Whole-grain (WG) intake is associated with beneficial health effects and epidemiological studies have shown that it is protective against cancer, diabetes, obesity and in particular CVDReference Jacobs, Marquart, Slavin and Kushi1–Reference Kushi, Meyer and Jacobs3. Whole grains are rich in fermentable carbohydrates such as dietary fibre, resistant starch and oligosaccharides and one proposed protective mechanism is the effect on human gut microbiotaReference Slavin4. Diet–microbe interactions within the colon are now thought to play important roles in regulating mucosal physiology and may provide protection from invading pathogens, impact on liver function, bone health, satiety and chronic diseases such as some cancers, inflammatory conditions and heart diseaseReference Rastall, Gibson, Gill, Guarner, Klaenhammer, Pot, Reid, Rowland and Sanders5, Reference Fava, Gitau, Lovegrove and Tuohy6. Many of these health-promoting activities are likely to be mediated by dominant members of the gut microbiota which have co-evolved with the human colon. Bacteria now seen as beneficial for human health include species belonging to the genera Bifidobacterium and Lactobacillus Reference Cummings, Antoine and Azpiroz7. Similarly, maintenance of stable and diverse populations of commensal bacteria e.g. Eubacterium spp., Atopobium spp., and certain Bacteroides spp., characterises the gut microbiota in health and no doubt contributes greatly towards improved colonisation resistance and protection against gastrointestinal disorderReference Rakoff-Nahoum, Paglino, Eslami-Varzaneh, Edberg and Medzhitov8. Functional foods targeting the human colon aim to stimulate beneficial genera either directly by providing growth substrates which selectively promote the growth of an individual's autochthonous bifidobacteria and lactobacilli in vivo within the colon (prebiotics) or indirectly by introducing live exogenous bacteria in specially formulated foods (probiotics). Prebiotics are generally non-digestible soluble fibres such as inulin, oligofructose or galactooligosaccharides, and mimic the bifidogenic activities of non-digestible oligosaccharides present in human breast milkReference Gibson and Roberfroid9. There is a growing body of evidence that certain prebiotics may mediate important health effects such as improved mineral absorptionReference Abrams, Griffin, Hawthorne, Liang, Gunn, Darlington and Ellis10, lowering of blood TAG concentrations in the hyperlipidaemicReference Williams and Jackson11 and affecting colon cancer risk by reducing faecal water genotoxicityReference Burns and Rowland12, Reference Klinder, Förster, Caderni, Femia and Pool-Zobel13 and in animal studies, reducing the number and size of chemically induced colonic tumoursReference Pool-Zobel14. Currently, no information exists on the prebiotic potential of WG wheat.
WG cereals comprise three distinct physiological regions, the endosperm, germ and bran. The grain endosperm is composed mainly of starch, whose digestibility and subsequent fermentability will be affected by food processing (e.g. heating, drying, acid/enzymatic digestion). Grain germ, which is a minor fraction of the grain in wheat, is made up of a complex mixture of lipids, proteins and some mainly soluble carbohydrates, while wheat bran (WB) is composed of non-digestible, mainly insoluble and poorly fermented carbohydrates such as cellulose, hemicellulose, arabinoxylan as well as polyphenolic lignins all together indicated as dietary fibre. Whole grains contain many compounds such as antioxidants, lignans, vitamins and minerals that may protect against chronic disease. Particularly in cereal products, dietary fibre is composed of different compounds that may be co-responsible for many of its physiological effects. An important amount of phenolic compounds (500–1500 mg/kg), mainly ferulic acid, is linked to the dietary fibre and this may explain why wheat dietary fibre has a marked antioxidant activityReference Esposito, Arlotti, Napolitano, Vitale and Fogliano15. Dietary fibre in toto (carbohydrate and phenolic compounds) mediates their biological activity on host health through the colonic microbiotaReference Wong, de Souza, Kendall, Emam and Jenkins16, Reference Scalbert, Morand, Manach and Remesy17. There is currently no information on the impact of specific WG cereals on the microbial ecology of the human colon, and how this may impact upon chronic disease risk.
In the present study, the efficacy of WG wheat compared with WB alone to beneficially modulate the gastrointestinal microbiota and their activities was determined. The objective was to assess the ability of WG compared with WB to selectively increase numbers of bifidobacteria and alter colonic metabolic output. The secondary objective was to determine the relative impact of WG and WB on biomarkers of gut health (bowel habit and faecal water genotoxicity) and blood lipid parameters. We present the findings of a double-blind, placebo-controlled crossover study where thirty-one healthy subjects were randomised in two groups and were fed either WG wheat breakfast cereal (48 g/d), or WB breakfast cereal (48 g/d) as placebo for 3 weeks. After a 2-week wash-out phase volunteers were then crossed over to the other breakfast cereal treatment for another 3 weeks. Fasting blood, 24 h urine and single stool samples were collected before and after treatment with the cereals, and changes within the gut microbiota and its metabolic output in terms of SCFA profiles and plasma ferulic acid.
Material and methods
Subjects
Thirty-two healthy volunteers (20–42 years of age, average age of 25 years; sixteen females, fifteen males) were recruited from the Reading area. Written consent was obtained from each person and selection took place following determination of health status through a medical interview and adherence to the inclusion/exclusion criteria. The subjects all satisfied the following inclusion criteria: they were all between 18 and 50 years old with a BMI between 20 and 30 kg/mReference Anderson, Hanna, Peng and Kryscio2. Good general health was determined by medical questionnaire. Volunteers were excluded from the trial if there was evidence of physical or mental disease or major surgery as revealed by history or physical examination, which might limit participation in or completion of the study. Volunteers with a history of drug abuse, including alcohol, and smokers were excluded. Volunteers could not be pregnant, lactating or planning pregnancy, have severe allergy or a history of severe abnormal drug reaction. Intake of an experimental drug within 4 weeks prior to study, former participation in prebiotics or laxative trial within the previous 3 months, use of antibiotics within the previous 6 months, chronic constipation, diarrhoea or other chronic gastrointestinal complaint (e.g. irritable bowel syndrome) and intake of other specific prebiotics or probiotics, drugs active on gastrointestinal motility, or a laxative of any class for 4 weeks prior to study, were all prohibited. The study was approved by the Ethics Committee of the University of Reading.
Requirements for diet and medication during study
The intake of the following foods or substances was not permitted: confirmed prebiotics (such as oligosaccharides e.g. fructooligosaccharide or inulin), probiotics (e.g. live yoghurts, fermented milk drinks), high bran or WG breakfast cereals other than the test food, drugs active on gastrointestinal motility, antibiotic treatment or any class of laxative. Any medication taken was recorded in diaries. Volunteers were instructed not to alter their usual diet or fluid intake during the trial periods.
Study design
The dietary intervention study was performed as a double-blind, randomised, placebo-controlled crossover manner. Thirty-two healthy volunteers were recruited onto the study but one volunteer dropped out due to personal reasons (n 31). For a 2-week period prior to dietary intervention, volunteers followed a restricted diet as described earlier. Thirty-one subjects were randomly allocated into one of two groups. One group (n 16) consumed first the 100 % WG wheat breakfast cereal (48 g/d) for 3 weeks, and then after a 2-week wash-out period, they consumed the equivalent placebo (WB based breakfast cereal; 48 g/d) for 3 weeks. The other group (n 15) received first the equivalent placebo (WB based breakfast cereal; 48 g/d) for 3 weeks, and then after a 2-week wash-out, they consumed the 100 % WG wheat breakfast cereal (48 g/d) for another 3-week treatment period. Each product was given for 3 weeks, followed by 2-week wash-out periods during which no breakfast cereal was consumed (Fig. 1). All test materials were packaged, labelled and randomised by the Cereal Partners UK (Welwyn Garden City, Herts., UK) prior to the study, investigators were not aware of which of the treatments the volunteers were taking and the volunteers were unaware which of the two breakfast cereals they were given (WG or WB).
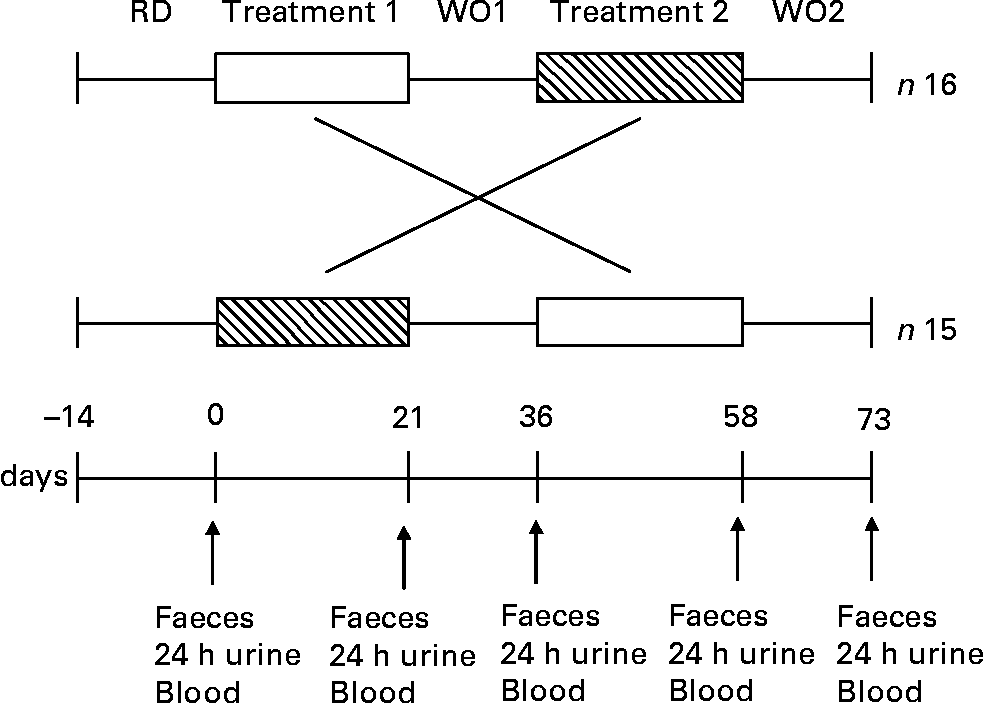
Fig. 1 Study design of a double-blind randomised placebo-controlled parallel study in which thirty-one subjects received WG (□) and WB (▧) for a period of 3 weeks each. One product was given over the first 3-week period followed by a 2-week washout period (WO), and then volunteers received the second product during the next 3 weeks, followed by a further 2-week washout period. A 2-week restriction diet (RD) preceded the start the trial. Faecal samples, 24 h urine and blood were collected from each volunteer at five different time points before and after each treatment and 14 d after the second treatment within 48 h (i.e. 0, 21, 36, 58, 73 d).
Volunteers were asked to keep diaries while ingesting cereals, to record stool frequency and consistency (constipation, hard, formed, soft or diarrhoea), abdominal pain (none, mild, moderate or severe), intestinal bloating (none, mild, moderate or severe) and flatulence (none, mild, moderate or severe) on a daily basis. Any concomitant medication and adverse events were recorded.
Faecal samples, 24 h urine and 30 ml fasting venous blood were collected from each volunteer at five different time points before and after each treatment arm and 14 d after the second treatment arm within 48 h (i.e. 0, 21, 36, 58, 73 d).
Composition of breakfast cereals
The energy intake of the cereals was not the same with WB containing 1184 kJ/100 g and WG containing 1442 kJ/100 g cereal, respectively. WG contained (per 100 g) 67·8 g carbohydrates, of which there are 0·9 g sugars; 11·6 g protein, 2·5 g fat and 11·8 g fibre. WB contained 48 g carbohydrate/100 g which was considerably lower than in WG; however, the proportion of sugars in the WB was higher at 17 g/100 g. The remaining 31 g carbohydrate in WB was starch. WB contained a considerably higher amount of fibre (27 g/100 g). The protein and fat content of WB were similar to those of WG, at 14 g and 3·5 g, respectively per 100 g.
Enumeration of faecal microbial populations by fluorescence in situ hybridisation
Faecal samples were stored in an anaerobic cabinet (10 % H2; 10 % CO2; 80 % N2) for no longer than 2 h prior to processing. The enumeration of faecal microbial populations was carried out using fluorescently labelled 16S rRNA targeted oligonucleotide probes and fluorescence in situ hybridisationReference Tuohy, Kolida, Lustenberger and Gibson18. Oligonucleotide probes (MWG-Biotech, Milton Keynes, UK) targeting the Atopobium group (5′-GGT CGG TCT CTC AAC CC)Reference Harmsen, Wildeboer-Veloo, Grijpstra, Knol, Degener and Welling19,
Bacteroides spp. (5′-CCA ATG TGG GGG ACC TT)Reference Manz, Amann, Ludwig, Vancanneyt and Schleifer20, Bifidobacterium spp. (5′-CATCCGGCATTACCACCC)Reference Langendijk, Schut, Jansen, Raangs, Kamphuis, Wilkinson and Welling21, Eubacterium rectale group (5′-GCTTCTTAGTCARGTACCG)Reference Franks, Harmsen, Raangs, Jansen, Schut and Welling22, Clostridium histolyticum group (5′-TTATGCGGTATTAATCTYCCTTT)Reference Franks, Harmsen, Raangs, Jansen, Schut and Welling22 and the lactobacilli/enterococci (5′-GGTATTAGCAYCTGTTTCCA)Reference Harmsen, Elfferich, Schut and Welling23 were used for enumeration of dominant members of the gut microbiota. The probes were labelled at the 5′ end with the fluorescent dye Cy3. For the enumeration of total cells, samples were stained with the nucleic acid stain 4′,6′-diamino-2-phenylindole (DAPI). Slides were enumerated using a Nikon microscope with an EPI-fluorescence attachment (Nikon UK, Kingston-upon-Thames, UK).
SCFA analysis
Aliquots (1 ml) of 100 g/l faecal suspension in sterile 1 m PBS, pH 7·0, were dispensed into 1·5 ml tubes and centrifuged at 13 000 g for 5 min to pellet bacteria and other solids. Supernatants were then filtered using a 0·2 μm polycarbonate syringe filter and added to four volumes of acetonitrile containing 3·7 mm 2-ethylbutyric acid as the internal standard. Calibration was done using standard solutions of acetic acid, propionic acid, i-butyric acid, n-butyric acid, i-valeric acid, n-valeric acid, n-caproic acid in acetonitrile. Standard solutions containing 20 mm, 10 mm, 5 mm, 1 mm and 0·5 mm of each external standard were used. Fatty acids were determined by GLC using a Hewlett Packard (Agilent) 5890 Series II GC system (HP, Crawley, West Sussex, UK) fitted with a Permabond FFAP column (25 m × 0·32 mm; Macherey-Nagel, Düren, Germany) and a flame-ionisation detector. The carrier gas, He, was delivered at a flow rate of 2·0 ml/min. The head pressure was set at 0·862 bar and the split ratio was 25:1. Injector, column, and detector were set at 220°C, 140°C (isothermic), and 220°C, respectively. After 5 min the column temperature was increased at 20°C/min increments to run for a further 15 min. Peaks were integrated using Atlas Lab managing software (Thermo Lab Systems, Mainz, Germany). SCFA concentrations were calculated by comparing their peak areas with those of the standards.
Faecal samples, plasma and 24 h urine preparation for LC–MS/MS analysis
Faecal sample preparation was carried out in conditions according to a procedure published by Kroon et al. Reference Kroon, Faulds, Ryden, Robertson, Williamson and Garcia-Conesa24 with some modifications. Briefly, faecal samples (500 μl) were treated for 2 h in the dark with 2 m NaOH at 35°C. The solutions were acidified to pH 2·0 with 4 m HCl. Phenolic acids were extracted twice with ethyl acetate (10 ml) under agitation and 5 min centrifugation at 4000 g. The ether phases were collected in amber test tubes and completely evaporated under N2. The dry extracts were dissolved in 1 ml 50 % aqueous methanol and filtered (PTFE membrane, 0·45 μm Millipore, Bedford, USA). A 20 μl aliquot of the filtrate was then analysed by LC–MS/MS. Plasma samples (500 μl) were spiked with 0·5 μg o-coumaric acid as internal standard. Following acidification (pH 6·5, 0·5 m HCl), plasma samples were incubated with β-glucuronidase (7·5 × 10Reference Slavin4 units/l) and sulfatase (1·5 × 10Reference Slavin4 units/l) for 1 h at 37°C in a shaking water bath. This mixture was further acidified with HCl to pH < 2 and then extracted with ethyl acetate (3 × 3 ml), and the organic phase rotary evaporated to dryness. The residue was dissolved in 500 μl 50 % methanol, centrifuged for 5 min at 15 000 g, and filtered through a 0·45 μm syringe filter prior to analysis by LC–MS. For the collection of urine for the study, volunteers were provided with a 24h-urine container (Fisher Scientific Ltd, Loughborough, Leicestershire, UK) containing 2 g ascorbic acid. The total volume of urine was recorded after which the urine samples were dispensed into 1·5 ml microcentrifuge tubes and frozen at − 20°C. The urine samples solution (2 ml) were mixed with 450 μl 50 m sodium acetate buffer (pH 5·5) and 1000 μl 0·6 m CaCl2 solution. These solutions were incubated in a manner similar to that of the plasma samples, followed by an acidification step with 20 μl 6 m hcl solution. Then the urine was extracted twice with 4 ml ethyl acetate and the organic phase was rotary evaporated to dryness. The samples were dissolved in 500 μl 50 % methanol, centrifuged for 5 min at 15 000 g, and filtered through a 0·45 μm syringe filter prior to LC–MS/MS analysis.
HPLC–ESI–MS/MS analysis
HPLC–ESI–MS/MS analyses were carried out on a LC–MS/MS System (API 3000, MDS SCIEX). LC analyses were performed using a system consisted of a series 200 binary pump (Perkin Elmer, USA). The mass spectrometer was equipped with a Model 11 syringe pump (Harvard, Apparatus, Holliston, MA, USA). A turbo Ionspray source, with the nebuliser temperature set at 450°C, was used. The collision-induced dissociation was carried out using N2 as collision gas. Analyses were performed using a Prodigy column 5 μ ODS3 100A, 250 × 4·60 mm (Phenomenex, USA). The solvent system was water (0·1 %), formic acid (solvent A) and methanol (solvent B); only in urine analyses solvent B was acetonitrile. The linear solvent gradient was as follows: 0–10 min 95 % A 5 % B, 10–12 min 55 % A 45 % B, 12–15 min 45 % A 55 % B, 15–17 min 0 % A 100 % B, 17–22 min 0 % A 100 % B, 22–24 min 95 % A 5 % B returned to initial conditions. The acquisition was carried out by a multiple reaction monitoring system, in negative mode, monitoring the transition of parent and product ions specific for each compound with a dwell time of 500 ms. Thirteen phenylacetic, phenylpropionic and cinnamic acid derivates, were simultaneously detected (see Table 1). To promote ionisation of the precursor ion, the voltage applied was 4500 while the collision energy and collision cell exit potential was optimised for each transition.
Table 1 Parent ions and ions of phenolic acids monitored in the multiple reaction monitoring mode by HPLC–ESI–MS/MS

Data acquisition and processing were performed using Analyst software 1·4. The amount of hydroxycinnamates compounds was expressed in μg/l.
Faecal water preparation
Faecal samples were diluted 1:2 (w/v) in 1 m ice-cold PBS (1 g faeces plus 1 ml PBS) in a stomacher bag. Samples were homogenised in a stomacher for 2 min at high speed or until a uniform consistency was achieved. Aliquots (10 ml), in duplicate for each sample, were then transferred into ultracentrifuge tubes (Beckman Ultra-clear tubes, Beckman Ltd, High Wycombe, Bucks., UK) and the tubes stored at − 70°C. Samples were defrosted on ice prior to centrifugation, then ultracentrifuged at 64 000 g for 2 h at 4°C (Beckman Optima L90K Ultracentrifuge). Following centrifugation the tubes were placed on ice and the supernatants (faecal water) were carefully removed and placed into 7 ml Sterilin tubes. The faecal waters were then filtered through a 0·2 μm syringe filter, dispensed into 0·5 ml aliquots and frozen at − 70°C and used for single cell gel electrophoresis.
Treatment of HT29 cells
The human colon carcinoma cell line HT29 was maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10 % fetal bovine serum and penicillin (50 IU/ml)/streptomycin (50 μg/ml) in a humidified 5 % CO2 incubator at 37°C. Prior to incubation with faecal water, HT29 cells were harvested and adjusted to a concentration of 3·2 × 106 cells/ml in un-supplemented DMEM. In order to determine faecal water genotoxicity, faecal water was added to the cell suspension giving a final concentration of 30 % faecal water and 2 × 106 cells/ml. Then cell suspensions were incubated for 30 min at 37°C. 30 % PBS served as a negative control.
Determination of DNA damage (Comet assay)
Numbers and viabilities of the treated cells were determined with Trypan Blue (Sigma-Aldrich) in aliquots. Briefly, following incubation with faecal water, 20 μl cell suspension was mixed with 20 μl Trypan Blue and counted under a light microscope using a Neubauer hemacytometer. Evenly blue stained cells were identified as dead cells while round, shiny cells were counted as viable cells. Viability is expressed as percentage of viable cells compared to the total cell count. The remaining suspensions were centrifuged at 200 g for 5 min at 4°C and the resulting cell pellets were mixed with agarose and distributed onto microscope slides. H2O2 (obtained as a 30 % aqueous solution from Merck & Co. Inc., Global Headquarters, USA) was used as genotoxic model substance. Cell suspensions (2 × 106 cells/ml) were incubated with 75 μm for 5 min on ice. Further steps of single cell gel electrophoresis (alkaline version of the ‘Comet assay’) were carried out as originally described by Singh et al. Reference Singh, McCoy, Tice and Schneider25. In summary, the slides were placed into the lysis solution (100 mm Na2EDTA, 1 % Triton X 100, 2·5 m NaCl, 10 mm Tris, pH 10) for at least 60 min at 4°C and further processed in the microgel electrophoresis chamber containing alkaline solution (1 mm Na2EDTA, 300 mm NaOH, pH 13). After 20 min incubation for DNA unwinding, electrophoresis was carried out at 1·25 V/cm and 300 mA for 20 min. Slides were then washed with neutralisation buffer (0·4 M Tris, pH 7·5) and stained with ethidium bromide (Sigma-Aldrich,). The microscopic analysis revealed images of more or less damaged DNA (‘comets’)Reference Pool-Zobel, Bub, Müller, Wollowski and Rechkemmer26. The proportion and extent of DNA migration were used as basis for evaluation. These parameters were quantified using the image analysis system of Komet 5·5 (Kinetic Imaging Ltd.). Fifty DNA spots were evaluated per slide. For each faecal-water sample three gels were processed for determination of DNA strand breaks.
Blood samples collection and analysis
For each volunteer, blood samples were collected into one 10 ml EDTA tube (BD Vacutainer® EDTA Tube, BD, Cowley, Oxon., UK) for the analysis of fasting TAG, total cholesterol (TC) and HDL-cholesterol concentrations; into one 2 ml fluoride/oxalate tube (BD Vacutainer® Fluoride/Oxalate Tube) for the analysis of fasting glucose concentration and into one 10 ml heparin tube (BD Vacutainer® Plasma Tube) for the analysis of fasting insulin concentration and ferulic acid. Following collection all samples were kept on ice until centrifugation. Plasma samples were recovered by centrifugation at 1700 g for 10 min, dispensed into 1·5 ml microcentrifuge tubes and frozen at − 20°C within 1 h from the collection. Plasma samples were defrosted and centrifuged for 5 min at 1500 g prior to analysis.
Determination of plasma TAG, glucose, total cholesterol and HDL-cholesterol concentration
Plasma TAG, glucose, TC and HDL-cholesterol concentrations were determined using the Monarch Automatic Analyzer ILab 600 (Instrumentation Laboratories Ltd, Cheshire, UK). Test kits (IL Test triacylglycerols, IL Test cholesterol, IL Test HDL cholesterol and IL Test glucose (hexokinase)) supplied by Instrumentation Laboratories were used according to instructions for the determination of plasma TAG, TC, HDL-cholesterol and glucose, respectively. Two quality control samples, Wako Control Serum I and Wako Control Serum II (Alpha Laboratories Ltd, Eastleigh, Hants., UK), containing normal and abnormal known concentration of TAG, TC, HDL-cholesterol and glucose were included at the beginning and at the end of each batch analysis. Sample results were accepted if quality control values were within the range specified by the manufacturers.
Determination of plasma insulin concentration
The determination of plasma insulin concentration was carried out using a specific commercial ELISA kit (DAKO Diagnostic Ltd, Cambridgeshire, UK). Prior to analysis plasma samples were thawed on a roller mixer. Calibrators and reagents were prepared according to manufacturer's instructions. A 25 μl aliquot of plasma, calibrator or control was pipetted onto a 96-well plate and 100 μl anti-insulin antibody, horseradish peroxidase, conjugate was added. The plate was shaken for 1 h at room temperature. Following incubation, the plate was washed three times with wash buffer. A 100 μl aliquot of substrate solution was added to each well and the plate shaken for 10 min at room temperature. The reaction was stopped by addition of 100 μl stop solution and the plate read immediately at 450 nm using an automated ELISA plate spectrophotometer (Tecan GENios; Process Analysis and Automation Ltd, Farnborough, Hants., UK). Insulin concentrations of the samples and quality controls were determined automatically by reading from the standard curve using Magellan (version 5.01) computer software.
Statistical analysis
All statistical analyses were performed using either generalised linear modelling or one-way ANOVA. Subsequent investigation of the three pairwise comparisons (i.e. baseline v. WG, baseline v. WB, and WG v. WB) used Tukey's post test with significance set at P < 0·05. We used GenStat® for Windows® 8th Edition Released for analysis.
Results
Faecal bacteriology
Population levels of the dominant members of the human gut microbiota in thirty-one volunteers were determined using fluorescence in situ hybridisation and are expressed as log10 cells/g faeces (mean value with sd, n 31).
It was confirmed that there was no carry-over from the first leg of the crossover study by statistical evaluation using a generalised linear model as there were no significant differences regarding the sequence of WB (placebo) or WG (treatment) for all of the tested bacterial groups. Using this statistical analysis we also found highly significant treatment effects for bifidobacteria and lactobacilli (P < 0·001) and to a lesser extent for Atopobium spp. (P = 0·024) compared with placebo. In order to have a more in-depth analysis and to compare WB and WG to pre-intervention samples (pre-WB or pre-WG) we used one-way ANOVA with Tukey's post test. Pre-WB and pre-WG data are either time point 1 (0 days) or time point 3 (36 days) values depending whether one or the other immediately preceded the respective treatment, WG or WB. The results from this comparison are presented in Table 2.
Table 2 Faecal bacterial numbers for thirty-one volunteers over the trial period. Bacterial counts in stool samples as determined by fluorescence in situ hybridisation are shown expressed as mean log10 cells/g faeces
(Mean values and standard deviations)
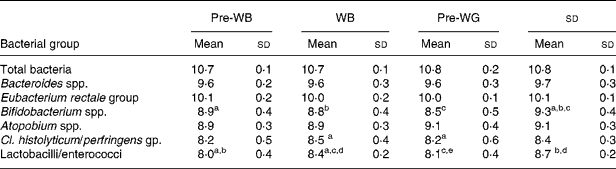
WB, wheat bran; WG, whole-grain.
abcdAll values in one row with a common letter are significantly different from each other (P < 0·05, Tukey's post test).
Numbers of bifidobacteria were significantly higher during the ingestion of the WG compared with the WB treatment period (P < 0·001). A significant increase in Bifidobacterium spp. numbers was also observed during WG intake compared with pre-WG (P < 0·01), while no changes were found between pre-WB and WB. Numbers of faecal lactobacilli/enterococci increased significantly with ingestion of either WB or WG compared with pre-treatment samples. However, the magnitude of change was significantly higher after WG compared with WB intake (P < 0·05). Numbers of clostridia increased significantly during WB intake compared with pre-WG (P < 0·05) but no significant difference was observed in this bacterial group between WG and WB feeding periods. No significant changes in numbers of total bacteria, Bacteroides spp., Atopobium spp. and Eubacterium rectale group were observed.
Colonic metabolic output (ferulic acid and SCFA concentrations)
Thirteen phenolic acids and their metabolites were quantified by LC–MS/MS in urine and plasma after treatment by β-glucuronidase and sulfatase. Concentrations of plasma ferulic acid, the main phenolic compounds in wheat, increased upon ingestion of either WG or WB, with higher concentrations found upon ingestion of WB (Fig. 2). The average concentration of plasma ferulic acid increased from 2·28 μg/l at baseline to 5·70 μg/l after WG and 6·22 μg/l after WB consumption, with great variability among subjects. Caffeic acid, 3-hydroxyl-phenylpropionic and hippuric acid were also detected, but at much lower concentrations.
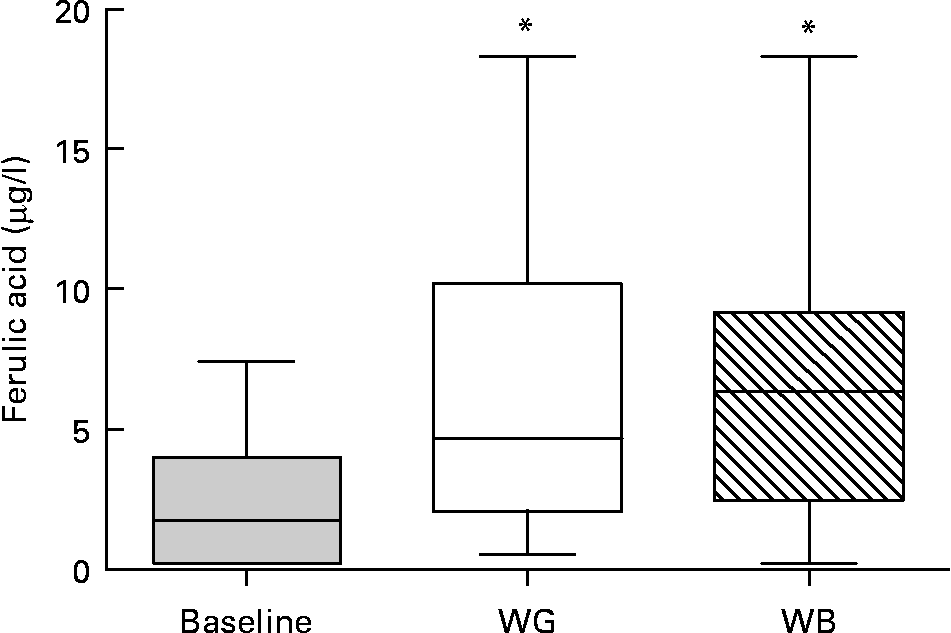
Fig. 2 Ferulic acid concentrations in plasma. There was a significant difference in the concentration of ferulic acid in blood at baseline compared to whole-grain (WG) or wheat bran (WB) treatment periods (*P < 0·05; one way ANOVA with Tukey's post test). Data are depicted as box plots with maximum value, 75th percentile (upper line of box), median (middle line of box), 25th percentile (lower line of box) and minimum (n 31).
In urine, two main microbiota metabolites of phenolic acids were identified: 3,4-dihydroxyphenylpropionic acid and 4-hydroxybenzoic acid, while other compounds such as caffeic acid and 3-hyrroxyphenilacetic acid, were present at trace concentrations. The concentration of 3,4-dihydroxyphenylpropionic acid tended to increase upon intake of both WG and WB (average value 24·7 μg/l) with respect to baseline (average value 10·7 μg/l), while that of 4-hydroxybenzoic acid increased from an average baseline value of 8·6 μg/l to an average value of 18·0 μg/l in WG and WB.
Fig. 3 shows the concentrations of faecal SCFA. No significant changes in faecal concentrations of acetic, propionic, butyric or caproic acid were observed over the course of the trial, neither between pre-WG and WG and pre-WB and WB nor when placebo (WB) and treatment (WG) were compared.

Fig. 3 SCFA concentrations in faecal samples collected from volunteers over the course of the trial measured by gas-chromatography. (a) Acetic acid; (b) Butyric acid; (c) Caproic acid; (d) propionic acid. Data are depicted as box plots with maximum value, 75th percentile (upper line of box), median (middle line of box), 25th percentile (lower line of box) and minimum (n 31).
Biomarkers of gut health
Table 3 summarises data on digestive tolerance and stool consistency, as recorded by the volunteers during intake of the breakfast cereals. Stool frequency was higher during ingestion of WB compared with WG (P < 0·05). Stool consistency, qualitatively graded by volunteers as hard, formed, or soft, varied greatly between individuals. A greater proportion of stools described as formed were reported during WG ingestion, while there was an increase in soft stools (P < 0·001) and in flatulence upon ingestion of WB (P < 0·001).
Table 3 Summary of bowel habit and gastrointestinal symptom data recorded by thirty-one volunteers over course of the trial. The percentage coverage of each category over the total number of responses given per volunteer was determined
(Mean values and standard deviations)
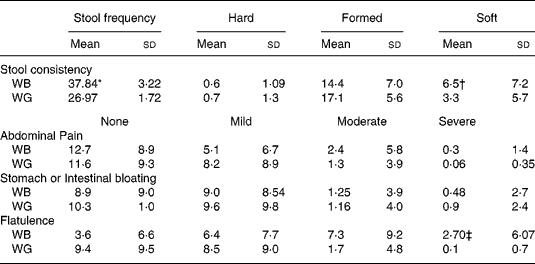
* Stool frequency was higher during ingestion of WB compared with WG (P < 0·05, P value from Tukey's post test).
† An increase in soft stools during WB intake (P < 0·001).
‡ The severity and frequency of reported changes in digestive tolerance varied greatly between volunteers, with neither treatment resulting in adverse symptomology. However, there was an increase of flatulence upon ingestion of WB (P < 0·001).11 851 Costabile Queries to author
The severity and frequency of reported changes in digestive tolerance varied greatly between volunteers, with neither treatment resulting in adverse symptomology.
Faecal water genotoxicity
Faecal water genotoxicity varied greatly between volunteers at the beginning of the study ranging from 6·3 % tail intensity to 66·1 % tail intensity (CV 74·4 %). Neither WG nor WB significantly influenced the potential of faecal water to induce strand breaks (Fig. 4). However, for the quartile of volunteers who had the highest genotoxic activity at baseline (genotoxicity ≥ 24·6 % tail intensity) there was a reduction in the faecal water genotoxicity after intervention with cereals, but this decrease was not significant (baseline, 42·90 (sd 16·68) %; WG, 28·39 (sd 16·88) % and WB, 24·35 (sd 16·56) % tail intensity, mean (SD), n 8, one-way ANONA, P = 0·0884). n 8, one-way ANOVA, P = 0·0884). There was no difference between cereal treatments.

Fig. 4 Faecal water genotoxicity as measured by single cell gel electrophoresis. No significant difference in DNA strand breaks measured as percentage of tail intensity was observed upon ingestion of whole-grain (WG) or wheat bran (WB) breakfast cereals. Data are depicted as box plots with maximum value, 75th percentile (upper line of box), median (middle line of box), 25th percentile (lower line of box) and minimum (n 31).
Blood lipid parameters
Fig. 5 shows data for TC, HDL-cholesterol, glucose and insulin, and NEFA measured in blood samples taken at baseline and following ingestion of WB and WG. Ingestion of neither breakfast cereal impacted upon blood lipid/metabolic parameters during the relatively short 3-week feeding period. No significant differences were observed in these parameters between WG and WB feeding periods. When the effect of the breakfast cereals was examined in those volunteers presenting with the highest starting TC concentrations (the top quartile of TC), a significant reduction in TC was observed from a baseline concentration of 5·576 (sd 0·421) mm, to 4·216 (sd 1·006) mm after WG ingestion and 4·385 (sd 1·093) mm, after WB ingestion (n 8, one-way ANOVA, P < 0·05).
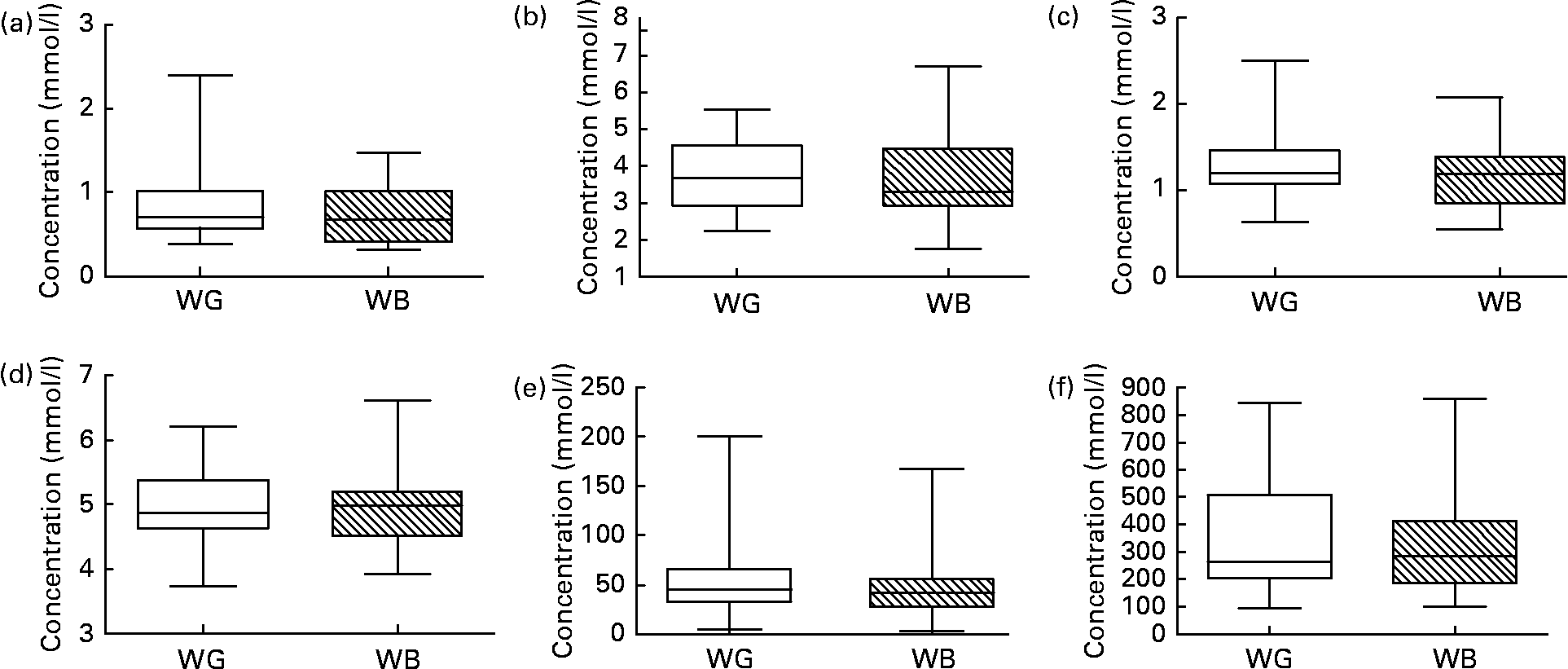
Fig. 5 (a) TAG, (b) total cholesterol, (c) HDL-cholesterol, (d) blood glucose, (e) insulin and (f) NEFA concentrations determined in fasting blood samples collected from each volunteer at baseline before ingestion of test breakfast cereals, after the 21-d intervention with whole-grain (WG) or wheat bran (WB) breakfast cereals, and after the 14-d post-WB and post-WG wash-out periods. No significant differences were observed upon ingestion of WG or WB breakfast cereals. Data are depicted as box plots with maximum value, 75th percentile (upper line of box), median (middle line of box), 25th percentile (lower line of box) and minimum (n 31).
Discussion
A prebiotic is defined as ‘a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microbiota that confers benefits upon host well-being and health’Reference Gibson, Probert, Van Loo, Rastall and Roberfroid27. There is a growing body of evidence supporting the beneficial health effects of prebiotics on bowel habit and constipationReference Chen, Lu, Lin and Ko28–Reference Wang, Lim, Kao, Chan, Lin and Wang32 and increased mineral absorptionReference Coudray, Bellanger, Castiglia-Delavaud, Remesy, Vermorel and Rayssignuier33–Reference Van den Heuvel, Muys, Van Dokkum and Schaafsma36 and recently, in reducing the risk for some chronic diseases (e.g. colon cancer and CVD). Prebiotics may have chemopreventive effects in colorectal cancer as it was shown that prebiotics prevented the occurrence of pre-neoplastic lesions and tumours in chemically-induced colon carcinogenesis in rodent modelsReference Jacobs, Marquart, Slavin and Kushi1. Recently, intervention with oligofructose-enriched inulin in combination with probiotics in human subjects favourably altered colon cancer biomarkers in high-risk individuals (polypectomised patients)Reference Rafter, Bennett and Caderni37. It was also reported that consumption of prebiotics lowered TC and TAG in plasmaReference Wang, Lim, Kao, Chan, Lin and Wang32, Reference Hidaka, Tashiro and Eida38–Reference Causey, Feirtag, Gallaher, Tungland and Slavin40 suggesting beneficial effects especially in hyperlipidaemic individuals who have an increased risk for CVD and type II diabetesReference Shaw, Hall and Williams41.
Likewise, epidemiological studies showed an inverse association between intake of whole grains and the risk of several chronic diseases including CVD, type II diabetes and cancerReference Kushi, Meyer and Jacobs3. As whole grains contain considerable amounts of fermentable carbohydrates it was proposed that one mechanism for the observed protection offered by whole grains is their impact on the gut microbiotaReference Slavin4. However, evidence to support the hypothesis that whole grains can influence bacterial populations and their activities in the gut is lacking.
To test this hypothesis, we measured the impact of WG wheat compared with WB on the human gut microbiota. The primary objective was to identify differences in the relative population levels of intestinal bacteria and their metabolic activities upon ingestion of WG compared with WB breakfast cereal. A secondary objective was to assess possible health effects by measuring biomarkers of gut health (bowel habit and faecal water genotoxicity) and CVD (blood lipid parameters). In particular the concentration of ferulic acid, 95 % of which is bound to the arabinoxylan present in the bran fraction of the wheat kernel and can be released or transformed by microbiota action, was determined.
The choice of this WG breakfast cereal was made following preliminary in vitro screening of three WG breakfast cereals compared with WB. In this screening, the WG breakfast cereal selected performed better than the others in terms of bifidogenicity and changes in SCFA production with increased concentrations of butyrate (data not shown).
Using direct molecular based enumeration of faecal bacteria, we have shown in this study that ingestion of a WG breakfast cereal results in significantly higher numbers of Bifidobacterium spp. in stool samples compared with an equivalent quantity of the WB based breakfast cereal after a 21-d feeding period. Although numbers of lactobacilli increased upon consumption of both breakfast cereals, there were significantly higher numbers of lactobacilli upon ingestion of WG compared with WB. It cannot be determined whether the increase in clostridia after WB intake should be seen as detrimental as the applied probe also recognises commensal bacteria such as Clostridium butyricum and Clostridium beijerinckii Reference Franks, Harmsen, Raangs, Jansen, Schut and Welling22. That WB is indeed an energy source for clostridia has been observed by Leitch et al. Reference Leitch, Walker, Duncan, Holtrop and Flint42 who showed that mainly unknown bacteria belonging to clostridia cluster XIVa selectively colonise WB. The different effects of WG and WB cereals on gut microbiota may be due to the different composition, as WG contained a considerably higher content of non-sugar carbohydrates, which might represent fermentable carbohydrates. This is the first report that consumption of a whole plant food, which has undergone little processing, may act as a prebiotic. However, there were no differences in faecal SCFA after ingestion of WG cereals. This is not surprising as SCFA are readily absorbed in the large intestine. Thus, an increase in acetic and butyric acid was only observed in colostomy effluent after intervention with a high concentration of resistant starch in human subjectsReference Ahmed, Segal and Hassan43, while other human intervention studies with proven prebiotics failed to show changes in the fermentation profile in faecesReference Alles, Hartemink, Meyboom, Harryvan, Van Laere, Nagengast and Hautvast44–Reference Kruse, Kleessen and Blaut48. Similarly, an increase of SCFA after prebiotic intervention in rats was mainly observed in the caecum, but not in faecesReference Campbell, Fahey and Wolf49, Reference Kleessen, Stoof, Proll, Schmiedl, Noack and Blaut50.
A significant increase of the ferulic acid plasma concentrations was observed upon ingestion of both WG and WB, without significant differences between the two types of breakfast cereals. It should be noted that this increase was observed in the plasma of fasting subjects. This indicates that the rapid absorption from the upper gut, of the small fraction of free ferulic acid present in cereal fraction is followed by colonic absorption of ferulic acid released through microbiotal metabolism. The concentration of ferulic acid found in the subjects is in agreement with those reported in the studies by Kern et al. Reference Kern, Bennett, Mellon, Kroon and Garcia-Conesa51 who measured the bioavailability of ferulic acid after a single dose of WG breakfast cereal.
The fact that a regular consumption of WG tripled the ferulic acid concentration in fasting plasma may be of physiological relevanceReference Andreasen, Landbo, Christensen, Hansen and Meyer52. In fact, food phenolic compounds in plasma are usually detected for a limited window-time of 0·5–3 h after the consumption of fruit or other free-phenolic-rich food such as coffeeReference Scalbert, Morand, Manach and Remesy17, Reference Kroon, Faulds, Ryden, Robertson, Williamson and Garcia-Conesa24, Reference Scalbert and Williamson53–Reference Cremin, Kasim-Karakas and Waterhouse55. Our data extend the results of the studies by Rondini et al. Reference Rondini, Peyrat-Maillard, Marsset-Bagliéri and Berset56, Reference Rondini, Peyrat-Maillard, Marsset-Baglieri, Fromentin, Durand, Tomé, Prost and Berset57 who showed that in rats plasma ferulic acid concentration is constant up to 24 h after bran consumption. Therefore our finding demonstrates for the first time that in human subjects the regular consumption of WG and WB is followed by a slow and continuous release of phenolic acids into the bloodstream. The continuous presence of these antioxidants could efficiently preserve some oxidation targets such as LDL and TAG thus contributing to the well known association between WG consumption and prevention of CVD.
A secondary objective of this study was to assess the relative impact of WG and WB breakfast cereals on health effects by measuring changes in bowel habit or symptomology and faecal water genotoxicity, blood lipid and metabolic profiles, specifically, blood TAG, TC, HDL-cholesterol, NEFA, glucose and insulin concentrations. An improvement in bowel habit was observed upon ingestion of both breakfast cereals, with WB increasing stool frequency (without diarrhoea), while WG stool form was more commonly described as formed than WB stools and flatulence was recorded as less severe during WG ingestion. Although there was a trend towards reduced faecal water genotoxicity upon ingestion of both breakfast cereals in volunteers with high faecal water genotoxicity before intervention, this effect was not significant. Taking all volunteers into account there was no difference in faecal water genotoxicity after intervention due to high inter-individual variation. Similar high inter-individual variation in faecal water genotoxicity was reported from other human studiesReference Osswald, Becker, Grimm, Jahreis and Pool-Zobel58–Reference Klinder, Karlsson, Clune, Hughes, Glei, Rafter, Rowland, Collins and Pool-Zobel62 making it difficult to observe an intervention effect in a small study populationReference Cross, Greetham, Pollock, Rowland and Bingham61. Further studies with greater numbers of volunteers are warranted to fully establish the impact of WG cereals or prebiotics on faecal water genotoxicity, especially in individuals with high faecal water genotoxicity who may be at a heightened risk of developing colon cancer. It is not altogether surprising, that there are no obvious differences considering the similarities in the test foods (many of the complex polyphenolic compounds present in WB are also present in WG wheat, including those likely to lead to the formation of signature phenolic derivatives, such as ferulic acid, often used as biomarkers of cereal grain ingestion) and the fact the volunteers were on an open diet, the only restriction being on consumption of other breakfast cereals, probiotics or commercial prebiotic functional foods.
Similarly, no significant differences in blood lipid or metabolic (glucose or insulin) parameters were observed. This too is not surprising for a short-term feeding study in healthy individuals and longer-term trials in high-risk populations are warranted to investigate the efficacy of WG and WB breakfast cereals in impacting on biomarkers of CVD. However, when the effect of breakfast cereal ingestion was examined in those within the top quartile for total serum cholesterol, a significant reduction in TC was observed for both breakfast cereals, with WG apparently having slightly greater cholesterol-lowering effect. No significant change in serum HDL-cholesterol was observed for these individuals during cereal intake, indicating that this reduction on TC may be viewed as beneficial in terms of CVD risk. Although the mean TC (5·576 (sd 0·421) mm) of this top quartile was still within the normolipidaemic range, these findings do suggest that intake of either breakfast cereal may have a beneficial impact on blood lipid profiles. So far, significant decreases in blood lipids after ingestion of prebiotics have usually only been observed in hyperlipidaemic individualsReference Wang, Lim, Kao, Chan, Lin and Wang32, Reference Hidaka, Tashiro and Eida38–Reference Causey, Feirtag, Gallaher, Tungland and Slavin40 while studies in the normolipidaemic failed to show a reductionReference Chen, Lu, Lin and Ko28, Reference Kruse, Kleessen and Blaut48, Reference Luo, Rizkalla and Alamowitch63–Reference Alles, De Roos, Bakz, Van de Lisdonk, Zock and Hautvast65. Another study with healthy volunteers which reported a lowering effect on TAG after intervention with inulin chose subjects with moderately raised TC and TAG levelsReference Jackson, Taylor, Clohessy and Williams66. These subjects would correspond to the volunteers in the upper quartile in this study supporting the beneficial effect observed here in this sub-group. In order to prove a beneficial effect of WG further studies involving larger numbers of individuals over a longer intervention period would be a prerequisite, especially in those with hyperlipidemia.
In conclusion, this study has, for the first time, demonstrated the differential impact of WG wheat and WB on the microbial ecology of the human gut. Additionally, it has established a prebiotic mode of activity for the WG breakfast cereal investigated with increased populations of bifidobacteria and lactobacilli compared to starting levels and the WB. Finally, the increase of the ferulic acid concentration in fasting plasma suggests that the WG intake caused a continuous release of antioxidant in the bloodstream. This study has established a prebiotic mode of action for a WG cereal, which together with antioxidant activities may contribute to the underlying mechanisms of protective health effects of WG wheat.
Acknowledgements
The authors thank Dr Julie Lovegrove, Dr Kim Jackson and Ms Jan Luff of the Hugh Sinclair Clinical Unit, Department of Food Biosciences, The University of Reading for use of their facility and their help with the study, and visiting students Elodie Duclos and Nicolas Pion, for technical assistance.










