Introduction
Contact heat is a medium through which phasic or tonic thermal or thermo-nociceptive stimuli can be applied, often for the assessment of thermal somatosensory functioning in experimental research. Evoked neuronal waveforms observed in response to contact heat are referred to as Contact Heat Evoked Potentials (CHEPs) and are well explored in EEG research alongside tonic stimuli. Reference Atherton, Facer and Roberts1 A commonly used stimulator for contact heat is the PATHWAY CHEPS (Contact Heat Evoked Potential Stimulator) (Medoc Ltd., Ramat-Yoshai, Israel), a commercially available stimulus system with an external heating foil that is surrounded by an electrically isolated plastic layer to protect the skin. Not to be confused with the evoked potential itself, the PATHWAY CHEPS thermode has a standard temperature range of 30–55°C (a lower boundary of 20°C is possible with a software licence), a rising temperature rate of 70°C/s and a cooling rate of 40°C/s. The PATHWAY CHEPS can ramp and hold targeted temperatures for extended durations, but also produce brief pulses of thermal stimuli, which facilitate the recording and analysis of event-locked waveform data. It can also be used in somatosensory assessments in clinical settings due in part to its ease of use, making its utility across settings especially valuable. Reference Atherton, Facer and Roberts1
Somatosensation is a physiological and psychological experience that is cortically processed; methods that elicit sensations in combination with functional neuroimaging methods that have a high temporal resolution can provide valuable insights into how sensation is represented temporally, spectrally and spatially in the brain – which can facilitate the identification of biomarkers for the development or progression of conditions of altered sensation, such as chronic pain. It is important to consider how well a stimulus emulates sensations that might be experienced outside of a laboratory or in clinic. In contrast to some experimental pain techniques used in neuroimaging, contact heat stimuli elicit an experience more reflective of sensations one might perceive during day-to-day life, as opposed to direct electrical stimulation that circumvents mechanical, thermal or chemical transduction of the nerve and laser radiation. As an alternative to laser stimuli in experimental pain studies, contact heat is more accessible and affordable – depending on the equipment, it also does not require as extensive safety precautions for experimenters or participants, poses a significantly reduced burn risk and is not impacted by skin reflectance. Reference Frahm, Gervasio, Arguissain and Mouraux2
There is a sizeable body of research exploring contact heat in EEG, Reference Savignac, Ocay, Mahdid, Blain-Moraes and Ferland3 but it has been explored to a lesser extent in magnetoencephalography (MEG). MEG and EEG brain recordings share similarities in temporal resolution and, it is assumed, their underlying sources, but their data must be interpreted differently. Source reconstruction of event-related potentials (e.g., CHEPs) recorded by EEG can be distorted by tissue conductivity, resulting in poorer spatial resolution and smaller signal-to-noise ratios than MEG recordings; Reference Singh4 MEG sensors commonly utilise gradiometry to reduce environmental noise and record magnetic fields that are not influenced by volume conduction, resulting in more accurate localisation of tangential sources in the cortex. Sensor-level MEG analysis has identified temporal components of evoked waveforms that were not present in EEG recordings, which underscores its potential for improved detection of neural correlates at sensor and source level. Reference Fardo, Vinding, Allen, Jensen and Finnerup5 MEG systems also have much reduced preparation time and up to 320 sensors in some configurations, which is of benefit for high-density recordings and patients that may struggle to sit still for long sessions – though MEG systems are insensitive to radial source generators, are much more expensive than EEG systems and require additional data cleaning techniques when used in conjunction with equipment that produce electromagnetic fields. Though the hardware specifications of the PATHWAY CHEPS are proprietary, researchers have suggested that thermode feedback components contribute to significant electromagnetic signal interference that MEG sensors are sensitive to. Reference Gopalakrishnan, Machado, Burgess and Mosher6 Advances in temporal signal space separation (tSSS) Reference Taulu and Simola7,Reference Taulu and Hari8 and research exploring beamforming techniques have demonstrated the ability to reduce this artefact, Reference Adjamian, Worthen and Hillebrand9 but despite the potential advantages for the identification of electromagnetic components in thermal somatosensory research, studies combining the methods appear sparse.
This systematic review aims to identify and critically appraise current literature that explores the use of contact heat in combination with MEG, highlighting findings and methodological implications for the study of sensation. Due to its value across clinical and research settings and its prevalence in the literature, the PATHWAY CHEPS is the primary focus of this narrative – though the search strategies were not limited to a system; the PATHWAY CHEPS system is capable of cooling configurations, which were excluded from the review.
Literature search methods
This systematic review is reported following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, to elucidate the literature combining MEG and contact heat methodologies. Reference Page, McKenzie and Bossuyt10 The protocol for this review is registered on PROSPERO as CRD42020178324.
Search Strategy
Electronic databases searched included MEDLINE, The Cochrane Library (CENTRAL), Embase, CINAHL, PsycINFO, SportDISCUS, Scopus and Google Scholar, searched from inception until 1st March 2020 (Appendix A). An experienced information specialist (MM) conducted the searches. The search strategy included a combination of free-text and indexing terms and was restricted to the English language. Reference lists and literature that cited included studies were hand-searched for additional relevant items. Force-directed graphs based on co-citation and bibliographic coupling were then created for all included studies via ConnectedPapers (www.connectedpapers.com), and these graphs were scrutinised for possible inclusions.
Study Selection
Two reviewers (TGD and RD) independently screened all titles and abstracts to identify potentially relevant studies. Any papers with methods that were unclear in their abstract were included for assessment of the full text. Full texts of potentially relevant studies were retrieved, and the same reviewers evaluated their eligibility, using the criteria outlined in Table 1. Disagreements at each stage were resolved through discussion, with a third author available to consult on any disagreements (SW).
Table 1: Eligibility criteria

Data Extraction
Due to the predicted heterogeneity across studies, a broad style of data extraction was implemented. The extraction of data from the included studies focused on any significant event-related fields (ERF), time-frequency and source localisation characteristics in response to sensory stimulation; the expected outcomes for which were direct findings or implications for pain processing or methodology in clinical or experimental settings. Additional data regarding the parameters of contact heat stimulation, individual stimulus adaptation, participant sample, study designs, MEG acquisition and analysis were collected and presented to evaluate consistency and any missing information.
Risk of Bias Assessment
Risk of bias was evaluated by one reviewer (TGD) and checked by a second (RD). Risk of bias was assessed using a version of the National Heart, Lung and Blood Institute Study Quality Assessment Tools for Case Series studies that were altered to reflect the studies identified (National Heart, Lung and Blood Institute, 2019; see Supplement A). The alterations refocused the questions to experimental procedures and randomisation. A third reviewer was available to consult on any disagreements if necessary (SW).
Data Synthesis
Due to heterogeneity across the included studies, a narrative synthesis was conducted. A meta-analysis was not considered appropriate given the aim for this review and the numerical data presented within the studies being inadequate for pooling. The included studies are aligned with the type of research (i.e., somatosensory or methodological research) and methods of analysis (i.e., ERF or time-frequency analysis). Study outcomes were synthesised and discussed in relation to cortical processing and implications for combined MEG and contact heat research.
Results
Study Selection
The database searches produced 646 results. Five additional papers were identified through other sources. After the removal of duplicates, 275 abstracts were screened, and 58 studies were identified as requiring full-text evaluation. Of these, seven were identified as meeting the eligibility criteria. Reference Gopalakrishnan, Machado, Burgess and Mosher6,Reference Adjamian, Worthen and Hillebrand9,Reference Gopalakrishnan, Burgess, Plow, Floden and Machado11–Reference Gopalakrishnan, Burgess, Lempka, Gale, Floden and Machado15 Fifty studies were excluded because they did not use MEG in their design. The PRISMA flow chart outlining the process is shown in Figure 1.

Figure 1: PRISMA flowchart for article selection.
Study Characteristics
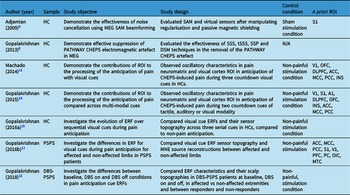
Table 2 outlines the included study characteristics. Included studies were experimental and primarily recruited healthy controls. Two of the seven studies investigated Post-Stroke Pain Syndrome (PSPS; or “Central Post-Stroke Pain”), Reference Gopalakrishnan, Burgess, Plow, Floden and Machado11,Reference Gopalakrishnan, Burgess and Malone12 five used the PATHWAY CHEPS as a noxious stimulus to elicit pain anticipation, Reference Gopalakrishnan, Burgess, Plow, Floden and Machado11–Reference Gopalakrishnan, Burgess, Lempka, Gale, Floden and Machado15 and two papers outlined artefact rejection methods to combat the electromagnetic noise generator by the PATHWAY CHEPS. Reference Gopalakrishnan, Machado, Burgess and Mosher6,Reference Adjamian, Worthen and Hillebrand9
Table 2: Characteristics of studies included in this review

ROI = Region(s) of Interest; TF = Time-frequency; PSPS = Post-stroke Pain Syndrome; HC = healthy control; SEF = Sensory Evoked Field; DBS-PSPS = Deep Brain Stimulation – Post-Stroke Pain Syndrome; ERF = Event-Related Field; V1 = Primary visual cortex; S1 = Primary sensory cortex; A1 = Primary auditory cortex; DLPFC = Dorsolateral prefrontal cortex; ACC = Anterior cingulate cortex; MCC = Medial Temporal Cortex; PCC = Posterior Cingulate Cortex; INS = Insula; OFC = Orbitofrontal Cortex; OIC = Operculo-insular Cortex; MTC = Medial Temporal Cortex; PFC = Prefrontal Corte; SAM = Synthetic Aperture Magnetometry.
Equipment, stimulation, titration and trial parameters are outlined in Table 3, as are MEG data preprocessing steps. All studies used the PATHWAY CHEPS (Medoc, Ramat-Yoshai, Israel) as their stimulus generator. All but one study recorded data using an Elekta Neuromag TRIUX MEG system with 204 planar gradiometers and 102 magnetometers; the other used a CTF 275-channel axial gradiometer MEG (VSM MedTech, Canada). Reference Adjamian, Worthen and Hillebrand9 Results directly linked to contact heat, somatosensory outcomes, or utility in MEG are highlighted.
Table 3: CHEPS and MEG parameters of the included studies

1 Same participants, though the dataset presented is different; *After any participant attrition; ǂ Estimated from figures; VAS = Visual Analogue Scale; NR = not reported; NA = not applicable; M = Male; F = Female; LP = Low pass; BP = bandpass; SSP = Signal Space Projection; tSSS = Temporal Signal Space Separation; DSM = Damped Sinusoid Model; ERF = Event-Related Field; ICA = Independent Component Analysis; DC = Direct Current; TFA = Time-frequency analysis. CD = Could not Determine.
Risk of Bias Assessment
Details of the risk of bias assessment are presented in supplement A. All studies were judged to have a low risk of bias in the domains of clear experimental procedure, research questions, statistical methodology and outcomes. None of the studies identified consecutive case samples, and of the experimental studies, only Gopalakrishnan et al. Reference Gopalakrishnan, Burgess, Plow, Floden and Machado14 sufficiently counterbalanced or randomised conditions – adding a considerable risk of desensitisation of thermoreceptors to four studies, as discussed later in this review.
Findings of Included Studies
The included studies consist of methodological, Reference Gopalakrishnan, Machado, Burgess and Mosher6,Reference Adjamian, Worthen and Hillebrand9 and pain anticipation research. Reference Gopalakrishnan, Burgess, Plow, Floden and Machado11–Reference Gopalakrishnan, Burgess, Lempka, Gale, Floden and Machado15 The pain anticipation findings are grouped by ERF and time-frequency analyses.
Methodological Research
Two studies identified by this review, specifically evaluate the utility of preprocessing and analysis techniques in combined MEG and contact heats research. Adjamian et al. Reference Adjamian, Worthen and Hillebrand9 demonstrated significant attenuation of PATHWAY CHEPS artefact by using a third-order synthetic gradiometer. In addition, the effectiveness of Synthetic Aperture Magnetometry (SAM) beamforming in localising PATHWAY CHEPS event-related power change in the 13–20 Hz frequency band was evaluated and found to have similar accuracy and greater precision in localisation of peak activation in S1 when compared to somatosensory evoked potentials following electrical stimulation. Blocking the thermal element of the PATHWAY CHEPS confirmed pain processing was responsible for the recorded output. Comparison of time-series and beamforming output showed that regularisation undoes the beamformer’s spatial filtering and re-introduces the PATHWAY CHEPS artefact.
Gopalakrishnan et al. Reference Gopalakrishnan, Machado, Burgess and Mosher6 explored different artefact rejection techniques used to suppress the electromagnetic noise associated with the PATHWAY CHEPS’ presence in magnetically shielded chambers and with its activation. It was demonstrated that signal space projection Reference Uusitalo and Ilmoniemi16 failed to adequately remove the artefacts using any variety of the method in any phase of the stimulus, while tSSS Reference Taulu and Hari8 performed well in removing temporal and spectral artefact components, but reduced the dimensionality of the data significantly. The authors piloted a Damped Sinusoid Modelling (DSM) technique and showed similar artefact removal with a lesser reduction of data dimensionality, as well as tighter control of the sinusoids being removed; though some of the artefacts did remain in all methods, the authors concluded that the artefact was sufficiently attenuated. The DSM method is not widely available, and this research group did not go on to explore post-stimulus time windows.
Somatosensory Research
Six studies were conducted by groups comprised of researchers across institutes at the Cleveland Clinic in Cleveland, Ohio Reference Gopalakrishnan, Machado, Burgess and Mosher6,Reference Gopalakrishnan, Burgess, Plow, Floden and Machado11–Reference Gopalakrishnan, Burgess, Lempka, Gale, Floden and Machado15 ; one of these explored the effectiveness of data cleaning methods, Reference Gopalakrishnan, Machado, Burgess and Mosher6 five were designed to elucidate the mechanisms of processing pain anticipation, Reference Gopalakrishnan, Burgess, Plow, Floden and Machado11–Reference Gopalakrishnan, Burgess, Lempka, Gale, Floden and Machado15 and as such, most analyses were focused on the time windows following anticipation cues before painful stimulation. The studies published by this group use a consistent protocol: in the first paradigm, noxious PATHWAY CHEPS or innocuous sensory stimulus was presented following three visual cues; in the second paradigm, an alternative individually adapted innocuous electrical stimulus was presented. Thermal stimuli were individually adapted by presenting them on the volar forearm for 2s in steps of 1°C until reaching an equivalent to 8/10 VAS score for each individual. Cluster permutation analyses were performed on post-cue time windows to evaluate significant between- and within-condition differences in time-locked waveform component amplitudes across all sensors. Time-frequency analysis was performed using virtual channels in regions of interest. All relevant findings below were reported as statistically significant at P < 0.05 or P < 0.01.
ERF Analysis
In the first of this series of pain anticipation studies, Machado et al. Reference Machado, Gopalakrishnan, Plow, Burgess and Mosher13 observed significantly greater power for V1 ERFs time-locked to the first cue in pain stimuli (PS) compared to no-pain (NPS) or no-stimulus conditions. Using the same dataset, Gopalakrishnan et al. Reference Gopalakrishnan, Burgess, Lempka, Gale, Floden and Machado15 performed a non-parametric cluster analysis to test for significant differences between conditions across sensors: this analysis identified central and frontal sensor groups as determinants for pain-specific between-condition differences in first cue visually evoked fields (VEF); additional analysis identified significantly greater power in components evoked by the first cue over later cues. These findings implicate a fronto-central association with pain-specific anticipation in healthy controls.
Additional studies using this study design and these analysis methods were performed on Post-Stroke Pain Syndrome (PSPS) patients, before and after undergoing Deep Brain Stimulator (DBS) surgery. Reference Gopalakrishnan, Burgess, Plow, Floden and Machado11,Reference Gopalakrishnan, Burgess and Malone12 Gopalakrishnan et al. Reference Gopalakrishnan, Burgess, Plow, Floden and Machado11 observed cue VEFs in PSPS patients at baseline and found significant differences in frontal and central sensors only when comparing second and third pain cues to innocuous or no-stimulation cues in unaffected limbs. In limbs affected by the chronic pain condition, no significant differences were found between any cues or conditions, elucidating a lack of cue saliency as a result of the chronic pain. After circumventing DBS stimulation artefacts with a bipolar configuration, the follow-up DBS study demonstrated restoration of affected limb cue saliency in conditions with the DBS turned on and off: greater amplitude in first pain cue N1 components were observed, and their differences localised to parietal and midline sensors. Further exploration of the role of responder (n = 4) vs non-responder (n = 3), as operationalised by a change in their Montgomery Åsberg Depression Rating Scale Reference Montgomery and Asberg17 score, showed responders had significant differences in frontal N1 components within both painful and non-painful cues that correlated with affective benefits. Non-responders also showed a difference between PS and NPS P2 that they claim is a biomarker for the maladaptive hypervigilant attentional processes that are not successfully modulated by DBS in these PSPS patients.
Time-Frequency Analysis
Gamma and beta VEF time-frequency representations were explored by three studies within this category of anticipatory research. Machado et al. Reference Machado, Gopalakrishnan, Plow, Burgess and Mosher13 used virtual sensors in dorsolateral prefrontal (DLPFC), orbitofrontal (OFC), calcarine, cingulate and insula cortices, observed increased gamma oscillation power throughout pain cues in the left calcarine and right DLPFC and increased beta oscillation power in the right OFC. A follow-up study that compared visual, auditory and somatosensory methods of cueing stimulations showed that visual cues entrained greater high beta and low gamma oscillations in the calcarine cortex than any other modality in their associated brain area; cross-modal gamma activations in primary sensory cortices were present only in pain conditions and to a lesser extent in A1.
The baseline assessment of PSPS patients in Gopalakrishnan et al. Reference Gopalakrishnan, Burgess, Plow, Floden and Machado11 demonstrated significant beta and gamma activity in the supramarginal gyrus and frontal polar region during cues two and three when comparing PS to NPS, but no significant effects in the non-affected extremity – consolidating their previous conclusions about the lack of saliency.
Discussion
Contact Heat in MEG
The results of this review highlight the scarcity of research utilising contact heat in MEG. Using broad search terms, only seven papers were identified and all of them used the PATHWAY CHEPS system. The paucity in this area of literature is likely due to challenges associated with the significant electromagnetic noise generated by the thermode: This review highlights two papers that specifically aim to evaluate analysis techniques that facilitate artefact removal – the results of which demonstrated adequate artefact rejection using SAM beamforming, Reference Adjamian, Worthen and Hillebrand9 tSSS or DSM methods. Reference Gopalakrishnan, Machado, Burgess and Mosher6 Indeed, most studies selected here use tSSS to remove external artefacts – though this method is not readily available for all MEG systems and some frequency components may remain. Though the following is only mentioned in two studies, the manufacturers have recommended the MRI-compatible thermode for MEG recording – implying there are possibly unsuccessful research projects that have failed to collect useful data because of using the standard, less expensive MRI-incompatible thermode.
Nevertheless, the findings of this review demonstrate the utility of the PATHWAY CHEPS when cleaned with tSSS or DSM in sensor and source-level analysis. The success of SAM, MNE and Bayes source localisation methods implies the possible utility of spatial filtering in other beamformers. With evidence suggesting MEG data can identify temporal components of evoked waveforms that are not present in EEG recordings Reference Fardo, Vinding, Allen, Jensen and Finnerup5 and the improved spatial resolution and localisation accuracy of its source reconstruction, future research utilising contact heat or exploring the neural correlates of its perception could benefit from MEG acquisition.
Methodological Considerations
The designs of the identified contact heat studies vary in parameters that are noteworthy in the literature and should be considered (Table 3). Baseline thermode temperature has been shown to influence the amplitude and latency of evoked responses in EEG, Reference Kramer, Haefeli, Jutzeler, Steeves and Curt18–Reference Rosner, Hubli and Hostettler20 as have stimulation location, Reference Rosner, Hubli and Hostettler20,Reference Granovsky, Matre, Sokolik, Lorenz and Casey21 fixed vs variable placement, Reference Greffrath, Baumgärtner and Treede22 hairy vs glabrous skin Reference Granovsky, Matre, Sokolik, Lorenz and Casey21,Reference Granovsky, Raz and Defrin23 and inter-stimulus interval. Reference Broadbent, Christina and Schoth24 Baseline temperature in the studies we have reported on ranged from 32 to 35°C, though the alternative of 42°C has revealed lower latency and higher amplitude responses over multiple stimulation locations, Reference Rosner, Hubli and Hostettler20 and it is possible that baseline temperature maintenance could contribute to the noise floor in a MEG environment. Reference Gopalakrishnan, Machado, Burgess and Mosher6 The heat stimuli in the studies identified here were most often individually adapted to 8/10 on a Visual Analogue Scale (VAS), with an average temperature of 48°C – this variable, however, is not comparable across all studies: In at least three of the identified studies the stimulation was held for as long as 2 s – as opposed to the range of 200–500 ms for a standard PATHWAY CHEPS pulse – which could significantly influence the experience of pain. In addition, no studies in this review reported or mentioned the overshoot control values that can be set within the PATHWAY CHEPS software. Overshoot parameters can result in the thermode reaching temperatures higher than their target value, then cooling down; this can possibly result in offset-analgesia or otherwise impact how the target temperature is reached. This is especially necessary to report if different settings are used in different laboratories. It is important to report parameters and record pain outcomes throughout contact heat paradigms and reflect upon study findings with the stimulus parameters in mind. Recommendations for designing and reporting on these are found in Table 4.
Table 4: Recommendations for designing and reporting on combined contact heat MEG studies

With each of the five studies that investigated anticipation cues, a minimum of 480 trials were collected (one study recorded 945) in one session. To avoid enduring pain effects, four of these studies presented non-painful stimuli and painful stimuli (with no-stimulation control trials) as separate experiments and did not counter-balance them. This lack of randomisation or counterbalancing leaves the data vulnerable to the effects of participant exhaustion, which are a considerable concern for the integrity of data in long recording sessions – especially in pain research. Reference Gross, Baillet and Barnes26 Of particular note is the paper by Gopalakrishnan et al., Reference Gopalakrishnan, Burgess, Lempka, Gale, Floden and Machado15 that compares the no-stimulation control trials from paradigm one and two, identifying a difference between the two and deducing that the control linked with the painful condition was more susceptible to vigilance; though this may be the case, the authors did not suggest that the difference between the two may be due to exhaustion or cue learning after many trials.
Pain Anticipation
Most of the results presented focus on the anticipation of contact heat pain following visual cues in healthy controls. The studies presented consistently identify increases in relative power of gamma and beta oscillations in frontal regions such as the DLPFC and OFC in response to cues that signal pain when non-pain stimulation control data is subtracted; frontal cortices were associated with the greatest pain specificity in N1 components of ERF analyses, whilst central sensors demonstrated differences among cues. PSPS patients did not have significant differences between pain and non-pain anticipation when expecting pain to be presented to their affected limb, demonstrating a lack of saliency possibly due to chronic vigilance even for non-painful stimuli Reference Broadbent, Christina and Schoth24 ; this saliency was reintroduced by DBS, especially in those that scored better on depression scales after intervention. Presenting the contact heat stimuli for 2s may prove to be more effective at eliciting affective anticipatory components, entraining more saliency in those with restored affective capability; the PATHWAY CHEPS’ capability to maintain longer duration ecologically valid stimulations with lesser risk of injury remains an advantage over laser and electrical techniques here.
Limitations
Some limitations to this review should be noted. First, most of the studies presented in this review do not assess evoked potentials, instead opting to analyse data in a pre-stimulus time window where any stimulus-related electromagnetic artefact is of reduced field strength. Though we identified studies discussing the methodology of acquiring CHEPs in MEG, no participant studies analysing the post-stimulus time window following heat stimulations were identified in the search. Second, though thorough searches of adjacent literature, citing papers and reference lists were conducted, unpublished research was not explored.
Additionally, six of seven studies identified in this review were performed by the same group – introducing researcher bias that is not reflected in the risk of bias assessment. In context of this review, this further highlights the lack of depth and breadth of research assessing CHEPs in MEG – as does the homogeneity of stimulus systems. Whilst other systems were not excluded from the search, only PATHWAY CHEPS models were utilised in any identified studies. It is likely that the PATHWAY CHEPS is favoured due to the availability of an optional fMRI-compatible thermode that enables the effective suppression of electromagnetic noise. With newer MRI-compatible contact heat stimulus systems capable of ramping temperatures up to 300°C/s, Reference De Schoenmacker, Archibald, Kramer and Hubli25 it is hoped that this review encourages researchers to expand into this area of paucity.
Future Research
Though the literature is sparse, the studies identified in this review provide a good overview of some current methods of analysis when acquiring contact heat data in MEG. MEG analysis methods with proper preprocessing are capable of localising activity with improved spatial resolution in comparison to EEG. Future research would benefit from comparing fMRI and MEG methods, as demonstrated with EEG by Roberts et al. Reference Roberts, Papadaki and Gonçalves27 ; though simultaneous recording would not be possible, more precise virtual time-series data could more accurately elucidate the contribution of cortical subregions to pain processing. The capability of the PATHWAY CHEPS to generate long-duration stimulations without harming the participant facilitates thermal tonic pain experimentation, which opens doors to exploring affective, summative and sensitisation dynamics. Reference Staud, Godfrey, Mejia, Ramanlal, Riley and Robinson28,Reference Linde, Haefeli and Jutzeler29 No studies experimentally analysing post-stimulus noxious CHEPs epochs in MEG currently exist, but this avenue of research could elucidate mechanisms previously not detected by EEG. Reference Fardo, Vinding, Allen, Jensen and Finnerup5 Utilising this combination to explore treatment response is a promising avenue of research, Reference Gopalakrishnan, Burgess, Plow, Floden and Machado11,Reference Gopalakrishnan, Burgess and Malone12 and any additional benefits over standard or single-electrode EEG recordings for clinical diagnostic use should be properly explored by further study.
Conclusions
Contact heat is a well-validated thermoalgesic pain stimulus which emulates a sensation that could be experienced outside of the laboratory, can be used to evaluate thermoception and nociception in clinical settings and is a more accessible and safer alternative to laser methods. Few experimental studies combining contact heat and MEG exist. All studies identified by this review utilised the PATHWAY CHEPS system and none analysed the post-stimulus time window in participants. Studies demonstrating the effectiveness of artefact removal and source localisation techniques in clearing electromagnetic noise generated by the PATHWAY CHEPS were described, though the most effective methods are not available as standard for all MEG systems. A portfolio of research was presented in this review that demonstrates the utility of contact heat in MEG research. Though MEG can identify spectral components that are not identified by other imaging methods and contact heat is a commonly used experimental pain stimulus, there are few published studies exploring them in combination. Future analysis of contact heat MEG data could improve our understanding of spectral correlates underpinning pathophysiological pain and sensory conditions, and contribute to contemporary discourse surrounding chronic and neuropathic pain treatment response and mechanisms.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2023.25.
Statement of Authorship
MM conducted the searches. TGD and RD screened the search results for eligibility and conducted the risk of bias assessment. TGD extracted the data, conceptualised the study and wrote the manuscript, to which RD, SW, MM, MV and SK contributed. All authors approved the final version of the manuscript.
Funding
No funding was received in support of this study.
Conflict of Interest
No authors have any conflicts of interest to declare.