INTRODUCTION
Visceral leishmaniasis (VL) is one among the various neglected tropical diseases caused by an obligate intracellular protozoan Leishmania donovani and transmitted in Indian subcontinent by the bite of Phlebotomus argentipes (Sand fly) (Singh et al. Reference Singh, Hasker, Sacks, Boelaert and Sundar2014). Around 0·2–0·4 million cases are reported globally. Among these, more than 90% of cases are confined to six countries: India, Bangladesh, Sudan, South Sudan, Brazil and Ethiopia (Alvar et al. Reference Alvar, Velez, Bern, Herrero, Desjeux, Cano, Jannin and den Boer2012). A joint memorandum was signed in 2005 by governments of India, Nepal and Bangladesh to eliminate VL with the aim to reduce its incidence by 2015 to less than 1 per 10 000 people at sub-district level. However, this target has been recently extended to 2017 (Singh et al. Reference Singh, Hasker, Boelaert and Sundar2016). Another collaborative disease eradication programme, the London Declaration on Neglected Tropical Diseases was launched on 30 January 2012 in London. It was inspired by the World Health Organization 2020 roadmap to eradicate or prevent transmission of neglected tropical diseases (London Declaration*). The emergence of co-infection with HIV in VL is one of the major challenges, which makes treatment more complex and increase the chances of drug resistance. Initially, HIV-VL cases were seen from south-western Europe, but now cases are on increase in Ethiopia, South Asia and Brazil. Co-infection has been reported from more than 35 countries (Alvar et al. Reference Alvar, Canavate, Gutierrez-Solar, Jimenez, Laguna, Lopez-Velez, Molina and Moreno1997, Reference Alvar, Aparicio, Aseffa, Den Boer, Canavate, Dedet, Gradoni, Ter Horst, Lopez-Velez and Moreno2008; Desjeux and Alvar, Reference Desjeux and Alvar2003). The prevalence of human immunodeficiency virus (HIV) in Bihar, India (which, till recently, accounted for 40% of world's burden of VL) is considered low (0·2–0·3%) (NACO report 2014#). Around 2·4% of all VL patients of age ⩾14 years were unknowingly found to be co-infected with HIV in an Indian study (Burza et al. Reference Burza, Mahajan, Sanz, Sunyoto, Kumar, Mitra and Lima2014a).
VL clinically manifests as prolonged fever, hepatomegaly, splenomegaly, pancytopenia, progressive anaemia and weight loss and is fatal without treatment. About 50% of patients in Sudan and 1–3% in India after recovery of VL develop a cutaneous manifestation in the form of indurated nodules or depigmented macules known as post kala-azar dermal leishmaniasis (PKDL). These cases are difficult to treat and also serve as a reservoir of leishmania (Mukhopadhyay et al. Reference Mukhopadhyay, Dalton, Kaye and Chatterjee2014).
With no effective vaccine available, the only option for treatment of VL remains chemotherapy. With a limited inventory of drugs and emerging drug resistance, the treatment of VL remains challenging.
Chemotherapy of VL
Pentavalent antimonials (SbV)
Sodium stibogluconate (SSG) and meglumine antimoniate (MA) are two forms of available SbV. It is given in doses of 20 mg kg−1 subcutaneously for 28–30 days. With emerging resistance to this drug in Bihar and adjoining areas of Nepal alternative treatment strategy has been adopted for these areas (Sundar et al. Reference Sundar, More, Singh, Singh, Sharma, Makharia, Kumar and Murray2000; Rijal et al. Reference Rijal, Chappuis, Singh, Bovier, Acharya, Karki, Das, Desjeux, Loutan and Koirala2003). Its efficacy has been also found to be low in HIV-VL co-infected patients when compared with immunocompetent VL patients (Ritmeijer et al. Reference Ritmeijer, Dejenie, Assefa, Hundie, Mesure, Boots, den Boer and Davidson2006). However, in east Africa SSG monotherapy is still effective with 6 month cure rate of 93·9% (Musa et al. Reference Musa, Khalil, Hailu, Olobo, Balasegaram, Omollo, Edwards, Rashid, Mbui, Musa, Abuzaid, Ahmed, Fadlalla, El-Hassan, Mueller, Mucee, Njoroge, Manduku, Mutuma, Apadet, Lodenyo, Mutea, Kirigi, Yifru, Mengistu, Hurissa, Hailu, Weldegebreal, Tafes and Mekonnen2012). It was recommended by WHO as first-line drug for treatment of VL in east Africa along with the combination of SSG-Paromomycin (PM) as the first-line treatment of VL patients in Eastern Africa (WHO, 2010).
Its use is further limited by associated life-threatening toxicities like cardiac arrhythmias, prolonged QT interval (QTc), ventricular premature beats, ventricular tachycardia, ventricular fibrillation and torsades de pointes. Other adverse effects include arthralgia, myalgia, increased pancreatic and liver enzymes (Sundar and Chakravarty, Reference Sundar and Chakravarty2015b).
Amphotericin B and liposomal Amphotericin B
In the Indian subcontinent for the treatment of VL, Amphotericin B deoxycholate is recommended at doses of 0·75–1·0 mg kg−1 given for 15–20 intravenous infusions. For PKDL, AmB is recommended at a dose of 1 mg kg day−1 up to 60–80 intravenous infusions for 4 months (Mishra et al. Reference Mishra, Biswas, Jha and Khan1992; Thakur, Reference Thakur1997; Thakur et al. Reference Thakur, Singh, Hassan, Kumar, Narain and Kumar1999). Its toxicity profile includes infusion reactions (in most patients), nephrotoxicity, hypokalemia, myocarditis and occasional death, which mandate close monitoring. This along with prolonged hospital stay escalates the cost of therapy. The lipid formulation of AmB has been developed to minimize side effects and for delivery of drug at large daily doses. Liposomal amphotericin B (AmBisome; Gilead Sciences; LAmB), amphotericin B lipid complex (ABLC; Abelcet, Enzon pharmaceuticals) and amphotericin B colloidal dispersion (ABCD; Amphotec, InterMune Corp.) and several generic formulations are available with LAmB being the only drug, which has US FDA approval. Various trials on LAmB has been shown in Table 1.
Table 1. L-AmB trials in VL and HIV-VL co-infection
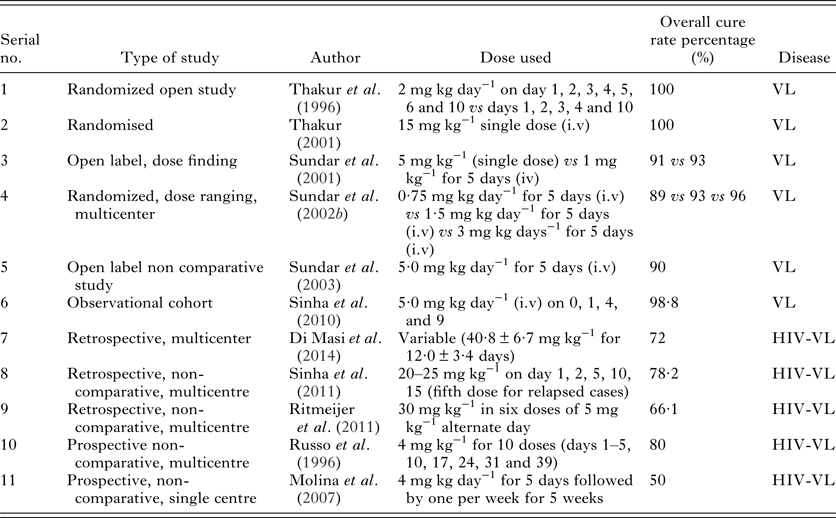
There is considerable geographical variation in the total LAmB dose (Sundar and Chakravarty, Reference Sundar and Chakravarty2015b). In the Mediterranean region and South America, 18–21 mg kg−1, administered in various regimens, has been recommended (Sundar and Chakravarty, Reference Sundar and Chakravarty2015b; Davidson et al. Reference Davidson, di Martino, Gradoni, Giacchino, Gaeta, Pempinello, Scotti, Cascio, Castagnola, Maisto, Gramiccia, di Caprio, Wilkinson and Bryceson1996; Freire et al. Reference Freire, Badaró, Avelar, Luz, Nakatani, Teixeira, Martins Netto and Badaró1997; Syriopoulou et al. Reference Syriopoulou, Daikos, Theodoridou, Pavlopoulou, Manolaki, Sereti, Karamboula, Papathanasiou, Krikos and Saroglou2003; Gradoni et al. Reference Gradoni, Gramiccia and Scalone2004). Single dose of 10 mg kg−1 has been shown to cure >95% VL cases in India (Sundar et al. Reference Sundar, Chakravarty, Agarwal, Rai and Murray2010). Another study showed that when 20 mg kg−1 of LAmB was given over 4–10 days, relapse rate was 2·4% (Burza et al. Reference Burza, Sinha, Mahajan, Lima, Mitra, Verma, Balasegaram and Das2014b). In Bangladesh, 10 mg kg−1 single dose of LAmB cured 97% patients (Mondal et al. Reference Mondal, Alvar, Hasnain, Hossain, Ghosh, Huda, Nabi, Sundar, Matlashewski and Arana2014). In another study, LAmB in three doses of 5 mg kg−1 each (total 15 mg kg−1) had a cure rate of >95% (Lucero et al. Reference Lucero, Collin, Gomes, Akter, Asad and Ritmeijer2015). In Sudan, LAmB is required a much higher dose, when given at 30 mg kg−1 over 10 days in primary VL cases, showed initial cure rate at 6 months of 92% with treatment failures, deaths and relapses of 1, 5 and 7%, respectively. In relapsed VL cases the initial cure was 94%, treatment failed in 4%, 1% died and 10% relapsed. Six percent were slow responders requiring 50 mg kg−1 of LAmB (Salih et al. Reference Salih, van Griensven, Chappuis, Antierens, Mumina, Hammam, Boulle, Alirol, Alnour, Elhag, Manzi, Kizito and Zachariah2014). In Europe, the total dose of 18–21 mg kg−1 is considered adequate (Sundar and Chakravarty, Reference Sundar and Chakravarty2013).
However, high cost remains the limiting factor for widespread LAmB use in most of the endemic regions. This was addressed by an agreement for preferential pricing with WHO (agreement between Gilead and WHO for donation of LAmB till 2020), which reduced the price of LAmB to $18 per 50 mg vial for endemic areas of developing countries (Reference Moon, Jambert, Childs and von Schoen-AngererMoon et al.). Following this, a study was conducted in India in which LAmB was used in a single dose of 10 mg kg−1 and compared with the conventional AmB administered in 15 infusions of 1 mg kg−1, given every other day during 29 days of treatment. At 6 months, cure rates were comparable in the two groups: 95·7% (95% CI 93·4–97·9) in the liposomal-therapy group and 96·3% (95% CI 92·6–99·9) in the conventional-therapy group (Sundar et al. Reference Sundar, Chakravarty, Agarwal, Rai and Murray2010). The decreased treatment cost and hospital stay made liposomal preparation as single infusion an excellent option for treatment of VL in the Indian subcontinent. It has been suggested by the WHO Regional Technical Advisory Group (WHO, 2009) and the WHO Advisory Panel for Leishmaniasis Control (WHO, 2010) to use single dose LAmB as the first line drug for the VL elimination program in the Indian subcontinent. The 10 mg kg−1 single dose regimen is being used in by the Control Programmes of India, Nepal and Bangladesh.
However, in East Africa low efficacy of LAmB in a randomized controlled trial lead to its midway termination when given as a single dose of 7·5, 10 mg kg−1 body weight, or multiple doses on days 1–5, 14 and 21. Definitive cure at 6 months was 85, 40 and 58% in patients treated with multiple doses, and single doses of 7·5 or 10 mg kg−1, respectively (Khalil et al. Reference Khalil, Weldegebreal, Younis, Omollo, Musa, Hailu, Abuzaid, Dorlo, Hurissa, Yifru, Haleke, Smith, Ellis, Balasegaram, EL-Hassan, Schoone, Wasunna, Kimutai, Edwards and Hailu2014)
An indigenous liposomal amphotericin B (Fungisome, developed by an Indian company Lifecare Innovations, Gurgaon, Haryana, India) was tested in a phase 2 study in two cohorts, cohort 1: 10 mg kg−1, cohort 2: 15 mg kg−1. The Initial cure rate of 100% at day 30 and a definitive cure rate of 93·3% at the 6-month follow-up was achieved in both the cohorts (Sundar et al. Reference Sundar, Singh, Rai and Chakravarty2015).
For HIV-VL co-infection, LAmB is given at doses of 4 mg kg−1 for 10 doses (days 1–5, 10, 17, 24, 31 and 38) up to a total dose of 40 mg kg−1 (WHO, 2010). Various trails using LAmB for HIV-VL is shown in Table 1.
In a study in Bihar, when intravenous LAmB (20–25 mg kg−1) was administered to 159 VL/HIV co-infected patients (both primary infections and relapses) in four or five doses of 5 mg kg−1 over 4–10 days, the estimated relapse risk at first year, second year and fourth year was 16·1, 20·4 and 25·9%, respectively (Burza et al. Reference Burza, Mahajan, Sinha, van Griensven, Pandey, Lima, Sanz, Sunyoto, Kumsr, Mitra, Kumar, Verma and Das2014c).
In a study in India, excellent initial response was seen with LAmB (a total dose of 20–25 mg kg−1 in 4–15 days) combined with antiretroviral therapy; however, the probabilities of VL relapse after treatment were 0, 8·1, and 26·5% at 6 month, 1 and 2 years, respectively (Mahajan et al. Reference Mahajan, Das, Isaakidis, Sunyoto, Sagili, Lima, Mitra, Kumar, Pandey, van Geertruyden, Boelaert and Burza2015). In a retrospective study in Bihar, India, a combination of LAmB and miltefosine was tested in 102 HIV-VL co-infected patients. LAmB was given at doses of 30 mg kg−1 divided into six infusions on alternate days, along with oral miltefosine for 14 days. At 6, 12 and 18 months follow-up, the cumulative incidence of all-cause mortality was 11·7, 14·5 and 16·6%, respectively. Relapse rates were 2·5, 6·0, 13·9%, at 6, 12 and 18 months, respectively (Mahajan et al. Reference Mahajan, Das, Isaakidis, Sunyoto, Sagili, Lima, Mitra, Kumar, Pandey, van Geertruyden, Boelaert and Burza2015). Secondary prophylaxis is also important and found to be effective in HIV-VL co-infected patients as with other opportunistic infections. It has been shown that Amphotericin B lipid complex (3–5 mg kg−1 per dose once) every 3 weeks for 12 months has 22% relapse rate as comparison with 50% in patients devoid of secondary prophylaxis at 1 year (Lopez-Velez et al. Reference Lopez-Velez, Videla, Marquez, Boix, Jimenez-Mejias, Gorgolas, Arribas, Salas, Laguna, Sust, Canavate and Alvar2004). LAmB at dose of 5 mg kg−1 administered every third week has been also studied for use as secondary prophylaxis with relapse-free probability of 89·7, 79·1, 55·9 and 55·9% at 6, 12 months, 24 and 36 months, respectively (Molina et al. Reference Molina, Falco, Crespo, Riera, Ribera, Curran, Carrio, Diaz, Villar del Saz, Fisa, Lopez-Chejade, Ocana and Pahissa2007). In a retrospective study from eastern India (2005–2015), the protective efficacy of monthly amphotericin B (AmB) for secondary prophylaxis in patients with HIV–VL coinfection was done. Secondary prophylaxis was provided in 27 HIV-VL with monthly 1 mg kg−1 AmB (15 liposomal, 12 deoxycholate). At 6 month, none in secondary prophylaxis group relapsed or died. Secondary prophylaxis remained the sole significant predictor against death in multivariate Cox regression. HIV–VL patients had higher 6-month relapse rate, less relapse-free 12-month survival and higher mortality than VL mono-infection. (Goswami et al. Reference Goswami, Basu, Ray, Rahman and Tripathi2017).
Miltefosine
Miltefosine, an alkylphospholipid compound, was the first effective oral anti-leishmanial drug in VL. Following a phase III trial, miltefosine was registered in India as a first oral antileishmanial drug in 2002. The cure rate of 94% was seen with 50–100 mg day−1 of miltefosine given for 28 days (Sundar et al. Reference Sundar, Jha, Thakur, Engel, Sindermann, Fischer, Junge, Bryceson and Berman2002a). Another phase 4 study showed a cure rate of 95% (Bhattacharya et al. Reference Bhattacharya, Sinha, Sundar, Thakur, Jha, Pandey, Das, Kumar, Lal, Verma, Singh, Ranjan, Verma, Anders, Sindermann and Ganguly2007). Because of its high efficacy and ease of administration, it was adopted by VL elimination programme in India, Nepal and Bangladesh (Sundar et al. Reference Sundar, Mondal, Rijal, Bhattacharya, Ghalib, Kroeger, Boelaert, Desjeux, Richter-Airijoki and Harms2008a). Unfortunately, after a decade of its use, the efficacy decreased and there was doubling of relapse rate (Sundar et al. Reference Sundar, Singh, Rai, Prajapati, Singh, Ostyn, Boelaert, Dujardin and Chakravarty2012; Burza et al. Reference Burza, Nabi, Mahajan, Mitra and Lima2013). In Nepal, relapse rates of 10·8 and 20% were seen at 6 and 12 months, respectively (Rijal et al. Reference Rijal, Ostyn, Uranw, Rai, Bhattarai, Dorlo, Beijnen, Vanaerschot, Decuypere, Dhakal, Das, Karki, Singh, Boelaert and Dujardin2013). The cure rate of only 85 and 75·8% was found in studies in Bangladesh and Ethiopia, respectively (Ritmeijer et al. Reference Ritmeijer, Dejenie, Assefa, Hundie, Mesure, Boots, den Boer and Davidson2006; Rahman et al. Reference Rahman, Ahmed, Faiz, Chowdhury, Islam, Sayeedur, Rahman, Hossain, Bangali, Ahmad, Islam, Mascie-Taylor, Berman and Arana2011). Similarly, for PKDL, miltefosine at doses of 50 mg thrice daily for 60 days or twice daily for 90 days was found to be effective (Ramesh et al. Reference Ramesh, Katara, Verma and Salotra2011). Another study showed that, miltefosine given as 100 mg day−1 for 12 weeks in PKDL produced high cure rates (Sundar et al. Reference Sundar, Sinha, Jha, Chakravarty, Rai, Kumar, Pandey, Narain, Verma, Das, Das, Berman and Arana2013). It is recommended for the treatment of PKDL in the Indian subcontinent at the dose of 50–100 mg for 12 weeks (WHO, 2010).
Miltefosine is associated with gastrointestinal adverse events chiefly vomiting and diarrhoea. Occasionally hepatotoxicity and nephrotoxicity might occur. It has a long half-life (~1 week), which renders it vulnerable for the development of its resistance in the parasites. It also has teratogenic potential, so women of child-bearing age are advised contraception for the duration of treatment and for three additional months after the end of therapy (Sundar and Chakravarty, Reference Sundar and Chakravarty2015b).
Paromomycin (aminosidine)
Paromomycin (PM) is an aminoglycoside antibiotic, which act by interference with protein synthesis in the ribosome of the target organism and inhibit the respiration (Chawla et al. Reference Chawla, Jhingran, Panigrahi, Stuart and Madhubala2011). In a phase II study of VL patients, PM at a dose of 16 mg kg day−1 for 21 days led to cure in 93% (Jha et al. Reference Jha, Olliaro, Thakur, Kanyok, Singhania, Singh, Singh, Akhoury and Jha1998). A Phase III trial of PM at a dose of 15 mg kg−1 (11 mg base) for 21 days showed 95% cure rate (Sundar et al. Reference Sundar, Jha, Thakur, Sinha and Bhattacharya2007). PM was approved by the Indian government for VL treatment in the Indian subcontinent in August 2006. However, PM was found to be ineffective in curing PKDL with the efficacy of only 37·5% (Sundar et al. Reference Sundar, Singh, Tiwari, Shukla, Chakravarty and Rai2014).
In Bangladesh, in an open-label Phase IIIb multicentre study where PM showed a cure rate of 94·2% at 6 months when administered at 11 mg kg−1 (base) intramuscularly once daily for 21 consecutive days (Jamil et al. Reference Jamil, Haque, Rahman, Abul Faiz, Rezwanul Haque Bhuiyan, Kumar, Hassan, Kelly, Dhalaria, Kochhar, Desjeux, Bhuiyan, Khan and Ghosh2015). In a phase II study in Sudan, Ethiopia and Kenya, its efficacy was low compared with SSG alone and the combination of SSG and PM (Hailu et al. Reference Hailu, Musa, Wasunna, Balasegaram, Yifru, Mengistu, Hurissa, Hailu, Weldegebreal, Tesfaye, Makonnen, Khalil, Ahmed, Fadlalla, El-Hassan, Raheem, Mueller, Koummuki, Rashid, Mbui, Mucee, Njoroge, Manduku, Musibi, Mutuma, Kirui, Lodenyo, Mutea, Kirigi and Edwards2010). In East Africa, PM efficacy was significantly lower than SSG (84·3% vs 94·1%) in a multi-centre randomized-controlled trial (Musa et al. Reference Musa, Khalil, Hailu, Olobo, Balasegaram, Omollo, Edwards, Rashid, Mbui, Musa, Abuzaid, Ahmed, Fadlalla, El-Hassan, Mueller, Mucee, Njoroge, Manduku, Mutuma, Apadet, Lodenyo, Mutea, Kirigi, Yifru, Mengistu, Hurissa, Hailu, Weldegebreal, Tafes and Mekonnen2012). In Sudan, in a dose-finding phase II study, PM showed efficacy of 80% (95% CI 56·3–94·3%) and 81% (95% CI 58·1–94·6%) when used for a longer duration (15 mg kg day−1 for 28 days) or at a higher dose of 20 mg kg day−1 for 21 days, respectively (Musa et al. Reference Musa, Younis, Fadlalla, Royce, Balasegaram, Wasunna, Hailu, Edwards, Omollo, Mudawi, Kokwaro, El-Hassan and Khalil2010). Pain at the injection site, reversible ototoxicity and hepatic transaminitis are common adverse effects. Although low cost of PM is an advantage, its parenteral route of administration limits its use in a control program. Monotherapy also poses a danger for the development of resistance.
Pentamidine
Pentamidine was used as an alternative treatment for refractory VL in India. However, side effects such as insulin-dependent diabetes mellitus and declining efficacy preclude its further usage (Jha et al. Reference Jha, Singh and Jha1991). Injection site pain, indurations and abscess, nausea, vomiting, myalgia, headache, dizziness, hypotension, syncope, transient hyperglycemia and hypoglycaemia are other adverse effects of this drug (Sundar and Chakravarty, Reference Sundar and Chakravarty2015b). It is currently recommended for secondary prophylaxis in HIV-VL co-infection as in a study from Ethiopia revealed relapse-free survival probability of 79 and 71% at 6 months and at 12 months, respectively (Diro et al. Reference Diro, Ritmeijer, Boelaert, Alves, Mohammed, Abongomera, Ravinetto, De Crop, Fikre, Adera, Colebunders, van Loen, Menten, Lynen, Hailu and van Griensven2015).
Multidrug therapy
Multidrug therapy advocated for other diseases like malaria, tuberculosis, leprosy, etc. has also been explored for VL treatment. The rationale behind it is to use drugs with synergistic or additive activity making the duration of therapy short with a decrease in drug doses, which lowers the occurrence of adverse effect and treatment cost (Sundar and Chakravarty, Reference Sundar and Chakravarty2015b). Further with monotherapy, there is always a higher chance of development of drug resistance.
In Sudan a study showed initial cure rate of 97% with combination therapy of PM at a dose of 15 mg kg−1 (equivalent to 11 mg kg−1 of PM base) plus Sbv at a dose of 20 mg kg−1 for 17 days compared with cure rate of 92·4% with Sbv monotherapy given for 30 days (Melaku et al. Reference Melaku, Collin, Keus, Gatluak, Ritmeijer and Davidson2007). It became the preferred regime in the region following another large multicentre, trial which showed the comparable efficacy of combination therapy for 17 days with SSG monotherapy (Musa et al. Reference Musa, Khalil, Hailu, Olobo, Balasegaram, Omollo, Edwards, Rashid, Mbui, Musa, Abuzaid, Ahmed, Fadlalla, El-Hassan, Mueller, Mucee, Njoroge, Manduku, Mutuma, Apadet, Lodenyo, Mutea, Kirigi, Yifru, Mengistu, Hurissa, Hailu, Weldegebreal, Tafes and Mekonnen2012). A prospective pharmacovigilance study sponsored by the Ministries of Health, Médecins Sans Frontières (MSF) and Drugs for Neglected Diseases initiative (DNDi) was recently conducted at 12 centres in a cohort from Sudan, Kenya, Uganda and Ethiopia for PM plus Sbv combination therapy in VL. The initial cure rate was 95·1% with no geographical variation. Thirty-four percent (34%) and 1·96% of patients had at least one adverse event (AE) and serious adverse event (SAE) during treatment, respectively. Mortality occurred in 1·0% of patients. HIV/VL co-infected patients had initial cure rates of 56%, which was significantly lower than that in VL patients without HIV (Kimutai et al. Reference Kimutai, Musa, Njoroge, Omollo, Alves, Hailu, Khalil, Diro, Soipei, Musa, Salman, Ritmeijer, Chappuis, Rashid, Mohammed, Jameneh, Makonnen, Olobo, Okello, Sagaki, Strub, Ellis, Alvar, Balasegaram, Alirol and Wasunna2017).
Combination of miltefosine and LAmB was recently evaluated in Sudan and Kenya where a phase II open-label, the non-comparative randomized trial was conducted. Three regimens [10 mg kg−1 single dose LAmB plus 10 days of SSG (20 mg kg day−1), 10 mg kg−1 single dose LAmB plus 10 days of miltefosine (2·5 mg kg day−1) and miltefosine alone (2·5 mg kg day−1 for 28 days)] were evaluated for efficacy and safety. Although safe, the definitive cure was <90% in all treatment arms so none regimen was evaluated for phase 3 trials (Wasunna et al. Reference Wasunna, Njenga, Balasegaram, Alexander, Omollo, Edwards, Dorlo, Musa, Ali, Elamin, Kirigi, Juma, Kip, Schoone, Hailu, Olobo, Ellis, Kimutai, Wells, Khalil, Strub Wourgaft, Alves and Musa2016).
In a multidrug randomized, non-comparative, group-sequential, triangular design study 181 patients were randomly assigned to 5 mg kg−1 of LAmB alone, 5 mg kg−1 of LAmB followed by MIL for 10 days or 14 days or 3·75 mg kg−1 of LAmB followed by miltefosine for 14 days. When efficacy of all regimens was apparent, 5 mg kg−1 of LAmB followed by miltefosine for 7 days were given 45 additional patients. All groups had similar cure rates (>95%) (Sundar et al. Reference Sundar, Rai, Chakravarty, Agarwal, Agrawal, Vaillant, Olliaro and Murray2008b).
In a Phase III study in India, three multidrug regimens were tested (Single injection of 5 mg kg−1 LAmB and 7-day oral miltefosine or 10-day 11 mg kg−1 intramuscular PM; or 10 days each of miltefosine and PM). The cure rate of >97% was found in all the three-drug regimens used (Sundar et al. Reference Sundar, Sinha, Rai, Verma, Nawin, Alam, Chakravarty, Vaillant, Verma, Pandey, Kumari, Lal, Arora, Sharma, Ellis, Strub-Wourgaft, Balasegaram, Olliaro, Das and Modabber2011a). The cure rate of 91·9% with a single dose of LAmB 5 mg kg−1 with miltefosine 2·5 mg kg day−1 for 14 days was shown in another study (Sundar et al. Reference Sundar, Sinha, Verma, Kumar, Alam, Pandey, Kumari, Ravidas, Chakravarty, Verma, Berman, Ghalib and Arana2011b).
Combination therapy is also an attractive option for the treatment of PKDL (Ramesh et al. Reference Ramesh, Avishek, Sharma and Salotra2014). Combination therapy is excellent and effective alternative strategy to decrease the cost of therapy by decreasing duration of treatment, side effect associated with drugs and hospital stay. Moreover, combination therapy delays drug resistance, thus prolongs use of the drugs.
Newer drugs, Immunotherapy and Vaccine development:
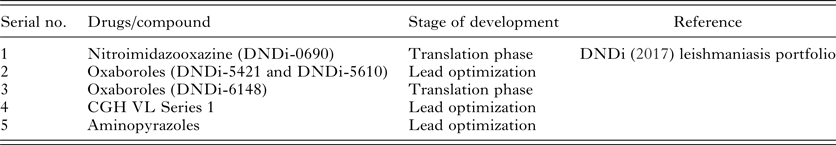
The various new compounds at various stages of development have been included in Drugs for Neglected Disease Initiative (DNDi) portfolio as shown in Table 2. Each of them is in preclinical stages.
Table 2. Newer compounds in pipeline for Visceral leishmaniasis in various stages of development
An alternative approach is to use immunotherapy and/or immunochemotherapy against VL. IFN-γ, a cytokine capable has been found to kill intracellular Leishmania by activating macrophages (Murray and Cartelli, Reference Murray and Cartelli1983). Accelerated clearance of parasite is seen on addition of IFN-γ. Treatment of VL with IFN-γ plus SbV showed a cure rate of >80% cure rate (Badaro, Reference Badaro1988; Badaro et al. Reference Badaro, Falcoff, Badaro, Carvalho, Pedral-Sampaio, Barral, Carvalho, Barral-Netto, Brandely, Silva, Bina, Teixeira, Falcoff, Rocha, Ho and Johnson1990; Sundar et al. Reference Sundar, Rosenkaimer and Murray1994) and enhanced the clinical efficacy of conventional SbV therapy (Sundar and Murray, Reference Sundar and Murray1995). Similarly, results were seen in a study in Kenya where SbV or SbV plus IFN-γ for 30 days was given in two groups for VL treatment with combination therapy showing quick parasite clearance (Squires et al. Reference Squires, Rosenkaimer, Sherwood, Forni, Were and Murray1993). In India in a large study, however, low cure rates of 36, 49 and 42% were obtained at 6 months of treatment with 30 days of SbV alone, or 30 days of SbV plus IFN-γ at dose of 107 U mg day−1, or 15 days of SbV plus IFN-γ respectively, with maximum efficacy in immunotherapy group (Sundar et al. Reference Sundar, Singh, Sharma, Makharia and Murray1997). Low cure rates in this study led to the stoppage of the development of IFN-γ as an adjunct to anti-leishmania drugs.
No vaccine is recommended for treatment of VL till now. To be an effective vaccine apart from eliciting prolong immunity it should be protective broadly against VL and CL. Infectious Disease Research Institute has developed vaccines such as Leish-F1, F2 and F3 based on selected Leishmania antigen epitopes, and has found to be immunogenic and safe in clinical trials with Leish-F2 completed phase 2 study. The Sabin Vaccine Institute is investigating on the immunogenicity by combination of sand fly salivary gland antigen with the recombinant Leishmania antigens (Chakravarty et al. Reference Chakravarty, Kumar, Trivedi, Rai, Singh, Ashman, Laughlin, Coler, Kahn, Beckmann, Cowgill, Reed, Sundar and Piazza2011). Recently, a first-in-human dose escalation Phase I trial to assess the safety, tolerability and immunogenicity of a prime-only adenoviral vaccine (ChAd63-KH) was conducted in 20 healthy volunteers for human VL and PKDL. ChAd63-KH was found to be safe and immunogenic and need further development to emerge as a novel third-generation vaccine for VL and PKDL (Osman et al. Reference Osman, Mistry, Keding, Gabe, Cook, Forrester, Wiggins, Marco, Colloca, Siani, Cortese, Smith, Aebischer, Kaye and Lacey2017). Various recombinant antigens have been studied to have a protective role against Leishmania infection however limited to experimental level with minimal experience in preclinical human studies.(Connell et al. Reference Connell, Medina-Acosta, McMaster, Bloom and Russell1993; Gurunathan et al. Reference Gurunathan, Sacks, Brown, Reiner, Charest, Glaichenhaus and Seder1997; Stober et al. Reference Stober, Lange, Roberts, Alcami and Blackwell2005; Rafati et al. Reference Rafati, Zahedifard and Nazgouee2006; Carrillo et al. Reference Carrillo, Crusat, Nieto, Chicharro, Thomas Mdel, Martinez, Valladares, Canavate, Requena, Lopez, Alvar and Moreno2008; Noazin et al. Reference Noazin, Khamesipour, Moulton, Tanner, Nasseri, Modabber, Sharifi, Khalil, Bernal, Antunes and Smith2009; Kumar et al. Reference Kumar, Goto, Gidwani, Cowgill, Sundar and Reed2010; Chakravarty et al. Reference Chakravarty, Kumar, Trivedi, Rai, Singh, Ashman, Laughlin, Coler, Kahn, Beckmann, Cowgill, Reed, Sundar and Piazza2011; Singh et al. Reference Singh, Stober, Singh, Blackwell and Sundar2012; Sundar and Chakravarty, Reference Sundar and Chakravarty2015a).
Concluding remarks
With meagre treatment options in chemotherapy and emerging risk of resistance to available drugs, the discovery of new drugs with anti-leishmania activity is the need of the hour. Combination therapy of Sbv with PM and LAmB are recommended in Africa and Mediterranean region, respectively. Newer approaches with other drug combination need to be investigated in these different geographical areas. PKDL patients serve as a reservoir of infection. Shorter and effective treatment is warranted for the success of VL Elimination programme. Drugs for Neglected Diseases Initiative (DNDi) have taken the initiative of finding new novel agents with anti-leishmania agents. Also, the feasibility of use of LAmB at district and public health centre in South Asia has also been explored by DNDi. The burden of HIV VL co-infection in increasing and LAmB found effective in Mediterranean region has limited efficacy in Ethiopia having high HIV-VL co-infection burden. Thus newer treatment regimes for this region need to be explored.
ACKNOWLEDGEMENT
This work was supported by National Institute of Allergy and Infectious Diseases, NIH grant number U19AI074321.
FINANCIAL SUPPORT
This work was supported by the Extramural Program of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) Tropical Medicine Research Centres [TMRC Grant No P50AI074321].





