Introduction
Forestry is a major industry in Canada, providing significant benefits to society by generating direct and indirect employment and contributing CDN$19.8 billion, or 1.25%, to Canada’s real gross domestic product (Natural Resources Canada 2014). Canadians also value the many ecosystem services provided by forests. These include water, recreation, habitat for wildlife, carbon sequestration, and others.
Forests are continuously subjected to natural disturbances, such as fire or insect epidemics, or to anthropogenic disturbances, such as harvesting and salvage logging, and reforestation is of paramount importance for maintaining a continuous productive cycle following disturbance. For example, restoration of areas of widespread tree mortality caused by the recent large outbreak of mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Curculionidae), which began in British Columbia (BC) in the 1990s, will need sustained investments in reforestation.
Lately, there is an increased interest in Canada, particularly in reforestation with fast growing plantations, such as poplars, for bioenergy production, and for climate change mitigation through increased carbon dioxide uptake by fast growing trees (Volney et al. Reference Volney, Alfaro, Bothwell, Hogg, Hopkin and Laflamme2005). These investments are bringing into focus the need for protecting young stands through pest management.
As it is the norm in forest ecosystems, most insects present in young stands have few negative effects on forests; many are beneficial, contributing to important ecosystem functions, such as nutrient cycling, or acting as pollinators, while others contribute to overall forest health by acting as effective parasitoids or predators of pests. However, some insects often escape natural controls and take advantage of the often favourable conditions created by silviculture, such as the creation of single species stands, to increase in numbers to the point where they became damaging to young stands, hindering reforestation efforts, forest productivity, and reducing economic benefits. These negative effects range from killing of seedlings and young trees, to causing growth reductions and stem deformities, which can significantly reduce the future merchantable timber value. Some insects, such as the white pine weevil, Pissodes strobi (Peck) (Coleoptera: Curculionidae), can produce substantial damage, particularly height growth losses through girdling of the apical tree leader (Alfaro et al. Reference Alfaro, Borden, Fraser and Yanchuck1995).
Management of young stands requires an understanding of the natural history and impacts of the pests affecting them. This paper reviews the natural history of the main groups of pests of young conifer (Pinaceae) stands in Canada and provides examples of damage assessment and management of particular pests, and proposes a framework for risk assessment of insects affecting regenerating forests.
Natural history and damage caused by insect pests of young stands
Pest insects of young forests are generally classified according to the part of the seedling or tree upon which they feed. This system allows for a better understanding of the relationship between the insect and its host.
Root and root collar insects
Root and root collar insects attack tree roots either from the outside or by boring inside the root. They can consume the entire root or just part of it. Normally it is the larvae of these insects that damage the roots, whereas the adults feed on the bark of small branches, twigs, foliage, or flowers, or do not feed at all (Furniss and Carolin Reference Furniss and Carolin1977; Coulson and Witter Reference Coulson and Witter1984; Drooz Reference Drooz1985). Removal of root tissue by these insects can induce several responses in their host plants, including changes in root:shoot ratios, with subsequent changes in carbon and nitrogen allocation that can ultimately result in the death of the plants (Hunter Reference Hunter2001). Injury to roots and root collars in temperate and boreal forest trees is mainly caused by insects belonging to the order Coleoptera and, more specifically, root-collar weevils in the genus Hylobius Germar (Coleoptera: Curculionidae), and root beetles in the genus Hylastes Erichson (Coleoptera: Curculionidae) (Hunter Reference Hunter2008). The effects of these insects are more severe on young trees than on old individuals (Coulson and Witter Reference Coulson and Witter1984).
Root-collar and seedling debarking weevils, Hylobius species
Warren root collar weevil (Hylobius warreni (Wood)) is a significant pest of pines in Canada. This insect occurs across Canada, from Newfoundland and Labrador, to coastal British Columbia (Furniss and Carolin Reference Furniss and Carolin1977; Cerezke Reference Cerezke1994). It can attack most pine and spruce species that are native to Canada, but the most susceptible hosts are lodgepole pine, Pinus contorta var. latifolia Engelmann; jack pine, Pinus banksiana Lambert; and white spruce, Picea glauca (Moench) Voss (all Pinaceae) (Cerezke Reference Cerezke1994). The life cycle of this insect typically is completed in two years, but adult weevils can live for up to five years (Cerezke Reference Cerezke1994). Developing larvae inflict the most damage to a tree by feeding on the phloem around the large lateral roots of the host or at the root collar. As the insects mature, their feeding galleries become deeper, and they may score the xylem tissues (Warren Reference Warren1956; Cerezke Reference Cerezke1994). Wounds that are caused by larvae can allow the entry and development of root-rotting fungal infections (Whitney Reference Whitney1961; Coulson and Witter Reference Coulson and Witter1984). The adults are flightless and thus vision plays an important role in host finding behaviour (Machial et al. Reference Machial, Lindgren and Aukema2012). They continue to feed on the bark of roots and twigs, and on the needles of the host (Furniss and Carolin Reference Furniss and Carolin1977). Healthy trees are susceptible to infestation at a young age, with these trees frequently being subject to repeated attacks at irregular intervals through to stand maturity or harvest (Cerezke Reference Cerezke1970, Reference Cerezke1994). In young trees, complete girdling can be accomplished by as few as one to three larvae (Cerezke Reference Cerezke1994). In trees with larger diameters, many more weevils are required to complete girdling, although insects rarely occur at high densities. Warren root collar weevils can cause mortality in stands as old as 30 years of age, although peak mortality occurs in five-year-old to 10-year-old stands (Ives and Rentz Reference Ives and Rentz1993).
Historically, Warren root collar weevil was thought to be of minor economic concern. For example, Hall et al. (Reference Hall, Bowers and Hirvonen1998) reported that weevil-related mortality in lodgepole pine stands was <1% per year in central interior British Columbia, and Ives and Rentz (Reference Ives and Rentz1993) also reported very low mortality (<0.5% incidence in five-year age classes) by this weevil in immature lodgepole pine stands (up to 25 years old) of west-central Alberta. In recent years, however, feeding activity and subsequent mortality from this insect has increased to levels as high as 16% in young stands in central British Columbia (Schroff et al. Reference Schroff, Lindgren and Gillingham2006). This trend may be related to the increase in areas replanted with young lodgepole pines in western Canada to replace dead trees that were salvaged following the outbreak of mountain pine beetle in this region. Increased migration of Warren root collar weevils that are in search of food has been observed, moving from unsalvaged stands killed by mountain pine beetle to young, reforested stands (Klingenberg et al. Reference Klingenberg, Lindgren, Gillingham and Aukema2010), leading to increased mortality of young trees. However, it has been observed that mortality in young stands tends to decrease with increasing distance from the stand edges (McCulloch et al. Reference McCulloch, Aukema, White and Klingenberg2009).
Pales weevil, Hylobius pales Herbst, is the most serious pest of pine seedlings in recently cut-over sites, reforested areas, and Christmas tree plantations (Drooz Reference Drooz1985). This insect is distributed throughout eastern North America. In Canada, this insect occurs from Nova Scotia to Manitoba (Finnegan Reference Finnegan1959). In southern Ontario, the weevil has been found feeding on white pine, Pinus strobus Linnaeus; red pine, Pinus resinosa Aiton; jack pine; Austrian pine, Pinus nigra Arnold; Scots pine, Pinus sylvestris Linnaeus; and tamarack or eastern larch, Larix laricina (Du Roi) Koch (all Pinaceae) (Finnegan Reference Finnegan1959). Damage is caused by the adults that feed on the bark of young seedlings, thereby girdling the stem above or below the ground surface, often leading to the death of the host. Although feeding occurs on the branches of large trees, injury caused by pales weevil is most serious on seedlings (Coulson and Witter Reference Coulson and Witter1984). Seedling mortality of 30–60% is not uncommon in plantations and mortality rates of over 90% have been reported (Ciesla Reference Ciesla2011). In Canada, damage produced by this weevil has been mostly associated with Christmas tree plantations where the insect can find a continuous supply of breeding material. For example, an epidemic outbreak of this insect killed or discoloured up to 40% of the branches of five-year-old to 10-year-old pines in Christmas tree plantation in southern Ontario (Finnegan Reference Finnegan1959).
Other species of Hylobius found in Canada include the seedling debarking weevil, H. congener Dalla Torre, Schenkling, and Marshall; the pine root collar weevil, H. radicis Buchanan; and the Couper collar weevil, H. pinicola Couper (Warren Reference Warren1960). The seedling debarking beetle feeds on red, white, and Scots pines (Martin Reference Martin1964) but it can also be found in larch and spruce (Drooz Reference Drooz1985). The adults feed on the inner bark, removing irregular patches of the bark (Martin Reference Martin1964), girdling the seedling. The attack of this insect does not always results in the death of the seedling because the debarked stem may form callus that covers the wounded area (Welty and Houseweart Reference Welty and Houseweart1985). This weevil has caused economic injury mainly in the Maritime Provinces of Canada. Damage produced by this insect was first reported in Nova Scotia in 1984 when seedling mortality exceeded 85% in some plantations (Magasi Reference Magasi1989). In 1986, the seedling debarking weevil caused high mortality in 29 sites in Newfoundland and Labrador and in Nova Scotia (Magasi Reference Magasi1995). The three Maritime Provinces reported damage produce by the weevil in 1988. However, Nova Scotia was the most affected of them, reporting losses as high as 90% of the seedling planted in some sites (Magasi Reference Magasi1989). The use of traps and lures for predicting expected levels of mortality produced by the weevil is currently recommended before planting stocks (Penny Reference Penny2007). The pine root collar weevil attacks many species of pines, including Scots pine; Austrian pine; mugo pine, Pinus mugo Turra; jack pine; pitch pine, P. rigida Miller; red pine; white pine; and lodgepole pine. Host mortality occurs from extensive damage on growth and transport tissues while larvae bore into the phloem and cambium of the root collar and roots (Wilson and Millers Reference Wilson and Millers1983). Economic damage produced by this insect has been observed in Canada. For example, pine root collar weevil incurred mortality in up to 25% of hosts in Manitoba and up to 90% in Scots and red pine plantations in southern Ontario (Prentice Reference Prentice1955; Finnegan Reference Finnegan1962). The Couper collar weevil attacks most coniferous trees, including members of the genera Pinus Linnaeus, Picea Dietrich, Abies Miller, and Larix Miller (all Pinaceae) (Warren Reference Warren1960; Grant Reference Grant1966). However, economic damage has been reported on balsam fir, Abies balsamea (Linnaeus) Miller; black spruce, Picea mariana (Miller) Britton, Sterns, and Poggenburg; tamarack, Larix laricina (Du Roi) Koch; and white spruce (Cerezke and Pendrel Reference Cerezke and Pendrel1995). The damage is produced by the larvae by feeding on the phloem around the large lateral roots of the host or at the root collar (Warren Reference Warren1960). Although this weevil is considered of minor economic concern, some stands can be heavily impacted. For example, this insect has been reported attacking balsam fir roots in stands 6–55 years old where 6–77% of tree root systems were injured. Furthermore, some injuries by this weevil can remain exposed to infection by wood-destroying fungi (Smerlis Reference Smerlis1961).
The weevil Steremnius carinatus (Boheman) (Coleoptera: Curculionidae) has also been reported to cause damage to conifer seedlings in plantations and young natural regeneration (Condrashoff Reference Condrashoff1968; Cerezke and Pendrel Reference Cerezke and Pendrel1995). The weevil occurs only in western Canada, mainly along the mainland of British Columbia, but also on Vancouver Island, the Queen Charlotte Islands, and interior southeastern wet belt of the province (Cerezke and Pendrel Reference Cerezke and Pendrel1995). It mainly attacks Douglas-fir, Pseudotsuga menziesii (Mirbel) Franco; Sitka spruce, Picea sitchensis (Bongard) Carrière; amabilis fir, Abies amabilis Douglas ex Forbes; and western hemlock, Tsuga heterophylla (Rafinesque) Sargent (all Pinaceae) (Condrashoff Reference Condrashoff1968; Cerezke and Pendrel Reference Cerezke and Pendrel1995). The weevil was considered a scavenger until it was observed damaging Douglas-fir seedling on Vancouver Island in 1961 (Lejeune Reference Lejeune1962). The economic damage is produced by the adult that girdles the main stem of the seedling from 1 cm below to 2–3 cm above ground level (Condrashoff Reference Condrashoff1968). The first reports of damage produced by the weevil in Vancouver Island indicated that the insect has killed or damaged over 40% of Douglas-fir seedlings planted (Lejeune Reference Lejeune1962; Condrashoff Reference Condrashoff1968). Furthermore, over 50% of young natural regeneration (cedar, hemlock, and Sitka spruce) was attacked and 25% of attacked Sitka spruce seedlings were completely girdled and killed in the Queen Charlotte Islands (Condrashoff Reference Condrashoff1968). In 1983, an average of 6% of recently planted conifer seedling were killed by the weevil in 17 of the 30 sites surveyed, being the seedlings of amabilis fir and western hemlock the most affected (12% and 10% mortality in average respectively). Four plantations were later surveyed that year, reporting that severe basal girdling by the weevil affected in average 29% of the amabilis fir, 18% of the western hemlock, and 5% of the Sitka spruce seedlings (Wood et al. Reference Wood, Van Sickle and Shore1984). In 1985, the weevil killed up to 9% of the seedling in 12 plantations on Vancouver Island, with amabilis fir being the most affected species reaching 40% mortality in one plantation. Other species affected by the weevil were Douglas-fir; western hemlock; western red cedar, Thuja plicata (Donn ex Don); Sitka spruce; and grand fir, Abies grandis (Douglas ex Don) Lindley (Wood and Van Sickle Reference Wood and Van Sickle1986).
Root-feeding insects attacking seedlings
Root feeding beetles in the genus Hylastes, along with seedling debarking weevils, are amongst the most important pests of coniferous seedlings in North America, producing high mortality.
The genus Hylastes has 15 known species in North America (Blackman Reference Blackman1941). Hylastes bark beetles infest conifers, mostly in the genera Pinus and Picea. These beetles are usually found in the roots and bases of dead or dying trees or in the stumps and roots of cut trees, where they breed (Drooz Reference Drooz1985). Adults of Hylastes can occasionally damage the roots, root collars, and stems of seedlings, resulting in the death of the individuals that have been attacked (Zethner-Møller and Rudinsky Reference Zethner-Møller and Rudinsky1967); otherwise, they have been considered economically unimportant. Recent reports, however, have indicated that Hylastes species play an important role in loblolly pine (Pinus taeda Linnaeus) decline in the United States of America by transmitting vascular stain fungi to the trees (Leptographium Lagerberg and Melin (Ophiostomataceae)) (Eckhardt et al. Reference Eckhardt, Goyer, Klepzig and Jones2004, Reference Eckhardt, Weber, Menard, Jones and Hess2007). In Canada, Hylastes nigrinus (Mannerheim) has been reported to be the vector of the black stain root disease pathogen Leptographium wageneri var. pseudotsugae Harrington and Cobb (Jacobi Reference Jacobi1992). This disease is a serious forest management problem in southern British Columbia. Jacobi et al. (Reference Jacobi, Zeglen and Beale2008) reported overall mortality that could be attributed to black stain root disease in lodgepole pine on Vancouver Island and mainland British Columbia rose from 11.4% in 1989 to 19.0% in 2000.
Other root-feeding insects that cause damage to tree seedlings are the non-native black vine weevil, Otiorhynchus sulcatus (Fabricius) (Coleoptera: Curculionidae) and the strawberry root weevil, O. ovatus (Linnaeus). These weevils are common horticultural pests and occasionally damage conifer seedlings in the field. Adults of both weevil species feed on the foliage and the tender bark of their host and lay their eggs in the soil near the seedling. The larvae, on the other hand, feed on the roots, which may severe the small roots and girdle the large one (Ives and Wong Reference Ives and Wong1988). The black vine weevil has been reported on black spruce and Douglas-fir seedlings, respectively damaging 10% of the former and 25% of the latter species at the Pacific Forest Research Centre in Victoria, British Columbia (Erickson and Hughes Reference Erickson and Hughes1982). Strawberry root weevil, which has been detected on conifer seedlings in British Columbia, can attack several conifer species and girdle the root collar under heavy infestations (Shrimpton Reference Shrimpton1985). In the Prairie Provinces, only strawberry weevil has been considered of any economic importance (Ives and Wong Reference Ives and Wong1988).
Insects causing damage to seedlings
Seedlings can be attacked either below ground by root-feeding insects (see previous section) or above ground by cutworms, bark-chewing beetles, caterpillars, sawflies, and aphids, among others (Furniss and Carolin Reference Furniss and Carolin1977). These attacks result in seedling mortality or reduced seedling quality. Given their small size, immature root systems, and small energy reserves, seedlings are more vulnerable than mature trees to insect attack; therefore, the presence of one or a few insects can inflict very serious damage to seedlings (Speight and Wainhouse Reference Speight and Wainhouse1989). The most common causes of seedling mortality are defoliation, cutting or girdling of the stem and injection of toxins. Sub-lethal effects, such as discolouration of foliage, stunting, bud damage, loss of foliage, and the production of multiple or crooked leaders, can significantly reduce seedling quality (Scarr et al. Reference Scarr, Smith, Turgeon and Howse2001).
Cutworms
Many of the insects that attack seedlings are polyphagous and sporadically become serious economic threats. One of the most prominent groups of insects damaging seedlings is the cutworms (Lepidoptera: Noctuidae). Cutworm caterpillars emerge from the soil at night to feed on seedling stems at or below the ground surface, usually cutting them down (hence the name cutworm) (Speight and Wainhouse Reference Speight and Wainhouse1989). These insects can pose serious problems for agricultural crops and occasionally damage nurseries and newly planted conifer seedlings (Coulson and Witter Reference Coulson and Witter1984). Occurring throughout Canada, the black army cutworm, Actebia fennica (Tauscher) is the most serious cutworm pest affecting conifer seedlings (Humble et al. Reference Humble, Shepherd and Maher1989). It was not considered a forest pest until 1973 when an extensive outbreak occurred on recently planted spruce, lodgepole pine and Douglas-fir seedlings in British Columbia (Ross and Ilnytzky Reference Ross and Ilnytzky1977). This insect is associated with burnt areas where its larvae appear 1–2 years after the fire (Humble et al. Reference Humble, Shepherd and Maher1989). Larvae defoliate seedlings, destroy terminal and lateral buds, and occasionally chew the bark (Maher and Shepherd Reference Maher and Shepherd1992).
Black army cutworm damage stunts root growth and new root establishment in the soil, leading to seedling mortality or height growth losses. Partial or complete replanting of seedlings may be required after severe damage (Humble et al. Reference Humble, Shepherd and Maher1989). According to Maher and Shepherd (Reference Maher and Shepherd1992), seedling mortality or height losses occur when defoliation is >60%, or when the terminal bud is killed. These authors also found that there is significantly lower mortality if defoliation occurs at least one year after seedlings were planted, probably because the root system has become established by then. Another cutworm species that causes damage to seedlings in Canada is the variegated cutworm, Peridroma saucia (Hübner). This insect is a sporadic pest of conifer seedlings, and has been detected damaging Douglas-fir seedlings in nurseries in British Columbia (Sutherland et al. Reference Sutherland, Shrimpton and Sturrock1989).
Sucking insects
Sucking insects of the genus Lygus Hahn (Hemiptera: Miridae) can injure tree seedlings by piercing the needles to secrete digestive enzymes in order to liquefy cell content for food (South Reference South1991). Attacks by these insects produce chlorosis of the needles, twisting and stunting of shoots, and the formation of multiple leaders that leads to stem defects, as a result of damaged apical buds (Sutherland et al. Reference Sutherland, Shrimpton and Sturrock1989; South Reference South1991; Scarr et al. Reference Scarr, Smith, Turgeon and Howse2001). Emerging needles from injured buds will also show signs of insect damage and will grow shorter and thicker, with some seedlings exhibiting brown lesions on the main stem (South Reference South1991). In Canada, Lygus bugs have been reported on seedlings of: jack pine, red pine, lodgepole pine, and white pine; white spruce, black spruce, and Norway spruce (Picea abies (Linnaeus) Karsten); and Douglas-fir (Shrimpton Reference Shrimpton1985; Scarr et al. Reference Scarr, Smith, Turgeon and Howse2001). One important species in Canada is the tarnished plant bug, Lygus lineolaris (Palisot de Beauvois). This insect occurs throughout Canada, from British Columbia to Newfoundland and Labrador (Kelton Reference Kelton1975). In 1983, it caused noticeable damage in several nurseries in British Columbia, attacking up to 20% of both container and bare-root stock (Shrimpton Reference Shrimpton1985). Given its wide distribution, it has been speculated to be responsible for abnormal bud development at other Canadian nurseries (South Reference South1991).
Shoot and tip insects
A number of insect species, especially in the orders Coleoptera and Lepidoptera, infest shoots and terminal buds of trees, consuming mostly meristematic tissues of their hosts. They seldom kill the trees, but their damage to the buds and lateral and terminal branches alters the shape and growth rate of the tree, resulting in deformity, multiple branching and growth loss (Ciesla Reference Ciesla2011). The two groups of insects of major concern are: bud and shoot-feeding moths, and the terminal and shoot weevils (Coulson and Witter Reference Coulson and Witter1984).
Shoot and bud-feeding insects
Twenty-four known species of Rhyacionia Hübner (Lepidoptera: Tortricidae) occur throughout the range of pines in North America, and many damage the shoots of conifers, especially pines (Coulson and Witter Reference Coulson and Witter1984). First instars usually mine needles and later instars feed on the buds and growing shoots of young pines, especially in plantations, even-aged natural stands, and ornamental trees (Furniss and Carolin Reference Furniss and Carolin1977; Knight and Heikkenen Reference Knight and Heikkenen1980). As a result of the destruction of terminal bud and shoots trees are deformed, have fewer branches than unaffected trees (one or more lateral buds killed), and their foliage may lose its colour. While the growth of young pines is retarded, trees are seldom killed (Furniss and Carolin Reference Furniss and Carolin1977; Knight and Heikkenen Reference Knight and Heikkenen1980; Coulson and Witter Reference Coulson and Witter1984). Young trees (up to 7.5 m in height), and seedlings, are more susceptible to injury by Rhyacionia species (Furniss and Carolin Reference Furniss and Carolin1977), but they may recover from their injuries and resume normal growth when feeding is terminated (Coulson and Witter Reference Coulson and Witter1984).
The most important member of this genus in Canada is the non-native European pine shoot moth Rhyacionia buoliana (Denis and Schiffermueller). This moth was first detected in 1925 in Ontario, Nova Scotia, and British Columbia (Syme et al. Reference Syme, Grant and Gray1995). The needle-mining larvae cause browning of the foliage, but this apparently has little effect on host vigour. Feeding on the terminal bud causes serious injury to the main stem, which affects its form. Repeated destruction of the terminal bud during the summer months initially causes profusion of shoot growth (witch’s broom) and, eventually, a new spike top form when the leader dies. Various degrees of crookedness will result as the lateral branches or buds assume dominance over the terminal shoots. Attacks usually become less intensive as the trees increase in height (Syme Reference Syme1984). In eastern Canada, red pine is the most seriously damaged species, whereas ponderosa pine (P. ponderosa Lawson and Lawson (Pinaceae)) and the exotic species Scots pine, Austrian pine, and mugo pine are moderately susceptible. In western Canada, European pine shoot moth is a serious pest of lodgepole and ponderosa pines (Syme Reference Syme1984; Syme et al. Reference Syme, Grant and Gray1995). The first recorded outbreak occurred in 1938, when this insect attacked lodgepole pine in Vancouver, British Columbia (Syme et al. Reference Syme, Grant and Gray1995). In Ontario, this insect has caused serious damage to red pine plantations (Pointing Reference Pointing1961), whereas in Québec it has been detected almost exclusively on mugo pine (Béique Reference Béique1960). During the 1970s, this moth became a major pest of red and Scots pine plantations in Nova Scotia and Prince Edward Island, where up to 80% of tree buds were destroyed (Magasi Reference Magasi1995).
European pine shoot moth infestations were suppressed in most of eastern Canada by extremely cold winters at the beginning of the 1980s, but low level populations persisted causing sporadic damage to pine plantations (Magasi Reference Magasi1995). After discounting the possibility that this moth could spread to native pine plantations in British Columbia, interest in this insect declined there until 1999, when R. buoliana larvae were detected in lodgepole pines of the Vernon Seed Orchard Company (Okanagan Valley, British Columbia, Canada) (Heeley et al. Reference Heeley, Alfaro, Humble and Strong2003). However, Heeley et al. (Reference Heeley, Alfaro, Humble and Strong2003) found no evidence of a serious threat to natural lodgepole pine stands from this pest at this time.
The pine tip moth, R. adana (Heinrich), is the other member of the genus Rhyacionia that has caused severe damage to pine plantations in Canada. This moth is native to North America and its larvae feed on young red, jack, and Scots pines, usually <1 m in height in nurseries, plantations, and natural stands. It will also attack the lower crowns of older trees up to 8 m in height (Martin Reference Martin1960; Drooz Reference Drooz1985).
Other important shoot moths are in the genus Eucosma Hübner (Lepidoptera: Tortricidae), especially the western pine shoot borer, Eucosma sonomana (Kearfott), and the eastern pine shoot borer, Eucosma gloriola (Heinrich) (Syme et al. Reference Syme, Grant and Gray1995). The former occurs in British Columbia where it feeds on its main host, ponderosa pine. Lodgepole pine, and Engelmann spruce, Picea engelmannii Parry ex Engelmann, are also attacked (Furniss and Carolin Reference Furniss and Carolin1977). The larvae mine downward through the centre of the terminal shoots, usually reducing shoot and needle elongation, thereby stunting and sometimes killing the shoot (Furniss and Carolin Reference Furniss and Carolin1977; Thier and Marsden Reference Thier and Marsden1990). Reduced tree height and a deformed crown result from repeated borer attacks (Stoszek Reference Stoszek1973). This insect has not been considered a significant pest in British Columbia even though it had been detected infesting 36–75% of lodgepole pines near Cascade, British Columbia, during the 1960s (Syme et al. Reference Syme, Grant and Gray1995).
The eastern pine shoot borer occurs in Manitoba, Ontario, and Québec. Its main host is eastern white pine but it also feeds on jack, Scots, red, Austrian, and mugo pines (DeBoo et al. Reference DeBoo, Sippell and Wong1971). Feeding by late instars on the terminal shoots causes loss of turgidity and internal structural support, which may result in bending or breaking, whereas damaged lateral shoots lose colour and gradually die as the season advances (DeBoo et al. Reference DeBoo, Sippell and Wong1971; Drooz Reference Drooz1985). Infested trees become bushy after repeated attacks (Drooz Reference Drooz1985). In Ontario, leader damage caused by this insect was detected in >50% of jack pine trees <6 m in height, but damage occurred on about 12% of trees higher than 6 m, suggesting that the overall incidence of this insect was low. In white pine, however, between 8% and 27% of plantations in Ontario were infested and resulted in significant loss of height growth and the deformity of the main stem, especially in trees 2–6 m tall (de Groot et al. Reference de Groot, Hopkin and Sajan2003).
Another group of insect that have caused economic damage in Canada is in the genus Petrova Heinrich (Lepidoptera: Tortricidae), the Pitch-blister moths. In Canada, five species of pitch-blister moths are present, being the two most important the northern pitch twig moth, P. albicapitana (Busck), and the metallic pitch nodule moth, P. metallica (Busck). The former moth occurs from Nova Scotia to British Columbia and attacks jack pine, lodgepole pine, and scots pine whereas the latter moth is distributed from Saskatchewan to British Columbia and attacks lodgepole pine (Wong et al. Reference Wong, Drouin and Rentz1995). Larvae of both species feed on the new and old growth of stem and branches of pines, causing branch deformity and breakage by wind and snow. The larvae form round nodules by combining pitch exuding from canals punctured during feeding with silk and frass where the overwinter (Martineau Reference Martineau1984; Wong et al. Reference Wong, Drouin and Rentz1985). Larvae of northern pitch twig moth frequently girdle and kill the stem or the branch while feeding beneath bark whereas damage to growing terminal shoots often causes the terminal shoot to grow in a crooked manner (Turnock Reference Turnock1953). Larvae of metallic pitch nodule moth do not girdle the cortical tissue, but tunnel in the xylem and pith, weakening the affected area that is subjected to wind or snow breakage (Wong et al. Reference Wong, Drouin and Rentz1995).
In Alberta, the northern pitch twig moth was highly abundant in nursery-grown lodgepole pine 0.3–2.0 m tall, weakening and occasionally girdling the stems that were more susceptible to wind breakage (Drouin and Kusch Reference Drouin and Kusch1981). In 1989, surveys conducted in the province reported that between 5.8% and 12.5% of pine host showed signs of damage produced by the moth (Moody Reference Moody1992). In Québec, this insect was not considered important before 1959 when outbreaks in plantations of jack pine were detected (Martineau Reference Martineau1984). In 1966, it was detected in several five-years-old to 10-years-old jack pine plantations, damaging 50% or more of the trees. The infestation level remained high up to 1972 when significant damage was observed all trees in certain plantations. In 1968, the moth was also found attacking natural jack pine forest, even in mature stands (McLeod and Tostowaryk Reference McLeod and Tostowaryk1971). From 1973, the population level of this insect was low until 1982 when increases in insect damage were observed in certain 12-year-old jack pine populations in Portneuf, Ville de Québec and Montmorency area (Lachance et al. Reference Lachance, Thibault and Monnier1991). Since then, reports of northern pitch twig moth were frequent but caused low damage (Moody Reference Moody1992). Occasional outbreaks of this moth have also been reported in the Maritimes Provinces (Martineau Reference Martineau1984; Moody Reference Moody1992) whereas low incidence of this insect on regeneration has been reported in Manitoba (Emond and Still Reference Emond and Still1974) and Saskatchewan (Petty et al. Reference Petty, Gautreau and Tidsbury1974). As for the metallic pitch nodule moth, its nodules are observed on trees from 0.3 to 25.0 m tall, being the most severe observed at 3 m high (Wong et al. Reference Wong, Drouin and Rentz1995). This moth has been detected attacking lodgepole pines in Alberta. However, it occurs at low level in all age classes of every forest (between 0.2% and 3.1% of trees attack) (Amirault and Pope Reference Amirault and Pope1989). Low population levels of this moth have also been observed in lodgpole pine stands in Saskatchewan (Cerezke et al. Reference Cerezke, Volney., Mallett and Emond1989).
Terminal and shoot weevils (Coleoptera: Curculionidae)
Weevils of the genera Pissodes Germar, Cylindrocopturus Heller, and Magdalis Germar (Coleoptera: Curculionidae) damage the terminals of young conifers, but Pissodes is the most important pest (Furniss and Carolin Reference Furniss and Carolin1977; Coulson and Witter Reference Coulson and Witter1984). Weevil larvae feed on terminal tissues producing unacceptable growth losses in young stands, together with crooking or branching of the trunks (Alfaro Reference Alfaro1989; Retnakaran and Harris Reference Retnakaran and Harris1995). Although weevil damage does not cause mortality of large trees, individuals that are <1 m in height may be killed. Timber volume and quality can be reduced through the production of cross-grained wood, larger knots, and compression wood (Coulson and Witter Reference Coulson and Witter1984). The two most important terminal weevil species in Canada are the white pine weevil and the lodgepole terminal weevil, Pissodes terminalis (Hopping) (Retnakaran and Harris Reference Retnakaran and Harris1995). These species are similar in appearance but they differ in their life histories, behaviour, hosts, and the damage that they incur (Drouin et al. Reference Drouin, Sullivan and Smith1963).
Despite its name, the white pine weevil attacks at least 20 species of pine and spruce in Canada (Furniss and Carolin Reference Furniss and Carolin1977). It occurs naturally across Canada, feeding upon white pine, Scots pine, jack pine, Norway spruce, and white spruce in the east (Nealis Reference Nealis1998), and on Sitka spruce, Engelmann spruce, and white spruce in the western part of its range (Wallace and Sullivan Reference Wallace and Sullivan1985). Adults overwinter in the duff near the base of trees and emerge in spring. Female weevils make feeding punctures with their snouts and later lay eggs in these punctures, which are then plugged with macerated phloem. Oviposition occurs in the cambium in the top half of the previous year’s leader. The larvae kill the leader by mining and consuming its phloem. Larvae pupate in chip cocoons under the bark. Newly developed adults start emerging in late summer and, before hibernation, feed on leaders of young trees (Silver Reference Silver1968). Weevil attack results in the death of the terminal leader, causing one or more lateral branches to assume dominance. Fast growing trees, on good sites, are able to develop a new leader in one year. In the process of recovery, branches below the damaged terminal compete for dominance and the tree remains for one or more years with multiple leaders, thus increasing food supply for future weevil generations. The attack results in major defects (crooks and forks) in the main stem (Alfaro Reference Alfaro1989). Repeated leader death can cause severe deformation of the main stem, rendering the tree non-merchantable. The weevil prefers vigorous trees, 2–6 m tall, with terminal shoots >4 mm in diameter (Gross Reference Gross1985). Although this weevil typically does not directly cause tree mortality, the stunted growth of susceptible trees – usually the most vigorous trees in the stand –may result in them being eliminated from the site by competing vegetation (Alfaro Reference Alfaro1982).
White pine weevil causes severe problems to reforestation programmes throughout Canada. In Québec, Norway spruce and white pine are the most affected species. In 1999, 90% and 85% of Norway spruce and white pine plantations, respectively, were attacked in some regions of the province (Boucher et al. Reference Boucher, Lavallée and Mauffette2001). In 2011, only 16% and 18% of trees in the respective plantations of these species were attacked at the provincial level (Ministère des Ressources naturelles et de la Faune 2012). In Ontario, white pine is the species most damaged by this weevil, followed by jack pine and Norway spruce (Scarr et al. Reference Scarr, Smith, Turgeon and Howse2001). A survey of Ontario white pine plantations indicated that, within a given year, 50% of stands were infested with this weevil, with up to 28% of trees being attacked (Gross Reference Gross1985). In coastal British Columbia, Sitka spruce had been so severely damaged that planting of this species, a valuable timber tree, was not recommended in most harvested areas (Alfaro et al. Reference Alfaro, Borden, Fraser and Yanchuck1995). However, a breeding programme in British Columbia succeeded in identifying tree genotypes with heritable and stable resistance against this weevil in Sitka and white spruce, allowing the establishment of operational seed orchards. Currently, the availability of resistant seed is contributing to increase the productivity of white spruce in the interior of British Columbia and has allowed the return of Sitka spruce as a timber species of choice in coastal British Columbia (Alfaro et al. Reference Alfaro, King and vanAkker2013).
Lodgepole terminal weevil is distributed from Manitoba to British Columbia, and mainly infests the terminal leaders of lodgepole and jack pine (Drouin et al. Reference Drouin, Sullivan and Smith1963; Langor and Williams Reference Langor and Williams1998). Unlike white pine weevil, the lodgepole terminal weevil attacks current growth, with feeding damage being concentrated above the nodal branches and intermodal shoots as the larvae move up the stem (Drouin et al. Reference Drouin, Sullivan and Smith1963). Attacks by lodgepole terminal weevil and white pine weevil kill the terminal leader, resulting in crooking or branching of stems (Drouin et al. Reference Drouin, Sullivan and Smith1963; Maclauchlan and Borden Reference Maclauchlan and Borden1996). Although it has not been completely assessed, the effect of the lodgepole terminal weevil on regenerating pine stands is undoubtedly less severe than that caused to spruce and pines by the closely related white pine weevil (Langor and Williams Reference Langor and Williams1998). In British Columbia, 40% and 50% top-kill was reported in two stands (Amman and Safranyik Reference Amman and Safranyik1984). Maclauchlan and Borden (Reference Maclauchlan and Borden1996) reported that height loss due to P. terminalis attack was 31.4% of the annual potential height increment in the year of attack, and 17% in the year following attack. In 2011, plot re-measurement revealed that the P. terminalis infestation increased by 27%, but its effects on tree growth and shape had decreased substantially since 2002 (Westfall and Ebata Reference Westfall and Ebata2011). From 1982 to 1985, the cumulative incidence of this weevil in Alberta was as high as 87% in lodgepole stands and around 32% in jack pine stands in Saskatchewan (Langor et al. Reference Langor, Drouin and Wong1992).
Other weevils can attack the terminal leaders and branches of pines, including Cylindrocopturus species and the defoliating weevil Magdalis gentilis (LeConte) (Coleoptera: Curculionidae). These weevils cause damage similar to that incurred by lodgepole terminal weevil (Kovacs and McLean Reference Kovacs and McLean1990), but their effects are not considered important because populations are never very high (Furniss and Carolin Reference Furniss and Carolin1977).
Gall insects
Many kinds of insects cause galls on forest trees. These insects inject a chemical substance into the plant that causes it to grow abnormally and produce a gall (Coulson and Witter Reference Coulson and Witter1984). Thus, gall-formers have the ability to manipulate the growth and development of plant tissues (Dreger-Jauffret and Shorthouse Reference Dreger-Jauffret and Shorthouse1992). Chemical compounds that stimulate gall formation are usually provided by the feeding stage of the insect, but the ovipositing female of some species injects the compounds when she lays eggs on the plant (Coulson and Witter Reference Coulson and Witter1984). The changes in cell structure are thought to improve the nutritional value of the attacked plant part for the insect, reduce chemical defenses, and provide shelter (Price et al. Reference Price, Fernandes and Waring1987). If the insect dies or leaves the host, the plant resumes normal growth (Coulson and Witter Reference Coulson and Witter1984). Galls can be found in all parts of a tree, from the foliage to the roots (Furniss and Carolin Reference Furniss and Carolin1977; Coulson and Witter Reference Coulson and Witter1984). They are caused by insects in the orders Thysanoptera, Hemiptera, Lepidoptera, Coleoptera, Diptera, and Hymenoptera (Dreger-Jauffret and Shorthouse Reference Dreger-Jauffret and Shorthouse1992). Gall insects are not considered economically important in forest stands, but they can inflict severe damage to ornamental trees, shrubs, nurseries, and young stands (Coulson and Witter Reference Coulson and Witter1984). The most problematic gall insects in Canada are the gall and pitch midges (Diptera: Cecidomyiidae), and the gall adelgids (Hemiptera: Adelgidae) (Humble and West Reference Humble and West1995).
Gall and pitch midges. The spruce bud midge, Dasineura swainei (Felt) (Diptera: Cecidomyiidae), occurs throughout the boreal region of Canada and attacks red spruce, Picea rubens Sargent (Pinaceae); Colorado blue spruce, P. pungens Engelmann (Pinaceae); as well as black, white, and Norway spruces (Clark Reference Clark1952; Cerezke Reference Cerezke1972). At the end of spring, newly hatched larvae bore into shoot tips, which leads to gall formation (West Reference West1989). Larvae feed within galled buds throughout the summer and mature at the end of autumn. Larvae overwinter in buds and transform into pupae in late April to early May. Adults emerge three weeks later, with the females ovipositing in flushing shoots in the late spring (Humble and West Reference Humble and West1995). When the terminal leader bud is damaged, subordinate buds compete for dominance and eventually one becomes the new leader or dominant shoot. Reductions in height growth of about 25% and 16% have been observed in white and black spruce, respectively, as a result of attacks by spruce bud midge (Cerezke Reference Cerezke1972; West Reference West1989). However, the effects of this insect are considered to be rather minor. For example, <4% of shoots in white pine plantations in the Maritime provinces were attacked by this midge in 1982 (Magasi Reference Magasi1983). Cozens (Reference Cozens1985) reported that spruce bud midge had attacked 2.2% of all spruce stems in plantations in Prince George, British Columbia. In Saskatchewan, this insect attacked 3.9% of all stems in young stands of white spruce (Cerezke and Brandt Reference Cerezke and Brandt1993). Higher levels of infestation (28%) and attack (13%) by the midge were observed in Québec spruce plantations in 2011 (Ministère des Ressources naturelles et de la Faune 2012).
Balsam gall midge, Paradiplosis tumifex Gagné (Diptera: Cecidomyiidae), forms ~3-mm-diameter galls at the base of the needles on mature and immature balsam fir, which is its main host. In Canada, the midge is found throughout the range of balsam fir, but it has also been observed attacking Fraser fir, Abies fraseri (Pursh) Poiret (Pinaceae), in British Columbia as well (Martineau Reference Martineau1984; Drooz Reference Drooz1985; Humble and West Reference Humble and West1995). After hatching, the larvae move to the bases of the needles to find a suitable feeding site. By late July, the gall is fully developed at the larval feeding site. At maturity, larvae leave the galls to overwinter in the duff or mineral soil (West and Shorthouse Reference West and Shorthouse1982; Humble and West Reference Humble and West1995). Galled needles gradually turn yellow and drop from the twigs in October (Drooz Reference Drooz1985; Humble and West Reference Humble and West1995). Since balsam gall midge damage is more serious in young balsam fir trees (<8 m tall) than it is in older and taller trees, this pest is of major concern in young plantations and Christmas tree plantations (Drooz Reference Drooz1985; Ives and Wong Reference Ives and Wong1988). Outbreaks of balsam gall midge have occurred in the Maritimes, Ontario, and Québec since 1938. Until the 1960s, outbreaks in the Maritimes and Ontario were severe. Since then midge population densities have remained relatively low, with occasionally localised outbreaks. For example, only one balsam fir plantation exhibited >20% needle infestation in the Maritime Provinces (Kondo and Moody Reference Kondo and Moody1987). Québec outbreaks, in contrast, have been localised, lasting a few years (Humble and West Reference Humble and West1995). A localised outbreak lasting three years occurred in Québec in 2000, causing 60% of buds infested in one balsam fir Christmas tree plantation (Cloutier et al. Reference Cloutier, Mailhot and Brodeur2006).
The jack pine resin midge, Cecidomyia resinicola (Osten Sacken) (Diptera: Cecidomyiidae), ranges from British Columbia to Québec. Since 1963, jack pine resin midge outbreaks have occurred in jack pine plantations in south-central Ontario and central Québec, and in natural stands in western Québec (Martineau Reference Martineau1984). This insect has also been reported causing moderate damage in jack pine plantations in localised areas near Grand Rapids, Manitoba (Kondo and Moody Reference Kondo and Moody1987). It also attacks pitch pine, and lodgepole pine (Gagné Reference Gagné1978; Ives and Wong Reference Ives and Wong1988). Adults emerge in late May or early June. Eggs are laid singly on the bark or needles of the new shoots, but usually on shoots that are only lightly resinous. The eggs hatch within about six days, and the young larvae burrow into pitch masses that accumulate on the new shoots. The larvae feed on the resin, as their mouthparts are not suited to rasping the tissues of the host. This feeding appears to irritate the young tissue and stimulates the flow of pitch until it forms a mass, in which the partially developed larvae can overwinter. Pupation occurs within the pitch masses, but the pupae wriggle out of the pitch before adult emergence (Martineau Reference Martineau1984; Ives and Wong Reference Ives and Wong1988).
Height growth reductions and rarely mortality can result from jack pine resin midge attacks. Although this midge can attack and destroy the shoots of older trees that grow on poor sites, its feeding causes greater damage to young pines and can kill up to 75% of the affected shoots (Martineau Reference Martineau1984). Yet, infestations are rarely severe enough to affect radial increment or cause mortality (Martineau Reference Martineau1984; Ives and Wong Reference Ives and Wong1988). In 2011, 29% of jack pine plantations in Québec were attacked by this midge, but the level of damage was low (Ministère des Ressources naturelles et de la Faune 2012).
Other midges that can cause minor damage on young stands in Canada are the jack pine midge, Cecidomyia piniinopis (Osten Sacken); the European pine needle midge, Contarinia baeri (Prell); the red pine midge Thecodiplosis piniresinosae (Kearby); other Contarinia Rondani species; the spruce gall midge, Mayetiola piceae (Felt); the false balsam gall midge, Dasineura balsamicola (Lintner); and Chamaediplosis nootkatensis Gagné and Duncan (Humble and West Reference Humble and West1995).
Gall adelgids. Adelgidae (Hemiptera) feed exclusively on conifers. This family contains two genera, Adelges Vallot and Pineus Shimer. In North America, the most important species within the family are the eastern spruce gall adelgid Adelges abietis (Linnaeus) (Hemiptera: Adelgidae) and the cooley spruce gall adelgid, A. cooleyi (Gillette) (Coulson and Witter Reference Coulson and Witter1984; Harris and Bowers Reference Harris and Bowers1995). Adelges abietis is found throughout Canada, mainly on Norway and white spruce, but Colorado, blue, and red spruces are also occasionally affected (Friend and Wilford Reference Friend and Wilford1933; Martineau Reference Martineau1984). The spruce gall adelgid is bivoltine (i.e., completes two generations per year). In the spring, after overwintering at the base of the buds, the nymphs become adults and females lay eggs on spruce needles. After hatching, the nymphs feed at the base of the needles and form pineapple-shaped galls on developing shoots of spruce trees in the spring (Coulson and Witter Reference Coulson and Witter1984; Martineau Reference Martineau1984). The presence of galls reduces the aesthetic value of ornamentals and Christmas trees, and can reduce tree growth in plantations when adelgid populations are high (Friend and Wilford Reference Friend and Wilford1933; Coulson and Witter Reference Coulson and Witter1984). The adelgid is not common in the Maritimes or Newfoundland and Labrador, but it has wreaked considerable damage in Québec and Ontario, most noticeably in the southern regions of both provinces (Martineau Reference Martineau1984). In 1982, the infestation rate of this insect in the Maritime Provinces was <1% of shoots, except for two New Brunswick sites and one site in Nova Scotia where 3% and 1% of shoots were affected, respectively (Magasi Reference Magasi1983). In 2011, 32% of spruce plantations in Québec showed signs of eastern spruce gall adelgid attack. The damage, however, was low in most plantations (Ministère des Ressources naturelles et de la Faune 2012).
The Cooley spruce gall adelgid occurs across Canada, but it is more abundant in the west where its alternate host, Douglas-fir, is present. The most seriously affected tree species are white spruce, Engelmann spruce, and Douglas-fir, but this insect will occasionally attack interior spruce, Picea engelmannii Parry×P. glauca (Moench) Voss, as well as black, red, and Sitka spruce. The Cooley spruce gall adelgid is indigenous to North America (Cumming Reference Cumming1959; Martineau Reference Martineau1984) and has natural history similar to that of the eastern spruce gall adelgid. In the west, however, it alternates between spruce and Douglas-fir, and goes through several generations annually on each host (Cumming Reference Cumming1959; Coulson and Witter Reference Coulson and Witter1984). The complete life cycle takes two years, including alternation of generations between hosts (Cumming Reference Cumming1959), but a shorter life cycle has been detected in Alberta and Saskatchewan (Cumming Reference Cumming1962). The elongate and pineapple-shaped gall of this insect may cover the entire length of the shoot or only one side, which can bend the shoot. Galls are not formed in Douglas-fir, but its needles will turn yellow and drop off (Martineau Reference Martineau1984). In natural forests, its damage is not serious except on seedlings and young trees, where the galls will kill the tips of branches, as well as stunt and deform the trees when they occur on the main stem (Furniss and Carolin Reference Furniss and Carolin1977). Damage can reduce the aesthetic value of ornamentals and Christmas trees, and reduces tree growth in plantations when insect populations are high (Coulson and Witter Reference Coulson and Witter1984; Martineau Reference Martineau1984). This adelgid is commonly observed throughout northern and western Alberta, and in northwest Saskatchewan, but its impacts have been considered low (Cerezke and Brandt Reference Cerezke and Brandt1993). In British Columbia, high infestation rates have been reported in some interior spruce plantations (up to 100%), but the insect rarely threatened tree health (Simard and Hannam Reference Simard and Hannam2000). The spruce gall adelgid, Adelges lariciatus (Patch), is native to North America and is distributed from the Maritimes provinces to British Columbia (Cumming Reference Cumming1968; Rose and Lindquist Reference Rose and Lindquist1994; British Columbia Ministry of Forests and Range 2009). This adelgid has been recorded in white spruce, black spruce, blue spruce, tamarack, subalpine larch (Larix lyallii (Parlatore)), and Siberian larch (Larix sibirica Ledebour). It alternates between spruce and larch and typically goes through six generations annually (Cumming Reference Cumming1968). This insect produces pineapple-shaped galls that cause a marked reduction of needle length on the galls (Ives and Wong Reference Ives and Wong1988) and damage and destroy current or future cones sites (British Columbia Ministry of Forests and Range 2009). This insect is considered of minor economic impact because it rarely affects the health of its hosts (Martineau Reference Martineau1984; British Columbia Ministry of Forests and Range 2009) but heavy gall formation can stunt or kill twigs, which can disfigure valuable trees. For example, high populations of this insect were detected on planted white spruce grown for genetic tree improvement and on shelterbelt trees within nurseries in Alberta in 1986 (Cerezke et al. Reference Cerezke, Dhir and Barnhardt2011). Although there is no evidence that this adelgid seriously affect seed production, it can make difficult seed extraction due to excessively gummed cones (Bennet Reference Bennet1994).
Defoliating insects
Foliage of young trees serves as the principal food source for a great number of folivorous insects from five orders: Coleoptera, Diptera, Hymenoptera, Lepidoptera, and Orthoptera. (Furniss and Carolin Reference Furniss and Carolin1977; Coulson and Witter Reference Coulson and Witter1984). Defoliation affects the tree by reducing its photosynthetic surface, and by interfering with transpiration and the processes of photosynthate translocation within the tree (Coulson and Witter Reference Coulson and Witter1984). The impacts of defoliators can be very serious, depending on their population densities. Trees can withstand defoliation without being seriously affected when the population densities are low to moderate. If population densities surpass a certain level, heavy feeding will reduce tree growth and may eventually kill the young tree, especially if it occurs in successive year and whether the feeding is confined to new or old needles. Factors such as tree species, tree age, site quality, tree health weather conditions, and secondary insects and diseases can modify insect defoliation effects by affecting tree resistance. Caterpillars of moths (Lepidoptera) and sawflies (Hymenoptera) are the most damaging insects that consume foliage (Furniss and Carolin Reference Furniss and Carolin1977).
Lepidoptera. The spruce bud moth, Zeiraphera canadensis Mutuura and Freeman (Lepidoptera: Tortricidae), is a native defoliator of spruce in North America, and occurs throughout Canada on its preferred host, white spruce (Mutuura and Freeman Reference Mutuura and Freeman1966). The bud moth is also found on black spruce, Sitka spruce, balsam fir, and other species of conifers (Drooz Reference Drooz1985). The female looks for small spruce trees about 2 m in height upon which to oviposit. From mid July to late July, she oviposits on the scales at the base of current-year shoots, and diapausing eggs overwinter (Turgeon Reference Turgeon1985). Egg hatch usually occurs in May and is well synchronised with budburst (Turgeon Reference Turgeon1986; Quiring Reference Quiring1993), because the temporal window for bud colonisation is only four to five days long (Quiring Reference Quiring1992). First instars colonise newly burst buds (Quiring Reference Quiring1993). After completing development, the fourth instars drop to the ground and pupate in the litter (Turgeon Reference Turgeon1985). Adult eclosion starts about three weeks later, with a flight period usually lasting two to three weeks (Turgeon et al. Reference Turgeon, Kettela and Jobin1995). The damage is caused by third instars and fourth instars, which feed on the cortical tissues of growing shoots and frequently destroy them, resulting in the production of multiple leaders, reduction in growth, and stem deformity. Carroll et al. (Reference Carroll, Lawlor and Quiring1993) reported that spruce bud moth caused immediate and substantial reductions in height increment, but volume increment was not affected unless white spruce sustained two to three consecutive years of heavy damage. Quiring (Reference Quiring1993) observed that 15-year-old white spruce trees that had sustained continual attack had more than five leaders, were <2 m in height, and had been “overtopped” by undamaged conspecifics of the same age.
This moth had been considered of minor economic importance until 1980, when it infested >16 000 ha of white spruce plantations in New Brunswick, causing shoot distortion and tree deformation. Since then, it has become widespread throughout the Maritime provinces, attaining shoot damage levels as high as 96% in New Brunswick, 55% in Nova Scotia, and 70% in Prince Edward Island in 1985 (Magasi Reference Magasi1995). In 1991, the bud moth caused severe defoliation of white and black spruce stands in Newfoundland and Labrador (Raske et al. Reference Raske, Sutton, Banfield, Stone, O’Brien and Pardy1992). In the early 1980s, it caused considerable damage to white pine plantations of the Lower St. Lawrence-Gaspé Peninsula of Québec, and by 1987, it was present in 88% of white pine plantations causing 32% terminal shoot damage (Lachance Reference Lachance1995). Spruce bud moth infestations decreased considerably in Québec during the 1990s; provincial monitoring of spruce bud moth populations in white spruce plantations was discontinued in 1999 (Ministère des Ressources naturelles 2000). No severe damage caused by this moth has been detected in Ontario, probably because plantations of its main host, white spruce, are not common in this province (Scarr et al. Reference Scarr, Smith, Turgeon and Howse2001).
The genus Choristoneura Lederer (Lepidoptera: Torticidae) includes several important defoliators that cause serious damage, mainly to mature forests, but they can also damage young stands (see Nealis Reference Nealis2015 in this special issue for further information on genus Choristoneura). The most notorious member of this genus is the spruce budworm Choristoneura fumiferana (Clemens). This insect occurs throughout Canada, where it feeds preferably on balsam fir, followed by white spruce, red spruce, and black spruce. This budworm is the most destructive insect pest in the maritime and boreal forests of North America. Populations of this forest defoliator have reached outbreak densities over extensive forested areas on a fairly regular basis over the past three centuries, at the very least (Blais Reference Blais1965; Burleigh et al. Reference Burleigh, Alfaro and Borden2002). Mature stands of balsam fir are the most vulnerable to spruce budworm (MacLean Reference MacLean1980), but this insect also attacks young stands, although it rarely causes mortality until the stands are over 20 or 25-year-old (Scarr et al. Reference Scarr, Smith, Turgeon and Howse2001). During the 1970s–1980s outbreak in eastern Canada, spruce budworm defoliation not only affected mature trees, but also naturally regenerated and plantation forests. After two years of severe defoliation, young balsam fir sustained a 50% reduction in volume growth (Piene Reference Piene1980), whereas young white spruce exhibited a 27% reduction in volume growth (Piene Reference Piene1991).
The western spruce budworm, Choristoneura occidentalis (Freeman), is a major pest of Douglas-fir, grand fir, subalpine fir (Abies lasiocarpa (Hooker) Nuttall), Engelmann spruce, white spruce, and western larch (Larix occidentalis Nuttall), in western Canada and the United States of America, with Douglas-fir being the most damaged species (Coulson and Witter Reference Coulson and Witter1984). Similar to other members of this genus, the western spruce budworm periodically reaches outbreak levels throughout its range (Alfaro et al. Reference Alfaro, Berg and Axelson2014). Budworm defoliation causes tree mortality, reduction of growth rates, dieback, and reduced lumber quality. Young stands are the most vulnerable to damage; dead tops on young trees can result in future stem deformities (Heppner and Turner Reference Heppner and Turner2006). Mortality is more prevalent among trees in the lower canopy and understorey regeneration than in the dominant or co-dominant strata (Alfaro et al. Reference Alfaro, van Sickle, Thomson and Wegwitz1982; Alfaro Reference Alfaro1986). If the understorey regeneration is lost to budworm in forests managed in shelterwood silvicultural systems, the mature trees cannot be removed until a new understorey becomes established, which creates regeneration delays (Alfaro Reference Alfaro1991). A recent outbreaks of western spruce budworm occurred in Alberta between 2000 and 2012 when budworm populations in southwest Alberta collapsed. The outbreak reached its peak in terms of area in 2008 when over 30 779 ha of Douglas-fir stands were attacked. It was, however, in 2007 when the impact of this outbreak was greater because severe defoliation was observed in over 16 201 ha in Porcupine Hills (Environment and Sustainable Resource Development Alberta 2008, 2010, 2013).
The jack pine budworm, Choristoneura pinus Freeman, feeds mainly on jack pine foliage, but it can also consume red pine, white pine, and Scots pine, especially when these species are minor components of jack pine stands (Howse and Meating Reference Howse and Meating1995). This budworm occurs from New Brunswick to British Columbia, but has caused the most damage in Ontario, Manitoba, and Saskatchewan (Martineau Reference Martineau1984). Outbreaks of this defoliator typically occur at 6-year to 12-year intervals and persist for two to three years (Volney Reference Volney1988). Jack pine budworm attack can result in growth loss, reduced seed production, top kill, and tree mortality (Howse and Meating Reference Howse and Meating1995). Usually, the most severe damage is found in overstocked, overmature stands (Coulson and Witter Reference Coulson and Witter1984), but this insect can also damage young stand and plantations. Young stands are immune to jack pine budworm attack until they start to flower, which probably occurs for the first time when they are 20 years old (Volney Reference Volney1988). High densities of this defoliator may be found in plantations younger than 40 years old, but tree mortality is rather low (Scarr et al. Reference Scarr, Smith, Turgeon and Howse2001).
Hymenoptera. Sawflies are one of the most destructive groups of insects in young conifer stands. They receive their name from the sawlike ovipositor of the female. The most destructive sawflies belong to the families Diprionidae and Tenthredinidae. Diprionid sawflies feed mainly on pines, whereas tenthredinid sawflies feed on spruce, fir, larch, and hardwoods (Coulson and Witter Reference Coulson and Witter1984). Sawflies are often present in plantations at low population levels but can occasionally increase to levels that cause significant damage.
The redheaded pine sawfly, Neodiprion lecontei (Fitch) (Hymenoptera: Diprionidae), is one of the most damaging insects that attack young pine plantations in Ontario, Québec and, to a lesser extent, in New Brunswick, Nova Scotia, and Prince Edward Island (Wallace and Cunningham Reference Wallace and Cunningham1995). The redheaded pine sawfly produces one generation per year in Canada, whereas it may have as many as five generations farther south (Benjamin Reference Benjamin1955). In Canada, adult sawflies emerge from mid-June to early July. The emerging larvae are gregarious and preferentially feed on previous year’s foliage and overwinter in the soil. Red pine is the principal host, but can include jack pine and Scots pine. Damage can be severe, especially on trees less than two to three years old (Cunningham and de Groot Reference Cunningham and de Groot1984). This sawfly is a voracious feeder that readily strips small trees (1–5 m tall) of part or all of their foliage. Young trees may be deformed or killed during an outbreak, which can last two to three years (Averill et al. Reference Averill, Wilson and Fowler1982). In Québec, the last outbreak occurred from 1974 to 1978 in red pine plantations (Martineau Reference Martineau1984; Lachance Reference Lachance1995). Since then, it has been present at endemic levels until 1999, when it caused increased damage. No serious damage, however, has been observed since this sawfly population was controlled using a nuclear polyhedrosis virus (Ministère des Ressources naturelles 2000). In Ontario, redheaded pine sawfly populations have fluctuated since the last outbreak was detected in 1974 (Martineau Reference Martineau1984; Howse Reference Howse1995) but caused 33% mortality at one site in 1981 (Howse Reference Howse1995). In southern Ontario, heavy sawfly damage was recorded in young 1-m tall red pine plantations in 1996 (Ingram et al. Reference Ingram, Curry, Francis and Rowlinson1997) and 80% infestation levels in one plantation of 4-m tall red pine in 2002 (Evans et al. Reference Evans, Lawrence, Ingram and Czerwinski2002)
The yellowheaded spruce sawfly, Pikonema alaskensis (Rohwer), (Hymenoptera: Tenthredinidae) is a pest of young, open-grown spruces across Canada (Martineau Reference Martineau1984). Although it has been reported to attack several spruce species (Ives and Wong Reference Ives and Wong1988; de Groot Reference de Groot1995), this sawfly is particularly harmful to plantations of black spruce and white spruce (Hall et al. Reference Hall, Bowers and Hirvonen1998). It can be found in nurseries, cutover areas, and in young natural regeneration (Martineau Reference Martineau1984). Larvae feed mainly on current-year foliage, although older age classes may be eaten if new foliage becomes sparse (Johns and Quiring Reference Johns and Quiring2010). This insect causes serious defoliation, growth reduction, and tree mortality, especially since this sawfly tends to concentrate its attacks on trees previously defoliated (Ives and Wong Reference Ives and Wong1988; Katovich et al. Reference Katovich, McCullough and Haack1995). In 1995, moderate-to-severe defoliation by this sawfly was reported in black and white spruce plantations in Newfoundland and Labrador, New Brunswick, Prince Edward Island, and Ontario. Only low-level infestations were observed in Nova Scotia and Québec (Hall et al. Reference Hall, Bowers and Hirvonen1998). In 2011, defoliation by this insect in Ontario was localised to some districts and mainly affected trees <3-m tall. These individuals exhibited up to 50% defoliation (Scarr et al. Reference Scarr, Ryall and Hodge2012). This sawfly was observed in 13% of spruce plantations in Québec, but signs of attack were evident in 10% of the trees (Ministère des Ressources naturelles et de la Faune 2012).
The balsam fir sawfly, Neodiprion abietis (Harris), occurs from coast to coast in southern Canada (Drooz Reference Drooz1985), where its preferred host is balsam fir. This sawfly also feeds on white spruce, black spruce, and, less frequently, red spruce (Martineau Reference Martineau1984). Larvae hatch in late June to early July, and feed first on one-year-old foliage, but later continue to feed on the remaining foliage, except the current-year needles (Piene et al. Reference Piene, Ostaff and Eveleigh2001). Damage may result in reduced vigour and growth, and tree mortality. Outbreaks of this sawfly have historically been of short duration and have been observed from Saskatchewan eastward (Cunningham Reference Cunningham1984; Martineau Reference Martineau1984). However, the current outbreak in western Newfoundland, which began in 1991, is unprecedented in severity and duration, having affected young managed stands of balsam fir (Piene et al. Reference Piene, Ostaff and Eveleigh2001; Moreau Reference Moreau2006). During 1991–2011, a cumulative area of 582 790 ha was moderately-to-severely defoliated in Newfoundland (Canadian Council of Forest Ministers 2013). In New Brunswick, balsam fir sawfly was detected over a 181 800 ha area in 2010; of which 7282 ha of crown forest was aerially sprayed with the biological insecticide AbietivTM (Balsam Fir Sawfly Polyhedrovirus, Sylvar Technologies Inc., Fredericton, New Brunswick, Canada). By 2012, its population had collapsed and only light scattered defoliation was detected within a small geographic area (New Brunswick Department of Natural Resources Forest Pest Management Section 2013). In 1995, this insect caused moderate to high damage in Québec, while 250 ha were heavy defoliated in Nova Scotia (Hall et al. Reference Hall, Bowers and Hirvonen1998). In Ontario, balsam fir exhibited up to 60% defoliation in 1995 (Hall et al. Reference Hall, Bowers and Hirvonen1998) and 20–65% defoliation in 2011 (Scarr et al. Reference Scarr, Ryall and Hodge2012).
The larch saw fly, Pristiphora erichsonii Hartig (Hymenoptera: Tenthredinidae), occurs in all Canadian provinces (Drooz Reference Drooz1985). Although this sawfly is specific to larch, its preferred host in Canada is tamarack (Martineau Reference Martineau1984). This univoltine insect reproduces by obligatory parthenogenesis and males form only 2% of the population (Turnock Reference Turnock1960; Martineau Reference Martineau1984). Females lay their eggs in slits that they cut with their ovipositor on new shoots (Turnock Reference Turnock1960). The oviposition injury retards the growth on the side of the shoot containing eggs but not in the opposite side, curling the tip of the shoot (Lejeune Reference Lejeune1947). After hatching, the larvae feed in light clusters, completely stripping the foliage (Lejeune Reference Lejeune1947; Turnock Reference Turnock1960). Defoliation is concentrated in certain parts during light outbreaks but the entire crown can be defoliated during severe outbreaks (Martineau Reference Martineau1984). The first recorded outbreak of this insect began in the early 1880s near Ville de Québec and then it spread to Ontario, New Brunswick, and Nova Scotia, wiping out millions of ha of tamarack (Lejeune Reference Lejeune1947; Coppel and Leius Reference Coppel and Leius1955; Martineau Reference Martineau1984). In 1938, a serious outbreak began in Manitoba and then extended to Saskatchewan and Ontario, mainly affecting young stands of tamarack (Lejeune Reference Lejeune1947). Most tamarack stands sustained up to three consecutive years of severe defoliation, and almost 20% mortality of tamarack in several stands in Manitoba and Saskatchewan. By the mid 1960s, population levels of this insect gradually declined and remained low with minor fluctuations until 1984 when small areas of moderate to severe defoliation where observed in west-central Manitoba (Cerezke and Volney Reference Cerezke and Volney1995). In 1957, increases in the population level of this insect were observed in western Québec and extended throughout the province during the next 10 years (Martineau Reference Martineau1984), causing severe defoliation in several stands of tamarack (Lachance et al. Reference Lachance, Thibault and Monnier1991). Since 1968, this insect has been present in the province at endemic levels (Lachance Reference Lachance1995). From 1973 to 1975, this insect caused very severe defoliation in the eastern part of Prince Edward Island. In Nova Scotia, most of the southern mainland and much of Cape Breton Island surface was severely defoliated in 1975. New Brunswick was also affected by this outbreak, but its impact was not as high as it was in Prince Edward Island and Nova Scotia. The outbreak collapsed in 1980 (Magasi Reference Magasi1995). Outbreaks of this insect in Newfoundland are very common (Hudak and Raske Reference Hudak and Raske1995). In 1942, dramatic population increases were recorded in two areas and persisted until 1949 when the outbreak collapsed. A second wave of population increases began in 1953 causing severe defoliation until 1968 when the outbreak collapsed due to unfavourable weather conditions (Martineau Reference Martineau1984). Since 1970, several larch sawfly outbreaks have persisted in Newfoundland but their impact has not been considered important because larch is currently not a commercial forest species in this province, and the tree is only a scattered component within coniferous stands (Hudak and Raske Reference Hudak and Raske1995).
Other sawflies that can affect young conifers in Canada are the non-native European pine sawfly, Neodiprion sertifer (Geoffroy), and European spruce sawfly, Gilpinia hercyniae (Hartig) (Hymenoptera: Diprionidae) (Wallace and Cunningham Reference Wallace and Cunningham1995). In its native range, the European pine sawfly prefers mugo and Scots pine, feeding to a lesser extent on Austrian pine. In North America, however, it feeds mainly on jack pine and red pine, but also on white pine, ponderosa pine, pitch pine, shortleaf pine (Pinus echinata Miller), and Japanese red pine (Pinus densiflora Siebold and Zuccarini). Within its native range, European spruce sawfly feeds upon Norway spruce and plantations of introduced spruces, especially in the United Kingdom (Billany et al. Reference Billany, Carter, Winter and Fielding1983). Introduction of this sawfly species into North America in the 1930s initially caused great losses of merchantable timber in the Gaspé of Québec, but shortly thereafter diminished in economic importance as biological control measures were implemented (Griffiths et al. Reference Griffiths, Cunningham and Otvos1984).
Sucking insects
The damage caused by Hemiptera with their piercing and sucking mouthparts is normally less apparent and damaging than that caused by other groups of insects such as defoliators; however, some species in the families Adelgidae and Aphididae can severely damage and even kill trees (Coulson and Witter Reference Coulson and Witter1984). Adelgids and aphids undergo incomplete metamorphosis but tend to have complex life cycles. Some have alternate hosts and others have both sexual and asexual reproductive stages with winged and wingless forms (Ciesla Reference Ciesla2011). Feeding by these insects causes foliage discolouration, interferes with transport of photosynthate and water supplies within trees, reduces tree growth, and causes branch and tree mortality if there is severe and repeated damage over a long time (Coulson and Witter Reference Coulson and Witter1984). These insects are generally very small, which renders the detection of their presence difficult before damage occurs (Ciesla Reference Ciesla2011).
One of the most damaging members of this group in Canada is the balsam woolly adelgid, Adelges piceae (Ratzeburg) (Hemiptera: Adelgidae). Native to Europe, it was first detected in eastern Canada in 1908 (Balch Reference Balch1952), but now occurs in British Columbia, New Brunswick, Newfoundland and Labrador, Nova Scotia, Prince Edward Island, and Québec (Harris and Bower Reference Harris and Bowers1995). This adelgid is a pest of Abies, and attacks balsam fir in eastern Canada and subalpine fir, amabilis fir, and grand fir in western Canada (Martineau Reference Martineau1984; Harris and Bower Reference Harris and Bowers1995). Reproduction of this adelgid is parthenogenetic and adults are tiny (about 1 mm in length) and wingless. The newly hatched juveniles, or “crawlers”, are the only stage capable of directed movement or dispersal. Long range spread is accomplished mainly by wind. Throughout the remainder of the life cycle, the insect is anchored to the tree by its feeding mouthparts (stylets), which are inserted in the bark feeding on phloem sap (Greenbank Reference Greenbank1970; Martineau Reference Martineau1984). Trees of all sizes can be attacked by this insect, producing two forms of damage. Infestations on twigs and branches result in swelling and distortion of twigs, a condition known as “gout” (Balch Reference Balch1952). This process, which takes about 25 years to complete, causes crown decline, branch dieback, and “top kill” (Martineau Reference Martineau1984). Severe feeding damage on the bole causes swelling of the affected area and results in restriction of water flow. During heavy adelgid infestations, trees can die in three to four years (Martineau Reference Martineau1984).
This adelgid caused considerable damage to balsam fir stands in Newfoundland and Labrador from the late 1950s to early 1960s, but its population densities decreased dramatically by the late 1960s for unknown reasons (Magasi Reference Magasi1995). The adelgid caused significant damage in several regions of the province in 1995, especially in stands where thinning occurred (Hall et al. Reference Hall, Bowers and Hirvonen1998). In the Maritime Provinces this adelgid caused similar mortality as did spruce budworm during the 1950s and early 1960s. Since then, balsam woolly adelgid populations were reduced by cold winters until 1979, when this insect was again detected attacking trees in 11% of the forested areas of New Brunswick, 74% in Nova Scotia, and 25% in Prince Edward Island. Despite these attacks, damage remained low and stable until 1981, when a serious infestation affected 122 ha of Christmas tree plantations in Nova Scotia (Magasi Reference Magasi1995). Currently, low-level attacks occur in New Brunswick, with only ~0.5% of balsam fir trees showing moderate-severe damage in 2010; no significant areas of damage were observed in 2012 (New Brunswick Department of Natural Resources Forest Pest Management Section 2013). In Nova Scotia, monitoring of balsam woolly adelgid populations and damage was discontinued by 2007 (Penny Reference Penny2007). In Québec, a survey revealed that about 2500 km2 were infested by this adelgid on the Gaspé, but levels of damage were low (Lachance Reference Lachance1995). In British Columbia, the most serious infestation occurred in 1987, when over 150 ha of amabilis fir were attacked and tree mortality reached 30% (van Sickle Reference van Sickle1995). This adelgid seems to be present wherever fir seedlings are produced for reforestation on Vancouver Island. Tests of experimental adelgid infestations on one-year-old seedlings showed that the pest reproduced successfully after one growing season on containerised nursery stock (Hall et al. Reference Hall, Bowers and Hirvonen1998).
The balsam twig aphid, Mindarus abietinus Koch (Hemiptera: Aphididae), is found throughout the range of native fir across Canada, feeding mainly on balsam fir, but also on amabilis fir, grand fir, and subalpine fir (Dawson Reference Dawson1971; Martineau Reference Martineau1984). Although it can attack natural forests, its damage is more economically important on ornamental and Christmas trees because it reduces their aesthetic value (Coulson and Witter Reference Coulson and Witter1984). This aphid has three or four generations per year, which extend from early May to early July, overwintering as diapausing eggs on tree foliage (Martineau Reference Martineau1984). Feeding on newly opened buds and current-year shoots results in needle distortion and shoot stunting (Furniss and Carolin Reference Furniss and Carolin1977). In Canada, outbreaks of this insect generally develop on balsam fir, but are of short duration. In Newfoundland, this aphid has normally caused low to moderate damage to balsam fir (Raske et al. Reference Raske, Sutton, Banfield, Stone, O’Brien and Pardy1992; Magasi Reference Magasi1995), except when it caused serious damage in 1972 in the Bay d’Espoir region (Martineau Reference Martineau1984). In the Maritime Provinces, local and short-term outbreaks are detected every three to four years (Martineau Reference Martineau1984). In 2012, 13% of 281 plots in New Brunswick had detectable levels of damage attributable to this pest (New Brunswick Department of Natural Resources Forest Pest Management Section 2013). In Nova Scotia, monitoring programmes were suspended in the province, because of low incidence of the aphid from 2003 to 2006 (Penny Reference Penny2007). Population densities of this aphid in Ontario have been usually low, except for an outbreak in 1946 (Martineau Reference Martineau1984). Infestations in British Columbia have been most frequent in open stands of immature amabilis and grand fir on Vancouver Island and in young subalpine fir stands in much of the interior, producing heavy damage (Dawson Reference Dawson1971). In 1978, high densities of the insect were reported with significant damage to localised areas in the province, whereas the damage was low in 1986 (van Sickle Reference van Sickle1995). The last outbreak was reported in 1989 (Hall et al. Reference Hall, Bowers and Hirvonen1998).
Assessing the impacts of pests of young stands
Assessment of the impacts of pests of young stands is a critical prerequisite to determine the levels of expenditures to be committed in management and research on these pests. Damage to the young age classes of the forest can have serious impacts on the short-term to long-term development of a forest. In the short term, losses to regeneration negate the initial investments in activities such as site preparation, plantation, and silviculture, and extensive damage can lead to plantation failure, which causes rotation delays and additional investments in restoration. In the long term, these pests reduce the total harvest and, because of selective mortality, they can change the species mixture at rotation and thus disturb the timber supply process. In forests managed in a multi-age silvicultural system, such as shelterwood systems, damage to the young age classes will result in gaps in the expected timber supply.
Assessing the impacts of damage that occur at an early age requires stand and landscape models capable of forecasting growth and yield, while separating the effects of the pest from mortality due to natural causes, such as attrition during forest development. For example, natural mortality occurs due to the natural process of stem exclusion, which takes place when stands change from open-grown to a closed canopy forest.
Modelling impacts requires good knowledge of pest epidemiology and damage, as well as of the processes of stand and landscape dynamics. Volney et al. (Reference Volney, Alfaro, Bothwell, Hogg, Hopkin and Laflamme2005) developed a framework for assessing the risk of damage to young poplar plantations in Canada, which could be applied to other situations. Insect and diseases of poplars were categorised as annual or cyclical events, or chronic disturbances. The latter included those pests that, once established, may affect the plantation for extended periods, for example, gall aphids. The model also considered the temporal distribution of the disturbance, that is, the statistical distribution of its occurrence in time, and whether its effects would linger and affect plantation productivity for several years.
Impact models also require an understanding of how the pest causes its damage to young trees and stands, including expected levels of mortality, growth reduction, and potential trunk deformities resulting in the affected trees. In addition, these models require a description of how the stand dynamics is changed by the pest event, in the short and long term (at harvest).
This complex information can be integrated into a decision support system for pests of regenerating forests capable of estimating potential economic losses at the end of rotation, comparing yields with and without pest damage. For example, Yemshanov et al. (Reference Yemshanov, McKenney, de Groot, Haugen, Pedlar and Sidders2011) used the potential infringements on annual allowable harvest targets as an approach to estimate threats from the invasive wood wasp, Sirex noctilio Fabricius (Hymenoptera: Siricidae), to the forest products sector of Canada. The approach proposed by these authors uses present-day harvest levels as a reference level to estimate when and where the impact of this wasp could become economically damaging.
Detailed studies of the natural history of Pissodes strobi, and of the recovery and defect formation in its coastal host, Sitka spruce was used to develop a computer model, Spruce Weevil Attack (SWAT) (full details of model in Alfaro et al. Reference Alfaro, Brown, Mitchell, Polsson and McDonald1996), capable of comparing losses at the stand level under various weevil and forest management scenarios. Working as a subroutine of the British Columbia Ministry of Forests Tree and Stand Simulator (Mitchell Reference Mitchell1975, Reference Mitchell1988), the SWAT model, is able to integrate different management options, such as sanitation thinning, and use of genetic resistance to weevil to mitigate the damage, and of forecasting stand productivity at rotation (Figs. 1–3).
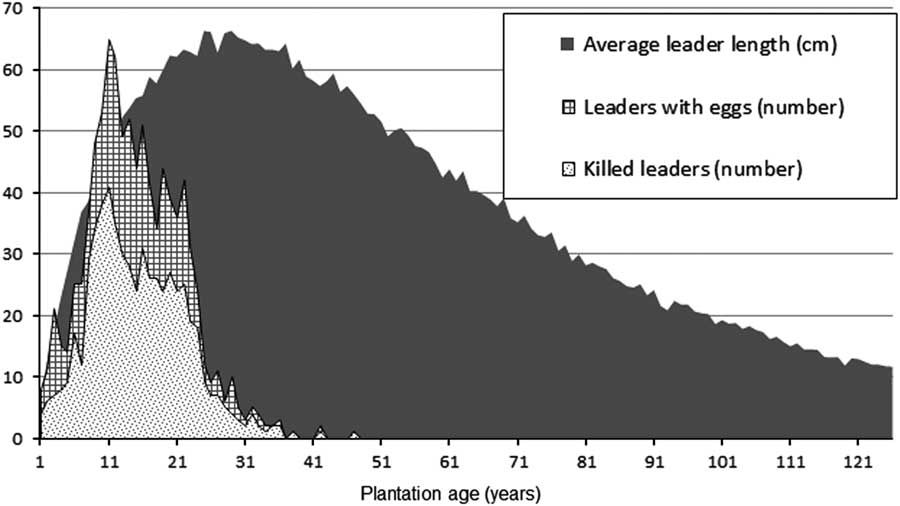
Fig. 1 Results of the Spruce Weevil Attack (SWAT) simulation model showing the dynamics of weevil population, represented by the number of eggs laid on leaders and resulting damage (number of killed leaders) through the life of a Sitka spruce forest from plantation to harvest. In this model, weevil population is regulated by the availability of long leaders (a proxy for food supply), which are more prevalent during the juvenile stage of the stand.
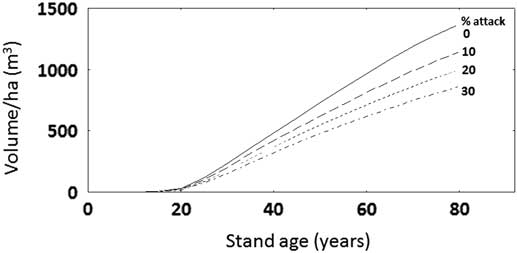
Fig. 2 Merchantable volume/ha versus stand age in a Sitka spruce plantation after simulated weevil infestations based on the Spruce Weevil Attack (SWAT) model. Weevil attack began at age six and lasted until age 40 years. Plantation was started at close spacing (2.74 m), on a site index of 30 m at breast height age of 50 years.
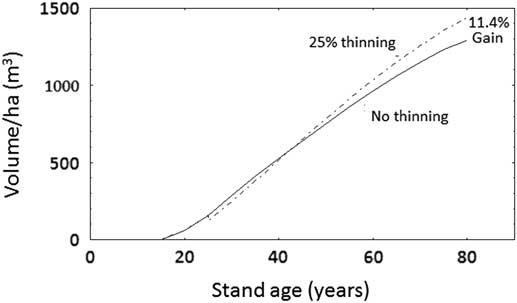
Fig. 3 Merchantable volume/ha versus stand age after simulated weevil infestations using the Spruce Weevil Attack (SWAT) model on Sitka spruce. Plantation was thinned at age 25 to remove 25% of the basal area, biasing the thinning to give priority to removal of trees with weevil-induced defects, such as major crooks and forks.
Risk assessment in regenerating forests
The framework proposed by Fuentealba et al. (Reference Fuentealba, Alfaro and Bauce2013) is used here to illustrate the usefulness of conducting a pest risk assessment before planting. The framework consists of four main steps. The first step is to evaluate the level of hazard or probability of occurrence of insect damage in the young stand. Factors such as geographic location, temperature, precipitation, humidity, seedling vulnerability, and proximity to infested plantations, can all affect the likelihood of insect attack in young stands. For example, low temperatures and humidity can negatively affect feeding and oviposition behaviour of adult white pine weevils, as well as, retard larval development, increasing exposure of weevil larvae to natural enemies (Alfaro et al. Reference Alfaro, Borden, Fraser and Yanchuck1995). White spruce trees that have a late bud break are less susceptible to spruce bud moth defoliation than those that have an early bud break (Quiring Reference Quiring1994). Young lodgepole pine mortality caused by Warren root collar weevil in reforested stands increases when unsalvaged, mature stands killed by mountain pine beetle are nearby (Klingenberg et al. Reference Klingenberg, Lindgren, Gillingham and Aukema2010). Climate-based hazard rating systems for specific pest insects can be of great use for reforestation planning, prioritising areas for control treatments, and deciding on the implementation of other pest management tactics, such as where to deploy resistant genotypes (Alfaro et al. Reference Alfaro, Borden, Fraser and Yanchuck1995).
Second, the level of risk exposure, or economic evaluation of potential impact must be determined, assuming different levels of pest activity (scenario building), to determine the economic threshold or pest level that will cause economic damages of greater value than the cost of the control actions. The impact models described above are useful in this phase of the risk assessment to guide practitioners in determining if the losses caused by the pests are tolerable, or whether control measures are required to avoid plantation failure. Pest control measures will be recommended in those situations where scenario building indicates, for the most likely pest level, that economic losses will significantly surpass the combined economic and ecological cost of control.
The third step consists of determining the vulnerability of the planted stock. The selection of tree species and subspecies to plant is critical to keep pest damage at low levels. The mechanisms of tree defence and genetic resistance to pest species found in the planting stock must be evaluated to determine what tree species or provenances are less prone to be damaged. Unfortunately, this information is missing for most pests of young stands, but when available, this assessment allows choice of planting stock with resistance according to risk level in the selected sites. For example, a successful breeding programme in Sitka spruce resulted in the commercial availability of stock with resistance to white pine weevil in British Columbia (Alfaro et al. Reference Alfaro, King and vanAkker2013). This programme succeeded in identifying sources of heritable and stable resistance against this weevil, allowing the establishment of seed orchards for the commercialisation of resistant seed. The deployment of resistant seedlings is expected to result in a reduction of over 80% in weevil attack levels (Alfaro et al. Reference Alfaro, King and vanAkker2013). In Québec, the observation of white spruce trees resistant to spruce budworm initiated a programme lead by researchers from Université Laval (Ville de Québec, Québec, Canada) to study the sources of this natural resistance and how to use it to mitigate spruce budworm damage. Preliminary results suggest that two phenolic compounds reduce the pressure of spruce budworm herbivory on specific host tree phenotypes (Delvas et al. Reference Delvas, Bauce, Labbé, Ollevier and Belanger2011).
The final step in risk assessment consists of determining the appropriate management response. The least expensive option for risk mitigation is prevention. Having established the hazard, exposure, and assessed tree species vulnerability to pest damage, it is necessary to prepare a plan for communicating and reducing risk. It is important to clearly communicate the level of risk under different situations to the public and to practitioners. This will effectively facilitate the acceptance and adoption of preventative control measures.
Management of pests of young stands
Responses to pest situations must be based on the management objectives for the plantation (i.e., values at risk, products expected at rotation), local pest status (e.g., pest is active in the vicinity), tree species vulnerability, and feasibility. Feasibility of pest control varies because options are never 100% effective, and thus, it is necessary to estimate the level of damage that will occur in spite of the control measures being applied.
The use of insecticides was often recommended in the past for direct control of pest damage. However, this tactic is now considered environmentally undesirable because of its potential impact on non-target organisms. Furthermore, the development of pest resistance to insecticides has encouraged researchers and practitioners to explore alternative pest control strategies. In the last decades there has been an increase in the use of biological insecticides such as Bacillus thuringiensis kurstaki (Bacillaceae) (Btk) to reduce the damage produced by Lepidoptera, such as spruce budworm (e.g., Fuentealba et al. Reference Fuentealba, Bauce and Dupont2015). Also, there has been an increased interest in classical biological control programmes for controlling pests of young stands, such as the European pine shoot moth in Chile, where the parasitoid Orgillus obscurator Ness (Hymenoptera: Braconidae) was used to control this invasive moth (Alfaro et al. Reference Alfaro, Hantula, Carroll, Battisti, Fleming and Woods2010).
The risk assessment framework described previously can be used as a basis for decision-making in an integrated pest management system (IPM). Integrated pest management is an ecological approach to pest control with the objective of using a combination of various tactics to reduce damage rather than to eliminate the pest. An effective IPM system could include the following components: use of planting stock with genetic resistance to the pest insect and tactics that reduce damage based on silviculture, such as shade conservation or increasing plantation density (Alfaro et al. Reference Alfaro, Borden, Fraser and Yanchuck1995, Lavallée et al. Reference Lavallée, Daoust, Mauffette, Audet and Coulombe2001). Integrated pest management aims at reducing the loss of productivity of a site by optimising the selection of the appropriate combination of control methods that lead to a profitable crop. The economic value of the various possible combinations of tactics, provided by the risk assessment, will assist foresters in their selection of the best control method combination.
Intensive research programmes in British Columbia on the natural history, damage, and control of the white pine weevil, permitted the development of an IPM system for this pest (Alfaro et al. Reference Alfaro, Borden, Fraser and Yanchuck1995), which was formulated to combine silviculture-driven and host resistance-driven tactics. The system relies on accurate risk assessment of plantation sites and requires continuous monitoring of attack levels and the forecasting of plantation productivity under various IPM tactics through the use of a decision support system (Alfaro et al. Reference Alfaro, Borden, Fraser and Yanchuck1995).
Conclusion
A number of insect species in a variety of families and orders cause damage to young Canadian forests. Successful management of pests of regenerating forests requires detailed information on the natural history of the damaging organism, including the factors that increase risk, and a careful assessment of the risk mitigation options. Decision support systems, in the form of stand or landscape models capable of incorporating pest management options are required to guide decisions in terms of the expected yields under various pest management scenarios. Risk assessment and IPM system are useful tools to guide practitioners in management of pests of regenerating forests. The fact that 154 of the 179 references cited in this review are by Canadian researchers indicate that Canadians have been active contributors to the research that advanced this field from the early descriptive studies to the development of practical tools to assist industry in managing pests of young forests.
Acknowledgments
The authors wish to thank to the two anonymous reviewers for their useful suggestions.





