
General measures
All patients need psychotherapy in the general sense of supportive listening, education and reassurance. Patients with chronic depression are often under standably demoralised, pessimistic and despairing. The treatment plan should therefore be developed as a collaborative exercise between the patient and the clinical team, and a stepped approach adopted (see Reference Thase, Friedman and HowlandThase et al, 2001). Patients appreciate a practitioner who makes it clear that pharmacological treatment will be used with care and skill and full, informed consent. Emergent adverse effects should be taken seriously and not minimised or attributed to underlying mental state.
Many patients with resistant depression are not at work and it is important to get a clear idea of how they are spending their time. Activity scheduling can be helpful, particularly if it is possible to find occupations that provide enjoyment or at least distraction from constant depressive ruminations. The role of structured psychotherapies in the treatment of resistant depression is being developed (Reference Thase, Friedman and HowlandThase et al, 2001). Cognitive techniques can be useful in helping patients to focus on achievement and interrupt negative thinking patterns.
Medication issues
The pharmacological treatment of patients who have failed to respond to antidepressant medication involves a number of different strategies:
-
1 optimisation of current treatment;
-
2 switching to another antidepressant;
-
3 combination treatment with antidepressants;
-
4 other augmentation treatments;
-
5 electroconvulsive therapy (ECT).
The Texas Medication Algorithm Project combines these strategies into a stepped approach, which is described in simplified form in Fig. 1. Although the use of this algorithm is associated with better results than treatment as usual (Reference Trivedi, Rush and CrismonTrivedi et al, 2004), many of the treatment suggestions are not backed by randomised trials. However, there is a good deal of evidence in the form of open studies and case series. The need for large-scale randomised trials of pharmacological treatment in resistant depression is increasingly recognised, and results from studies currently being conducted, such as the Sequenced Treatment Alternatives to Relieve Depression (STAR*D; Reference Rush, Fava and WisniewskiRush et al, 2004), which is sponsored by the US National Institute of Mental Health, are keenly awaited.
Generally, switching antidepressant medication is seen as the best initial step following failure with a first antidepressant medication. Augmentation strategies such as antidepressant combinations and lithium augmentation are recommended for patients who have not responded to two or more trials of single medications. However, application of this stepped approach should be flexible. For example, some patients may gain limited but definite benefit from an initial treatment and this will almost certainly be lost when the first preparation is withdrawn. A situation like this might recommend an earlier use of an augmentation strategy (Table 1). Finally, if an individual has, for example, depressive psychosis with active suicidal ideation it may be appropriate to consider the use of ECT before several lengthy trials of medication.
Table 1 Switching antidepressants v. augmentation
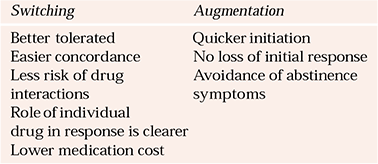
| Switching | Augmentation |
|---|---|
| Better tolerated | Quicker initiation |
| Easier concordance | No loss of initial response |
| Less risk of drug interactions | Avoidance of abstinence symptoms |
| Role of individual drug in response is clearer | |
| Lower medication cost | |
As noted in Box 1, where possible the aim of treatment should be to achieve remission, which in practice means that patients should be virtually asymptomatic. This is associated with lower rates of relapse to major depression. However, many pharmacological studies in treatment-resistant depression use a 50% reduction in a depression rating scale score to define a patient as a ‘responder’. This criterion can, of course, result in patients experiencing significant residual depressive symptomatology, despite being definitely improved. Overall, the current data suggest that clinicians need to use available treatment options (both pharmacological and psychological) to achieve symptomatic remission where this is at all possible (Reference Fava, Fabbri and SoninoFava et al, 2002).
Optimising current treatment
The optimisation of treatment centres in concordance with current medication and dose–response issues. It is well recognised that tricyclic antidepressants (TCAs) can be more effective in higher dose, and doses greater than 150mg daily of amitriptyline, imipramine and clomipramine can be used, provided that tolerance is satisfactory. Plasma monitoring of TCA levels is helpful at higher doses, to guard against toxicity, particularly if there is a possibility of pharmacokinetic interactions. It is prudent to avoid the use of high-dose TCA treatment in patients with a history of cardiac disorder or those who are taking other medications that might impair cardiac conduction.
Venlafaxine also appears to have greater efficacy at higher doses in treatment-resistant patients (Reference de Montigny, Silverstone and Debonnelde Montigny et al, 1999), but selective serotonin reuptake inhibitors (SSRIs) are said to have relatively flat dose–response curves. Despite this, if tolerance permits, increasing the dose of an SSRI can produce symptomatic improvement, particularly in patients who have shown a partial response (Reference Fava, Rosenbaum and McGrathFava et al, 1994).
Switching antidepressants
If a patient does not respond to one antidepressant, the first step is usually to stop this medication and try another. Most published studies of this approach have studied patients in an open sequential way; clearly, this cannot control for the placebo effect or the possibility of spontaneous remission. Overall, however, there is reasonable evidence that switching to a second antidepressant produces benefit in about 50% of patients unresponsive to an initial medication trial (see Reference NelsonNelson, 2003).
If a patient has not responded to one kind of antidepressant, it would seem sensible to switch to an antidepressant with a different pharmacological profile. However, it must be acknowledged that open studies have shown equally good response rates when patients who failed to respond to one SSRI were switched to another (see Reference NelsonNelson, 2003). Switching from a drug with serotonergic properties to another serotonergic compound (for example, from citalopram to venlafaxine) should be carried out cautiously because of the risk of serotonin toxicity. This can be particularly problematic when switching from fluoxetine, because of the long half-life of its active metabolite norfluoxetine. However, cross-tapering can be employed when switching between agents with different pharmacological properties (for example, from citalopram to mirtazapine or reboxetine). Useful instructions are provided by the Maudsley Prescribing Guidelines (Reference Taylor, Paton and KerwinTaylor et al, 2003).
For the broad range of patients with depression, currently marketed antidepressants have roughly equal efficacy. However, there is evidence from meta-analyses that amitriptyline (Reference Barbui and HotopfBarbui & Hotopf, 2001) and venlafaxine (Reference Smith, Dempster and GlanvilleSmith et al, 2002) may be a little more effective. It is therefore worth trying these drugs at some point in the management of patients unresponsive to initial medication.
Monoamine oxidase inhibitors
The use of non-selective, irreversible monoamine oxidase inhibitors (MAOIs) in patients resistant to TCAs and other antidepressants has some support from controlled trials. For example, Reference Nolen, Van de Putte and DijkenNolen et al(1988) studied 21 patients who had failed to respond to imipramine, fluvoxamine or oxprotiline (a selective noradrenaline reuptake inhibitor). Individuals were randomly allocated to double-blind treatment with nomifensine (a dopamine and noradrenaline reuptake inhibitor) or the MAOI tranylcypromine. Of 11 patients receiving tranylcypromine, 5 showed a clinical response. In a subsequent cross-over, 5 of 8 non-responders to nomifensine responded to tranylcypromine. Of the 10 patients who responded to tranylcypromine, 8 maintained their response for at least 6 months.
There is also evidence that patients with certain clinical features may have a preferential response to MAOIs. For example, patients with symptoms of atypical depression (Box 2) have significantly higher rates of response to phenelzine than imipramine, and the same may be the case for patients with ‘anergic’ bipolar depression (Reference Quitkin, McGrath and StewartQuitkin et al, 1989; Reference Himmelhoch, Thase and MallingerHimmelhoch et al, 1991).
Box 2 Clinical predictors of response to conventional MAOIs
-
• Atypical depression with mood reactivity, overeating, oversleeping, overwhelming fatigue, rejection sensitivity
-
• Bipolar depression with anergia, fatigue and hypersomnia
Although MAOIs are undoubtedly useful drugs in treatment-resistant depression, their adverse effect profile and liability to produce dietary and drug interactions makes their use unlikely until most other options have been exhausted. The reversible type-A MAOI moclobemide is relatively free from tyramine interactions and is better tolerated than conventional MAOIs. Whether moclobemide is effective as a sole agent in treatment-resistant depression is doubtful. However, Reference Kennedy and PaykelKennedy & Paykel (2004) described useful effects of moclobemide combined with tricyclic antidepressants and lithium in severely treatment-refractory patients in a tertiary referral centre.
Combination treatment with antidepressants
Combination strategies aim to supplement the antidepressant effect of an ineffective or partially effective medication with another antidepressant agent. This approach can therefore be considered an augmentation strategy, although if the patient’s condition remits it may be unclear whether the response is due to the combined effect of the two antidepressants or the second agent acting alone.
The pharmacological rationale of combination treatment is the use of two agents to produce a broader spectrum of activity on monoamine pathways than either agent could produce alone. In practice, this means that SSRIs or venlafaxine are usually combined with noradrenergic promoting agents such as TCAs, reboxetine or mirtazapine (which increases noradrenaline function through blockade of auto-inhibitory α2-adrenoceptors). In the USA, the combination of bupropion with SSRIs is also common. This has the aim of supplementing serotonin potentiation with dopamine and noradrenaline activation. The addition of the anxiolytic drug buspirone to SSRI treatment has also been found to be useful in open case series. However, results from two controlled trials have not been supportive (see Reference NelsonNelson, 2003)
The evidence for any of these strategies is limited, although they are endorsed in case series and expert reviews. The best evidence is probably for the addition of mirtazapine or its predecessor, mianserin, to ineffective SSRI treatment, although a large randomised trial in resistant depression failed to find a benefit of combined sertraline and mianserin over mianserin alone (Reference Carpenter, Yasmin and PriceCarpenter et al, 2002; Reference Licht and QvitzauLicht & Qvitzau, 2002).
Safety is obviously a key consideration in combination therapy. Of the SSRIs, fluoxetine, fluvoxamine and paroxetine can elevate levels of other psychotropic drugs through inhibition of the hepatic cytochrome P450 system. Therefore their use in combination with cardiotoxic TCAs requires special caution. Venlafaxine and citalopram are not significant P450 inhibitors and sertraline has relatively modest effects. Quite apart from the risk of drug interactions, the side-effect burden is likely to increase when two antidepressants are given together. For this reason it is wise to add a second agent cautiously at low dose and to increase the amount gradually according to tolerance.
Tricyclics and MAOI combination
The combination of TCAs and MAOIs has been in use since the 1960s, when the efficacy of this approach was strongly advocated by William Sargent. Although this combination is reported to be hazardous, the risks of significant interactions can be minimised if reasonable precautions are taken. The combination of amitriptyline and trimipramine with MAOIs appears to be safe, but imipramine and clomipramine should definitely be avoided because of the risk of fatal serotonin toxicity. It is usually thought best to start the MAOI and TCA treatment simultaneously at low dose or cautiously to add MAOI treatment to established TCA medication.
In patients not selected for treatment resistance, the combination of TCA and MAOI does not appear to confer additional therapeutic benefit over either drug used alone. In treatment-resistant patients, however, there is some evidence that combined treatment may be of value (Reference Tyrer and MurphyTyrer & Murphy, 1990). As noted above Reference Kennedy and PaykelKennedy & Paykel (2004) found that the combination of moclobemide and TCA (combined in some cases with lithium) produced benefit in a significant number of severely treatment-resistant patients.
Generally, the adverse effects of TCA–MAOI combinations are little worse than those of either drug given alone, although weight gain and postural hypotension may be more troublesome. Conversely, combination of an MAOI with trimipramine or amitriptyline may prevent MAOI-induced insomnia. Trazodone in low doses (50–150 mg) is also sometimes used to treat MAOI-induced insomnia and is generally well tolerated for this purpose. However, there are reports suggestive of serotonin toxicity where trazodone has been combined with SSRIs or MAOIs.
Lithium addition
Lithium given alone has modest antidepressant properties in patients with bipolar disorder, but other patients with depression generally show little response when lithium is used as a sole agent. There is now good evidence from randomised trials that lithium added to ineffective antidepressant treatment can produce useful clinical improvement in patients who fail to respond or only partially respond to antidepressant medication. In a meta-analysis, Reference Bauer and DöpfmerBauer & Döpfmer (1999) reported that the addition of lithium to antidepressant treatment increased the chance of responding threefold relative to placebo (odds ratio = 3.3; 95% confidence interval 1.5–7.5), yielding a number needed to treat (NNT) of 3.7.
Lithium appears to be effective in improving antidepressant response when added to different kinds of antidepressant treatment including TCAs and SSRIs. It has also been suggested that the combination of lithium with MAOIs may be particularly helpful in patients with severe refractory depression (Reference Price, Charney and HeningerPrice et al, 1985; Reference Kennedy and PaykelKennedy & Paykel, 2004). Lithium has the ability to increase the release of serotonin from pre-synaptic terminals; therefore, caution is needed when using lithium together with SSRIs and venlafaxine, because of the risk of serotonin toxicity. In view of this, it is usually best to initiate lithium addition at a low dose (for example, 200 mg daily) and increase it by 200 mg weekly. The plasma level of lithium required to produce an antidepressant effect in treatment-resistant patients has not been clearly identified, but 12-hour levels of 0.4–0.6 mmol/l are usually adequate; this typically requires doses of 400–800 mg daily. Used in this way, together with slow initiation, the tolerance of lithium in treatment-resistant depression is usually satisfactory.
Antipsychotic drugs
Depressive psychosis
Both clinical experience and controlled studies suggest that antidepressant agents are relatively unhelpful as a sole therapy for depressive psychosis and that effective treatment of this condition requires the concomitant use of antipsychotic drugs. In a much-cited study, Reference Spiker, Weiss and DealySpiker et al(1985) used a double-blind randomised design to allocate 51 in-patients with depressive psychosis to treatment with amitriptyline, perphenazine or both drugs used in combination. Remission rates were significantly higher in the combination treatment group (78%) than in patients taking amitriptyline (41%) or perphenazine (19%) alone. A meta-analysis of 597 patients who received combination treatment with TCAs and conventional antipsychotic drugs also suggested a high overall response rate (77%), and it seems likely that the combination of SSRIs and conventional antipsychotics is similarly effective (see Reference SchatzbergSchatzberg, 2003).
Atypical antipsychotic drugs are increasingly used in depressive psychosis and there have been suggestions that they may be effective as a monotherapy. However, in a randomised trial of patients with psychotic depression, Reference Muller-Siecheneder, Muller and HillertMuller-Siecheneder et al(1998) found that treatment with risperidone was less effective than a combination of amitriptyline and perphenazine. A more recent study demonstrated the value of combination treatment with fluoxetine and olanzapine, which produced a response rate of 56% in depressive psychosis, significantly greater than the response to either placebo (30%) or olanzapine alone (36%) (see Reference SchatzbergSchatzberg, 2003).
Atypical antipsychotics in non-psychotic depression
Although typical antipsychotic drugs have little value in non-psychotic depression except to ameliorate agitation, there is preliminary evidence that some atypical antipsychotic drugs may have antidepressant effects when used in combination with SSRIs. In a randomised controlled trial, Reference Shelton, Tollefson and TohenShelton et al(2001) found that, in patients resistant to fluoxetine treatment, the addition of olanzapine (5–20 mg) produced a significantly greater response rate than placebo addition or olanzapine monotherapy. Open studies also support the usefulness of low-dose risperidone augmentation for SSRI-resistant patients (Reference Ostroff and NelsonOstroff & Nelson, 1999). Large-scale randomised trials of these approaches are in progress.
The pharmacological mechanisms involved in the augmenting effects of atypical antipsychotic drugs in SSRI-resistant patients requires further study. It has been hypothesised that 5-HT2A/2C receptor blockade may play a role because this action might be expected to increase dopamine and noradrenaline release in cortical regions (Reference Marek, Carpenter and McDougleMarek et al, 2003). Mirtazapine and mianserin are also potent 5-HT2A/2C receptor antagonists and so this action might contribute to their utility in combination with ineffective SSRI treatment.
The use of atypical antipsychotics to augment SSRIs employs lower doses than would be used to treat schizophrenia, perhaps because effective 5-HT2A/2C receptor blockade occurs at lower doses than dopamine D2 receptor blockade. Despite this, olanzapine, even at low doses, can cause troublesome sedation and weight gain; concomitant use of risperidone produces a degree of weight gain together with hyperprolactinaemia.
L-tryptophan
There is evidence from controlled trials that the addition of the serotonin precursor, L-tryptophan, can improve the therapeutic effect of MAOI treatment in patients not selected for treatment resistance. However, there are no controlled trials to indicate that L-tryptophan produces therapeutic benefit in patients who have failed to respond to MAOIs or TCAs. Nevertheless, its use has been recommended to supplement the serotonin-potentiating effects of lithium–MAOI and lithium–clomipramine combinations (Reference Barker, Scott and EccelstonBarker et al, 1987; Reference Hale, Procter and BridgesHale et al, 1987).
L-tryptophan use has been associated with the development of the eosinophilia myalgic syndrome, a severe connective tissue disease that can have a fatal outcome. However, subsequent studies have shown that the syndrome was almost certainly caused by a contaminant that occurred in the production of L-tryptophan from a single manufacturing source (Reference Slutsker, Hoesly and MillerSlutsker et al, 1990). In the UK, it remains possible to prescribe L-tryptophan in combination with other antidepressant drugs for patients with chronic treatment-resistant depression. It should be noted, however, that the combination of L-tryptophan with MAOIs can lead to serotonin toxicity, so caution is needed. For the same reason the combination of L-tryptophan and SSRIs is not recommended.
Thyroid hormone
Some open and controlled studies have suggested that the addition of tri-iodothyronine (T3) in doses of 20–40μg daily to ineffective TCA treatment can bring about a useful clinical response. In one of the best studies of this approach, Reference Joffe, Singer and LevittJoffe et al(1993) studied 50 out-patients with non-psychotic unipolar major depression who had failed to respond to 5 weeks’ treatment with a TCA (daily dose 2.5 mg/kg). They were randomly allocated to double-blind addition of T3 (37.5μg daily), lithium carbonate or placebo for a further 2 weeks. At the end of treatment, 10 of 17 patients had responded to T3 addition and 9 of 17 to lithium; significantly fewer subjects (3 of 16) responded to placebo.
However, a meta-analysis of four published randomised trials that assessed the efficacy of T3 addition to ineffective TCA treatment was less encouraging (Reference Aronson, Offman and JoffeAronson et al, 1996) and, overall, T3 failed to show a significant treatment effect.
A dose of T3 of 20 μg is equivalent to a dose of thyroxine (T4) of about 100μg. The use of T3 augmentation treatment at the usual doses (20–40μg daily) rarely produces clinical evidence of hyperthyroidism, but occasionally mild tachycardia and sweating may occur. Thyroid function tests typically show plasma levels of T3 in the high normal range with subnormal T4 levels. Thyroid-stimulating hormone (TSH) levels are low, but usually not completely suppressed.
Another approach employed by some groups is to add high-dose T4 to ineffective antidepressant treatment, with the aim of suppressing TSH and increasing plasma T4 into the mild hyperthyroid range. Reference Bauer, Hellweg and GrafBauer et al(1998) described 17 patients with severe refractory depression (12 with bipolar depression) who were treated with thyroxine (mean dose 482 μg daily). Following 12 weeks of treatment, 10 individuals had remitted and 9 of these maintained a clinically important improvement over the next 2 years. Ten patients noted typical symptoms of hyperthyroidism, but these were reported as mild and tolerable. At 1-year follow-up there was no evidence of significant cardiovascular changes or bone demineralisation. Useful effects of high-dose thyroxine treatment have also been reported in patients with rapid-cycling bipolar disorder (Reference Bauer and WhybrowBauer & Whybrow, 1990).
The use of high-dose T4 in patients with pre-existing cardiovascular disease clearly raises concerns and requires regular clinical and biochemical monitoring. It is somewhat easier to use T3, but again it should be avoided in patients with cardiac disease. A major drawback of T3 augmentation is that there is little information about its efficacy in combination with newer antidepressants such as the SSRIs.
Other augmentation approaches
The literature contains many other augmentation approaches for the management of resistant depression. Many of these are of theoretical and practical interest; none currently has a strong evidence base.
Pindolol
The β-adrenoceptor antagonist pindolol has 5-HT1A receptor antagonist properties and there has been much interest in whether pindolol might augment the action of SSRIs by blocking the inhibitory action of 5-HT1A autoreceptors in the raphe nucleus. It does appear that pindolol addition can speed onset of therapeutic effect of SSRIs (Reference Artigas, Celada and LaruelleArtigas et al, 2001), although whether this occurs to a clinically useful degree is debatable. However, it seems doubtful that pindolol is able to augment the action of SSRIs in treatment-resistant patients (Reference Perez, Soler and PuigdemontPerez et al, 1999). The caveat to this conclusion is that the dose of pindolol generally used in augmentation studies (7.5 mg daily) is probably too low to provide effective blockade of 5-HT1A receptors (Reference Rabiner, Bhagwagar and GunnRabiner et al, 2001). Whether higher doses might be more effective is uncertain.
Omega-3 fatty acids
There has been much interest in the possible antidepressant effects of omega-3 fatty acids, particularly eicosapentaenoic acid (EPA) given in doses of 1–2 g daily. In a small double-blind randomised trial (n = 20), Reference Nemets, Stahl and BelmakerNemets et al(2002) found that 2 g daily of an EPA derivative produced a significantly greater response in antidepressant-resistant patients (60%) than did placebo (10%). At these doses EPA is usually well-tolerated.
Electroconvulsive therapy
Among the indications for ECT is failure to respond to adequate antidepressant drug treatment. However, a history of medication resistance may lower the response to ECT.
Reference Prudic, Sackeim and DevenandPrudic et al(1990) studied the effect of previous antidepressant drug treatment on the response of 53 patients who received bilateral ECT. They found that, among those who had received previous adequate pharmacotherapy (defined as a TCA at a dose of at least 200 mg daily for at least 4 weeks), the response rate to ECT was 50%. In contrast, the response rate in patients who had not received adequate drug treatment was significantly greater (86%).
Another point that needs to be considered is outcome after ECT. Reference Sackheim, Prudic and DevenandSackheim et al(1990) followed 58 patients who responded to ECT and found that 1 year after treatment, 50% had relapsed. The relapse rate in patients who had received adequate antidepressant treatment prior to ECT (64%) was significantly higher than in those who had not (32%). The relapse rate after ECT was only weakly influenced by whether or not patients had received adequate antidepressant treatment after the ECT. Development of appropriate pharmacological continuation therapy after ECT is clearly a priority. It seems that the common clinical practice of continuing with the same medication that the patient was taking before the ECT is not a generally effective strategy.
Bipolar depression
The management of bipolar depression is outside the scope of this article [for a review of the topic, readers are referred to Reference FrangouFrangou, 2005, this issue. Ed.] but patients with bipolar disorder who present with resistant depression can pose additional problems in terms of pharmacological management. These problems can extend to patients with bipolar II disorder. Among the relevant issues is the possibility that antidepressant drugs will induce mania or rapid cycling. Where patients are having what appears to be a rapidly relapsing depressive illness it is important to establish whether or not the clinical picture is, in fact, rapid cycling, with periods of mild hypomania interspersed with depression (Reference Hurowitz and LiebowitzHurowitz & Liebowitz, 1993). Daily mood charting can be helpful in diagnosis. If rapid cycling is confirmed, withdrawal of antidepressant treatment and institution of mood stabilising treatment can be a useful strategy (for guidelines describing the treatment of bipolar depression, see Reference GoodwinGoodwin, 2003).
The treatment of bipolar depression (Box 3) should generally include a mood stabiliser because this lessens the risk of manic upswing. Although lamotrigine may not be as effective as lithium in preventing mania, it probably has superior antidepressant efficacy in patients with bipolar disorder (see Reference Goodwin, Bowden and CalabreseGoodwin et al, 2004). However, it is not yet licensed for use in mood disorders in the UK. Atypical antipsychotic drugs are also gaining a growing role in the treatment of bipolar depression. Reference Tohen, Vieta and CalabreseTohen et al(2003) reported a randomised study of olanzapine and combined olanzapine and fluoxetine treatment in 833 patients with bipolar depression. Remission rates were best in the olanzapine–fluoxetine treatment group (48.8%), followed by olanzapine alone (32.8%) and then placebo (24.5%). Rates of mania onset did not differ between any of the treatment groups.
Box 3 Treatment of bipolar depression
-
• Antidepressants should be used in combination with a mood stabiliser
-
• Monitor for induction of mania or rapid cycling
-
• Lamotrigine may be effective as a monotherapy or in combination
-
• Atypical antipsychotic drugs may be effective in combination
Conclusions
General and old age psychiatrists need to have confidence in their ability to manage the pharmacological aspects of treatment-resistant depression. It is important to retain the belief that recovery is possible, because even several years of treatment-resistant depression can be followed by eventual clinical remission (Reference Mueller, Keller and LeonMueller et al, 1996). At the same time, it is necessary to recognise and acknowledge to the patient the limitations and discomforts of contemporary drug treatments.
Reference Thase and RushThase & Rush (1997) warn that it is important for the clinician not to become demoralised or frustrated when treatments prove ineffective and point out that even where promising pharmacological options appear to be limited, supportive psychological treatment has an important life-sustaining function.
MCQs
-
1 In the treatment of resistant depression, monoamine oxidase inhibitors:
-
a are not effective in patients who have failed to respond to TCAs or venlafaxine
-
b may be effective in patients with hypersomnia and hyperphagia
-
c can cause postural hypotension at higher doses
-
d should not be combined with lithium.
-
-
2 Controlled trials have shown that the following treatments can augment SSRIs in unresponsive patients:
-
a lithium
-
b mirtazapine
-
c pindolol
-
d tri-iodothyronine.
-
-
3 Lithium augmentation of ineffective antidepressant treatment:
-
a does not work in unipolar depression
-
b is contraindicated with SSRIs
-
c requires a plasma concentration above 0.8 mmol/l
-
d has an NNT between 3 and 4.
-
-
4 The following combinations can cause severe drug interactions:
-
a SSRIs and tricyclic antidepressants
-
b SSRIs and tryptophan
-
c amitriptyline and tri-iodothyronine
-
d mianserin and venlafaxine.
-
-
5 In the treatment of bipolar depression :
-
a mood stabilisers are not usually necessary
-
b antidepressant treatment can provoke rapid cycling
-
c lamotrigine has antidepressant properties
-
d anergic states respond well to TCAs.
-
MCQ answers
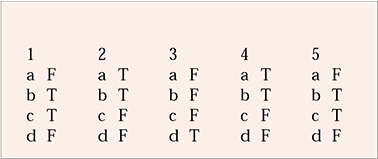
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | F | a | T | a | F | a | T | a | F |
| b | T | b | T | b | F | b | T | b | T |
| c | T | c | F | c | F | c | F | c | T |
| d | F | d | F | d | T | d | F | d | F |





eLetters
No eLetters have been published for this article.