INTRODUCTION
Vibrio spp. cause illnesses that result in substantial morbidity and mortality. Although cholera, caused by toxigenic V. cholerae O1 or O139, is a major global problem, it is rare in the USA. On the other hand, vibriosis – infection caused by any species or strain of Vibrionaceae other than those that cause cholera – is an important public health problem in the USA. Most notably, V. vulnificus infection causes high rates of mortality and severe illness, usually in persons with underlying health conditions. Vibriosis is frequently associated with bivalve mollusc consumption [Reference Cazorla1–Reference Weis8]. Infections with Vibrio spp. have frequently been associated with consumption of oysters [Reference Cazorla1, Reference Haq and Dayal5, Reference Weis8], but illness associated with clam consumption has also been well documented [Reference Chen2, Reference Han4, Reference Kirs6, Reference Vieira7]. Vibrios are ubiquitous in marine and estuarine environments. Vibrio abundance in the environment and seafood, along with vibriosis incidence, peak in the summer when water temperatures are warmest.
The Cholera and Other Vibrio Illness Surveillance system (COVIS) is a collaborative reporting system initiated in 1988 by Gulf Coast states (Alabama, Florida, Louisiana, Texas), the Centers for Disease Control and Prevention (CDC), and the US Food and Drug Administration (FDA). COVIS includes surveillance for toxigenic V. cholerae O1 and O139, which cause cholera. Surveillance also includes vibriosis caused by infection with V. alginolyticus, V. cholerae (all strains other than those that cause cholera), V. parahaemolyticus, V. vulnificus, and all other members of the genus Vibrio as well as Photobacterium damselae subsp. damselae (formerly V. damsela) and Grimontia hollisae (formerly V. hollisae). Nearly all states had begun reporting vibriosis to COVIS by the early 2000s. In 2007, the Council of State and Territorial Epidemiologists issued a position statement making all Vibrio infections nationally notifiable; in 2010 the position statement was updated to reflect taxonomic changes [Reference Dunn and Fullerton9].
METHODS
Information reported to COVIS by state and local health officials includes demographic, isolate (i.e. species and clinical site of specimen), clinical, and risk exposure information as well as seafood traceback information when available. Demographic information collected includes patient's age, sex, race, and ethnicity. The specific Vibrionaceae isolated and the date of specimen collection are also captured. Clinical information includes symptom onset date, duration of illness, pre-existing comorbid conditions, medications used within 30 days before illness onset, hospitalization, and mortality. Risk exposure information includes seafood consumed, interstate and international travel history of the patient, exposure to bodies of water and marine life – all within 7 days before illness onset – and whether the case was associated with an outbreak. For patients with seafood exposure, detailed information about its preparation (i.e. cooked vs. raw), source, and location consumed (i.e. home, restaurant, at a gathering, etc.) is collected. Harvest and shipment information from seafood tags (seafood traceback information) is also obtained from the location where suspect seafood was consumed.
We analysed data for cases of vibriosis reported to COVIS from 1988 to 2010. We excluded cases in which international travel within 7 days of illness onset was reported. We also excluded non-foodborne cases, those cases for which information on risk exposures was missing or the only reported risk exposures were direct contact with bodies of water, marine or estuarine life, or drippings from raw or live seafood. We classified the remaining cases (those reporting only seafood consumption, or seafood consumption and other risk exposures) as follows: for cases in which seafood consumption was the only risk exposure reported, we considered illness to be foodborne if (1) Vibrio was isolated only from a gastrointestinal or blood or other normally sterile site or, (2) if more than one specimen site was reported, a skin or soft tissue site was not reported and at least one site was gastrointestinal or other normally sterile site. For cases in which other risk exposures as well as seafood consumption were reported, we considered illness to be foodborne if Vibrio was isolated from a gastrointestinal site and a skin or soft tissue site isolation was not reported. Thus, the study population includes only domestically acquired foodborne infection. The COVIS surveillance form includes the following types of seafood consumed: clams, crab, fish, lobster, mussels, oysters, shrimp, and ‘other seafood’. In our analysis clams were defined as bivalve molluscs living in sand or mud.
Of foodborne cases, we categorized clam consumption for those patients for whom information was available as ‘no clams’ or ‘any clams.’ Patients in the ‘any clam’ category reported clam consumption and were not excluded if they also reported other seafood consumption. We also defined a subset of patients in the ‘any clam’ category, i.e. those who reported ‘only clams’ and no other seafood consumption. We also performed a subanalysis of patients who reported consuming ‘only oysters’ and no other seafood consumption. We compared demographics (gender, age, race, ethnicity) and clinical information (duration of illness, hospitalization rate, mortality, underlying comorbid conditions), stratified by species isolated. For patients whose only seafood exposure was clams, we analysed seasonality of infections, clam preparation [i.e. raw vs. cooked (including steamed, baked, boiled, or fried)], and clam harvest site information from seafood tags. Additionally, we performed detailed subgroup analysis for the most commonly reported species, V. parahaemolyticus. Analyses were conducted using SAS v. 9.3 (SAS Institute, USA).
RESULTS
In total, 8455 cases of vibriosis in patients without a history of international travel were reported to COVIS from 1988 to 2010. Of these reports, 5376 (64%) were not classified as foodborne resulting in 3079 (36%) domestically acquired foodborne seafood (foodborne transmission) cases of vibriosis being analysed. Of these, information on clam consumption was available for 2312 (75%). Only these reports were included in the full analysis described below.
To compare clam-associated vibriosis to vibriosis associated with other kinds of seafood, we compared patients who reported consuming clams to those who did not. Of all 2312 reports specifying one or more seafood items consumed and for which information on clam consumption was available, 1756 (76%) reported ‘no clam’ consumption, 556 (24%) reported ‘any clam’ consumption, and, of these, 93 (4%) reported consuming ‘only clams’ and no other seafood. Therefore, 17% of clam consumers reported only eating clams. Table 1 presents information on species of reports by clam consumption status. Persons who reported eating ‘only oysters’ and no other seafood were generally very similar to persons who reported eating ‘no clams’. V. parahaemolyticus was the species most commonly associated with clam consumption, accounting for 77% of reports of persons who reported eating ‘only clams’, somewhat higher than in the 65% who reported eating ‘no clams’. By contrast, V. vulnificus, the second most common species reported by persons with ‘any clam’ consumption, accounted for 7% of ‘any clam’ reports, somewhat less than the 10% in those with ‘no clam’ consumption (Table 1). V. vulnificus infections in persons with ‘only clam’ consumption were rare, with only four persons in this category. Vibrio illnesses in persons with ‘only clam’ consumption peaked in July and August (56% of reports were in these two months), as did illnesses in individuals with ‘any clam’ consumption (52% of reports) and ‘no clam’ consumption (46% of reports).
Table 1. Domestically acquired, seafood-associated vibrio infections, by species and by history of clam consumption, Cholera and Other Vibrio Illness Surveillance (COVIS), 1988–2010
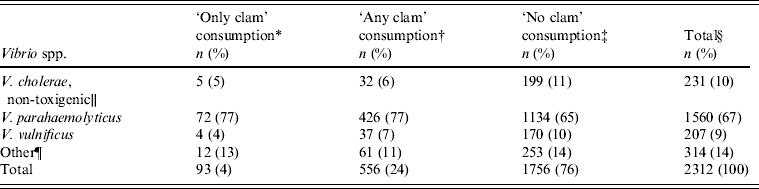
* Ill persons reported clam consumption and reported that they did not consume any additional seafood items.
† Ill persons reported clam consumption and were not excluded if they reported consuming any additional seafood items.
‡ Ill persons reported seafood consumption of crab, lobster, mussels, oysters, shrimp, crawfish, fish, and/or other shellfish, but not clams.
§ Sum of ‘any clam’ and ‘no clam’ consumption categories because ‘only clam’ is a subset of ‘any clam’.
∥ Includes non-toxigenic O1, O139, and non-O1, non-O139 V. cholerae.
¶ This category includes V. alginolyticus, V. cincinnatiensis, Photobacterium damselae subsp. damselae (formerly V. damsela), V. fluvialis, V. furnissii, Grimontia hollisae (formerly V. hollisae), V. metschnikovii, V. mimicus, Vibrio spp., multiple species, and other species.
Of foodborne vibriosis cases with information on clam consumption, isolation sites included gastrointestinal only (2049 reports, 89%), blood or other normally sterile site only (249 reports, 11%), and multiple sites (14 reports, 1%, including seven reporting both blood and stool, two blood and peritoneal fluid, two blood and skin, one blood and bile duct, one blood and cerebrospinal fluid, and one stool and urine). Isolation site varied by species isolated; for all foodborne V. parahaemolyticus, ∼99% of isolates were gastrointestinal, ∼1% were blood or other normally sterile sites, and <1% were multiple sources. Conversely, for foodborne V. vulnificus, 84% of isolates were from blood or other normally sterile sites, 14% gastrointestinal, and 2% multiple sources. In general, isolation site did not vary substantially by clam consumption within species categories.
Table 2 presents demographic and clinical characteristics for V. parahaemolyticus. A somewhat higher proportion of patients reporting ‘only clam’ consumption reported any pre-existing condition (43% in patients with ‘only clam’ consumption vs. 34% with ‘no clam’), notably with diabetes mellitus (12% vs. 7%). The proportion hospitalized was higher for those who consumed ‘only clams’ compared to ‘no clams’ (29% vs. 19%) although the median duration of illness was slightly shorter (5 days vs. 7 days).
Table 2. Demographic and clinical characteristics of persons with Vibrio parahaemolyticus infection associated with seafood consumption, Cholera and Other Vibrio Illness Surveillance (COVIS), 1988–2010
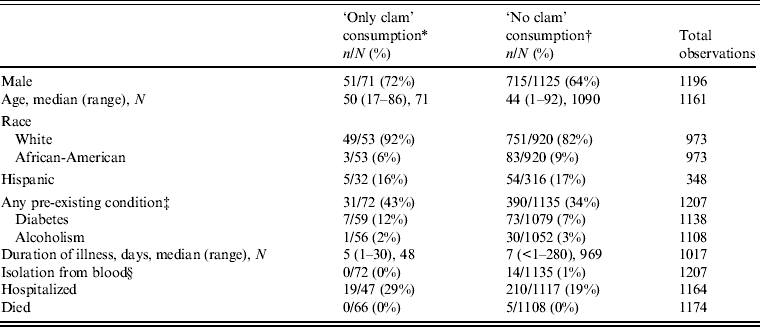
* Ill persons reported clam consumption and reported that they did not consume any additional seafood items.
† Ill persons reported seafood consumption of crab, lobster, mussels, oysters, shrimp, crawfish, fish, and/or other shellfish, but not clams.
‡ Defined as alcoholism, diabetes, peptic ulcer, gastric surgery, heart disease, haematological disease, immunodeficiency, liver disease, malignancy, and/or renal disease.
§ Includes persons with only blood isolates and persons with blood and other isolate.
To assess the harvest sites and preparation of clams consumed by patients with vibriosis who consumed ‘only clams’, we quantified these parameters. Of the 93 ‘only clam’ cases, 37 reports had harvest site information available. These reports showed that clams were most often harvested from the Atlantic Coast (29 reports, 83%), although harvest sites in the Pacific (four reports, 11%) and Gulf (two reports, 6%) coasts were also reported. For reports (29 reports) with harvest sites in the Atlantic, the species reported were: 23 V. parahaemolyticus, three non-toxigenic V. cholerae non-O1, non-O139 serogroups, two V. fluvialis, and one V. alginolyticus. All reports (four reports) from harvest sites in the Pacific were V. parahaemolyticus, and of the two reports from Gulf Coast harvest sites, one was V. parahaemolyticus and one was V. vulnificus. Two ‘only clam’ patients consumed clams that were imported: one patient consumed clams from Canada and one from clams from New Zealand. Of the ‘only clam’ persons for whom information about clam preparation was available (n = 76), 88% (67/76) reported eating clams raw, 5% (four persons) steamed, and 7% baked, boiled, or fried. The number of clams consumed varied widely (range 2–300, median 12 clams). Demographics of persons who reported consuming raw vs. cooked clams were similar: median age was 54 vs. 56 years, 77% vs. 71% male, and 85% vs. 83% white race, respectively. Of eight seafood tracebacks that included information about factors contributing to contamination, only a single case of ‘improper storage, cross-contamination, or improper holding temperature’ was reported.
DISCUSSION
Clam-associated infections account for a substantial minority of Vibrio illnesses. Of patients with foodborne seafood-associated vibriosis acquired in the USA, 24% of ill persons with any seafood consumption reported eating clams before becoming ill, and more than a sixth of those reported eating only clams. For persons with V. parahaemolyticus infection, the most commonly reported species causing vibriosis, the proportion with clam exposure was even higher. More than 80% of patients with only clam consumption and information available ate clams harvested in the Atlantic, and almost 90% ate them raw. These data demonstrate the contribution of clams to the burden of vibriosis in the USA and provide direction for prevention measures.
Clam-associated vibriosis has received little attention in the scientific literature, although well-documented outbreaks have occurred. From 1973 to 2009, seven Vibrio outbreaks associated with clam consumption were reported by states to the CDC Foodborne Disease Outbreak Surveillance System (FDOSS) [10]. Six were caused by V. parahaemolyticus, ranging from 2 to 26 illnesses (median, four illnesses). Three of these outbreaks occurred in 1982, one occurred in 1992, and the other two in 2006. Four outbreaks were associated with raw clams and two with steamed clams, indicating that even clams presented to the consumer as cooked can have associated health risks. Steamed clams may be undercooked or subject to cross-contamination by raw products. A seventh outbreak of two cases of non-toxigenic V. cholerae O1 infection associated with clam consumption in a private home was reported in 1977. No clam-associated V. vulnificus outbreaks have been reported.
Our analyses are subject to several limitations. First, persons with more severe illness outcomes may be more likely to be diagnosed and be reported to COVIS than persons with less severe illness outcomes. However, this reporting bias is likely to impact all illnesses associated with different seafood commodities equally, so it is unlikely to change our conclusions regarding comparisons of clam-associated vs. other foodborne seafood-associated vibriosis. Second, multiple seafood items are often served together at the same meal, and ill persons may not recall or report all seafood consumed. This may lead to misclassification of persons into ‘any’, ‘only’, and ‘no clam’ consumption categories. Nearly one quarter of reports were missing seafood exposure information and were not included in our analysis. Third, nearly two-thirds of reports from individuals who had consumed only clams did not have harvest site information available, which limits our ability to identify common harvest sites, and <10% had information on whether there was evidence of improper storage, cross-contamination, or improper holding temperature. Finally, vibriosis reporting practices vary by state and year. As a passive surveillance system, COVIS captures only illnesses that states report to CDC. Additionally, it is not known whether states not reporting cases to COVIS actually had no cases. This may lead to systematic under-reporting of vibriosis, and there may be regional variations in reporting over time.
Historically, many vibriosis prevention programmes have focused on risk reduction strategies for raw oysters [11]. In the USA, the Interstate Shellfish Sanitation Conference (ISSC) establishes policy for molluscan shellfish safety (including oysters, clams, mussels and scallops) [12]. ISSC began implementing stricter time and temperature requirements and consumer education in the 1990s primarily to address the risk of V. vulnificus infection from oyster consumption in persons with pre-existing conditions such as liver disease and alcoholism. The current V. parahaemolyticus and V. vulnificus control plans, which were implemented in 2008 and 2010, respectively, address these risks with the consumption of oysters only. Our analyses draw attention to the parallel risks associated with clam consumption, showing that clams, especially raw clams, are also an important source of vibriosis, especially V. parahaemolyticus infection. V. vulnificus infection was also associated with clam consumption, although rarely in patients with ‘only clam’ consumption.
The rarity of reported V. vulnificus infections associated with clams may reflect the fact that the major source of implicated clams was Atlantic rather than Gulf Coast waters. V. vulnificus illnesses are more frequently reported in residents of the Gulf Coast than the Atlantic Coast, regardless of type of seafood consumed (CDC, unpublished data). Moreover, a national study of bacterial pathogens in live oysters at retail establishments found that oysters harvested from Gulf Coast waters had significantly higher levels of V. vulnificus than oysters harvested from the Mid-Atlantic in the spring and autumn and from the North Atlantic year round [Reference DePaola13]. By contrast, V. parahaemolyticus levels were highest in Mid-Atlantic and Gulf oysters. Although this study focused on oysters, not clams, it suggests that environmental V. vulnificus levels in Gulf Coast waters may be higher than those in Atlantic waters. Since many species of clams for US consumption are primarily harvested from Atlantic waters, our findings may represent the distribution of pathogens in seafood beds [14]. Although data were limited, improper handling of clams was only documented in a single report of the eight reports that included information, suggesting that, as for other foodborne seafood-associated vibriosis, contamination was usually intrinsic. Risk reduction strategies focusing only on raw oyster consumption ignore an important vehicle for Vibrio illnesses. Comprehensive prevention programmes must address the risks associated with clams as well as oysters.
While our results are based on small numbers, they raise the question of whether clam-associated V. parahaemolyticus infections might be more severe, on average, than those due to other kinds of seafood. About one third of patients reporting ‘only clam’ consumption were hospitalized, compared to one fifth of those with ‘no clam’ consumption, although the median duration of illness was also 2 days shorter for ‘only clam’ consumers. The most plausible explanation for this intriguing but unproven possibility would be that consumers may, on average, ingest a higher dose of vibrios with clams than with other seafood. This could occur if clams concentrate vibrios more than other seafood, such as oysters, if vibrios replicate more rapidly in post-harvest clams than in other seafood, or if consumers tend to eat more clams or more raw clams in a sitting than other seafood. Studies have examined the growth rates of vibrios in post-harvest oysters and have shown variability in virulence of sub-populations [Reference Gooch15, Reference DePaola16], but similar studies have not been reported for clams. The ISSC is shifting from established illness reduction goals to a risk per serving approach for oysters [17], which could also be applied to clams. Our study results provide the strongest evidence to date that clams are an important vehicle of foodborne vibriosis in the USA.
ACKNOWLEDGEMENTS
We thank our colleagues in state and local health departments for reporting Vibrio infections. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.




