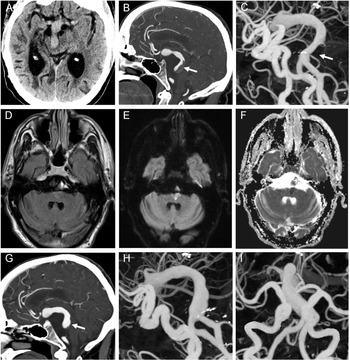
Fabry disease is an X-linked lysosomal storage disease resulting in reduced, or absent, alpha-galactosidase A activity. This leads to progressive accumulation of glycosphingolipids in the cerebral vascular endothelium and smooth muscle, which in part, contributes to cerebrovascular events and intracranial arterial dolichoectasia (IADE). Not much is known about the acute and secondary management of individuals with Fabry disease and IADE. Therefore, we highlight a unique case of a 51-year-old male with a known pathogenic c.511G>C (p. Gly171Arg) mutation in the galactosidase alpha (GLA) gene, not on enzyme replacement therapy and with comorbid hypertension, concentric left ventricular hypertrophy, chronic kidney disease and prior right thalamocapsular stroke, who presented to hospital with acute right-sided weakness and anarthria with a National Institutes of Health Stroke Scale (NIHSS) of 14. His last known normal was 2.5 hours prior, and his baseline NIHSS was 3 from his prior stroke. His CT head showed preserved gray-white matter differentiation. However, his CT angiography revealed a non-occlusive thrombus extending 2.8 cm in length in a significantly dolichoectatic basilar artery (Figure 1A-C). He was given IV tissue plasminogen activator (tPA) and his NIHSS returned to his baseline.

Figure 1: Axial noncontrast CT head demonstrating the presence of a dolichoectatic basilar artery with mass effect on the midbrain (A). With contrast administration, the sagittal CT angiography demonstrated a nonocclusive thrombus in the dolichoectatic basilar artery denoted by the arrow (B). Three-dimensional (3D) digital angiography reconstruction further highlighted the extent of intracranial arterial dolichoectasia in both the anterior and posterior circulation with the associated nonocclusive thrombus in the basilar artery denoted by the arrow (C). MRI axial T2 FLAIR brain imaging demonstrated a subtle hyperintensity in the left paramedian pontine region (D), with associated increased signal on diffusion-weighted imaging (E) and decreased signal on apparent diffusion coefficient imaging (F) consistent with an acute ischemic infarct. Repeat CT angiography showed an interval decrease in the size of the nonocclusive thrombus in the dolichoectatic basilar artery denoted by the arrow (G). There is also interval improvement in the caliber of the basilar artery on the 3D digital angiography reconstructions (H, I) in the setting of ongoing diffuse intracranial arterial dolichoectasia.
He was started on a heparin infusion given that the extensive clot burden in the dolichoectatic basilar artery remained relatively unchanged post-tPA. A subsequent MRI ruled out any acute ischemia and he was transitioned to warfarin. Eleven days after his initial presentation, while therapeutic on warfarin, he developed recurrent right-sided weakness and anarthria with an NIHSS of 10. Repeat CT angiography showed no interval change in thrombus size or intracranial hemorrhage, but his MRI showed a new left paramedian pontine infarct (Figure 1D-F). Given his new ischemic stroke, low dose aspirin was added to his anticoagulation. His 3-month follow-up imaging showed decreased size of the thrombus in the dolichoectatic basilar artery (Figure 1G-I) while on warfarin and low-dose aspirin.
Fabry disease (Online Mendelian Inheritance in Man [OMIM] phenotype number 301500) is an X-linked lysosomal storage disease with significantly reduced, or absent, alpha-galactosidase A activity, resulting in progressive accumulation of glycosphingolipids, specifically globotriaosylceramide and its deacylated form, globotriaosylsphingosine, in various cells of different organs, leading to progressive multiorgan dysfunction and early mortality Reference Ortiz, Germain and Desnick1 .
Ischemic stroke and transient ischemic attacks are the most common types of cerebrovascular events in Fabry disease Reference Kolodny, Fellgiebel and Hilz2 . In a large cohort of Fabry disease patients, the majority experienced their first stroke at less than 40 years of age Reference Sims, Politei, Banikazemi and Lee3 . Moreover, the relative risk of stroke between 35 and 45 years is 12.2 times higher in men and 4.2 times higher in women, compared to healthy controls Reference Kolodny, Fellgiebel and Hilz2 . The abnormal accumulation of glycolipids within endothelial and smooth muscle cells are thought to play a role in arterial vessel wall remodeling by initiating an inflammatory cascade, leading to the release of reactive oxygen species, cytokines and chemokines that triggers smooth muscle proliferation, resulting in intima-media thickening, potential accelerated atherosclerosis and weakening of the vessel wall Reference Kolodny, Fellgiebel and Hilz2,Reference Sims, Politei, Banikazemi and Lee3 . These vessel wall changes, in turn, are speculated to give rise to IADE, which most commonly affects the basilar artery in Fabry disease Reference Kolodny, Fellgiebel and Hilz2 .
Previous studies have demonstrated that the presence of IADE is associated with lacunar strokes, white matter disease and recurrent ischemic strokes Reference Pico, Labreuche and Amarenco4 . Moreover, males with Fabry disease, increased age and comorbid hypertension, were more likely to have IADE and acute ischemic infarcts occur in the vertebrobasilar territory Reference Thijs, Grittner and Fazekas5 . Despite available enzyme replacement therapy and small molecule therapy for amenable GLA mutations, the known impact of these agents on reducing the occurrence of cerebrovascular events and progression of intracranial vessel remodeling is limited. A longitudinal observational 10-year study investigating the long-term outcomes of patients with Fabry disease from the phase 3 clinical trial of agalsidase beta, extension study and Fabry Registry, demonstrated that the most frequent severe clinical event was stroke Reference Germain, Charrow and Desnick6 . Moreover, the impact of small molecule therapy on preventing cerebrovascular events and intracranial vessel remodeling is unknown.
In addition, not much is known about acute and secondary stroke management of IADE in the setting of Fabry disease. As such, selecting the most appropriate acute and secondary stroke management strategies can be challenging. It is speculated that the nonocclusive thrombus detected in the patient’s dolichoectatic basilar artery was likely due to a low flow state created by the dilated and tortuous basilar vessel. Although the extent of thrombus in the dolichoectatic basilar artery could be concerning for possible artery-to-artery thromboembolism in the context of IV tPA therapy, a retrospective study found that the administration of IV tPA was safe in individuals with anterior circulation strokes and associated vertebrobasilar dolichoectasia Reference Gocmen, Arsava, Oguz and Topcuoglu7 . Moreover, to our knowledge, our case is the first to demonstrate the use of IV tPA in the setting of a non-occlusive thrombus in a dolichoectatic basilar artery.
Secondary stroke management in the context of IADE can also be challenging. A prior case series outlined how aggressive anticoagulation for an intraluminal thrombus in a dolichoectatic basilar artery led to catastrophic hemorrhage, while the use of antiplatelet therapy in preventing stroke recurrence is uncertain due to small sample size and limited studies Reference Lin, Chen and Lai8 . As such, there is a fine balance between the risk of fatal complications with use of anticoagulation and stroke recurrence. The size of the non-occlusive thrombus and the risk for a top of the basilar syndrome prompted us to lean towards anticoagulation. Unfortunately, even with therapeutic anticoagulation, the patient experienced recurrent symptoms leading to an acute ischemic infarct. It is possible that the left paramedian infarct arose from artery-to-artery emboli, or vessel wall changes secondary to glycosphinoglipid deposition from the patient’s underlying Fabry disease, resulting in branch orifice obstruction of a basilar perforating artery. As such, low dose antiplatelet therapy was added to his full anticoagulation therapy, which was borrowed from the anti-phospholipid syndrome literature. Although there could be consideration for low dose rivaroxaban and aspirin therapy, atherosclerotic vascular disease in the patient population of the COMPASS trial was driven by conventional cardiovascular risk factors, which is not the case for patients with Fabry disease, as highlighted above. Moreover, low-dose rivaroxaban and aspirin therapy are most effective in preventing ischemic strokes secondary to cardiac embolism and stroke of undetermined source and not lacunar strokes.
Cerebrovascular complications are prevalent in individuals with Fabry disease and can lead to early morbidity and mortality. IV thrombolysis appears to be safe and effective in the setting of Fabry disease and IADE, while secondary stroke management involves weighing the risks and benefits of using anticoagulation and/or antiplatelets for future stroke prevention.
Author Contributions
GM contributed to writing the preliminary draft of the manuscript. GM, APP and KP contributed to reviewing the manuscript and were involved in the patient’s circle of care.
Conflict of Interest
All authors declare that they have no conflicts of interest to disclose.