Somatization is characterized by the tendency to experience somatic distress and multiple physical symptoms unaccounted for by pathological findings, to attribute them to physical illness and to seek medical help for them (Lipowski, Reference Lipowski1988). Although somatization symptoms manifest before the age of 30, initial symptoms are often present by adolescence (Oyama et al., Reference Oyama, Patoo, Greengold and Plant2007). Somatization is more common in females than in males: It has been estimated to occur in 0.2%–2% of females and 0.2% of males (Oyama et al., Reference Oyama, Patoo, Greengold and Plant2007). Albeit inconsistent, some studies have documented that somatization is more prevalent in Asians than in Western populations, and the symptom profiles are different across the two cultures. For example, Hsu and Folstein (Reference Hsu and Folstein1997) found that somatization was significantly more common among ChineseAmerican than Caucasian American patients referred for psychiatric consultation, and that Chinese American somatizers complained predominantly of cardiopulmonary and vestibular symptoms, whereas their Caucasian counterparts had symptoms listed in the Diagnostic and Statistical Manual of Mental Disorders (4th ed. [DSM-IV]; American Psychiatric Association [APA], 1994) such as headache and back pain. Recently, several researchers (e.g., Choi et al., Reference Choi, Chentsova-Dutton and Parrott2016; Ryder & Chentsova-Dutton, Reference Ryder and Chentsova-Dutton2012) suggested that Asians tend to express their psychological distress to excessive stress with somatic rather than psychological symptoms due to social stigma associated with expression of mental illness problems and concerns about possible loss of harmonious interpersonal relationships. Thus, somatization may be considered as effective coping strategies to secure social support and health resources in Asian cultures (Ryder & Chentsova-Dutton, Reference Ryder and Chentsova-Dutton2012).
Although heritability of somatization in Asians remains largely unknown due to a dearth of twin studies of somatization in Asians, it has been shown that genetic factors explain 25%–49% of the total variance in somatization in Caucasians (Bartels et al., Reference Bartels, van de Aa, van Beijsterveldt, Middeldorp and Boomsma2011; Gillespie et al., Reference Gillespie, Zhu, Heath, Hickie and Martin2000; Hansell et al., Reference Hansell, Wright, Medland, Davenport, Wray, Martin and Hickie2012; Kendler et al., Reference Kendler, Walters, Truett, Heath, Neale, Martin and Eaves1995; Vassend et al., Reference Vassend, Roysamb and Nielsen2012). Evidence for the presence of sex-specific genetic effects on somatization has not been reported in these studies. However, the magnitudes of genetic influences were found to vary by sex in some studies. For example, Bartels et al. (Reference Bartels, van de Aa, van Beijsterveldt, Middeldorp and Boomsma2011) found that heritability for the somatic complaints subscale of the Youth Self-Report (Achenbach & Rescorla, Reference Achenbach and Rescorla2001) was about 36% in females, whereas it decreased from approximately 49% at age 12 years to nearly zero at age 20 years in males. Kendler et al. (Reference Kendler, Walters, Truett, Heath, Neale, Martin and Eaves1995) also reported sex difference in heritability: genetic influences were 49% for males and 36% for females in the somatization subscale of the Symptom Checklist 90. Non-genetic influences were attributed to individual-specific environment and measurement error rather than shared environmental influences.
It has been well documented that somatization co-occurs with other psychiatric symptoms such as anxiety and depressive disorders (Lieb et al., Reference Lieb, Meinlschmidt and Araya2007) and personality disorders (Bornstein & Gold, Reference Bornstein and Gold2008), and that these relationships are mediated in part by shared genes (Hansell et al., Reference Hansell, Wright, Medland, Davenport, Wray, Martin and Hickie2012; Klengel et al., Reference Klengel, Heck, Pfister, Bruckl, Hennings, Menke and Ising2011). Although somatization is known to be comorbid with hwabyung (HB, anger syndrome; Min & Suh, Reference Min and Suh2010), genetic etiology of this comorbidity has been very rarely investigated, perhaps because HB is bound to Korean culture. HB, known as an anger syndrome, is characterized by chronic suppression of anger, continued feelings of unfairness and resentment and somatic complaints, including heat sensation, chest tightness and indigestion (APA, 1994; Min, Reference Min and Koh2013). The prevalence of HB syndrome has been estimated to be between 4.2% and 13.3% in the Korean general population and Korean immigrants in Western countries (Min, Reference Min and Koh2013). Similar to somatization, the prevalence of HB is known to be higher in females than in males (Lee & Lee, Reference Lee and Lee2008). Using the sample employed in the present study, we previously demonstrated that 44% of the variation in HB symptoms was due to additive genetic effects, with the remaining variance being associated with individual-specific environmental influences and measurement error, and that there were no significant sex differences in these estimates (Hur et al., Reference Hur, Choi, Kim, Jin and Lee2018). Somatic symptoms are a common element of somatization disorders and HB. However, somatic symptoms in HB are due predominantly to anger accumulated inside over a long period (Kim et al., Reference Kim, Chung, Suh, Jung, Lee, Kim and Kim2010), whereas somatic symptoms in somatization disorders are associated with unspecified psychological distress (Lipowski, Reference Lipowski1988). Thus, there may be genetic commonality as well as differences between HB and somatization because different genetic and environmental mechanisms may be involved in various emotions. The main objectives of the present study were to estimate genetic and environmental influences on somatization in South Korean adolescent and young adult twins and to explore shared genetic and environmental etiologies of the co-occurrence of HB and somatization in these twins.
Materials and Methods
Sample
The sample consisted of 1754 (367 monozygotic male [MZM], 173 dizygotic male [DZM], 681 monozygotic female [MZF], 274 dizygotic female [DZF] and 259 opposite-sex dizygotic [OSDZ]) twins drawn from the South Korean Twin Registry (Hur et al., Reference Hur, Jeong, Chung, Shin and Song2013). The mean age of the twins was 19.1 years (SD = 3.1 years, range: 12–29 years). Sixty-two percent of the sample was female. The preponderance of females in the present sample was due in part to the fact that males are required to serve in the military service in South Korea. Twins under 20 years were recruited mostly from schools throughout South Korea, while those older than 19 years were mostly recruited from Facebook, colleges throughout South Korea and twin clubs on the internet. Zygosity of the twins was assessed using a three-item zygosity questionnaire. When compared to DNA analysis, this approach has been shown to achieve over 90% accuracy (Ooki et al., Reference Ooki, Yamada and Asaka1993). The number of MZ twins was much greater than that of DZ twins in the present sample. These rates of MZ and DZ twins likely reflected the low DZ twin birth rates in the South Korean population for the birth cohorts in the present study (Hur & Kwon, Reference Hur and Kwon2005).
Measures
Somatization scale
A telephone interview was given to twins to complete a Korean version of the somatization scale of the Personality Assessment Inventory (PAI; Kim et al., Reference Kim, Kim, Oh, Im and Hong2001; Morey, Reference Morey2007) and the HB symptoms scale (Kwon et al., Reference Kwon, Kim, Park, Lee, Min and Kwon2008) explained below. The PAI has been used to screen individuals with psychopathology in both clinical and community settings (Morey, Reference Morey2007). The somatization scale of the PAI consists of eight items regarding routine and vague physical complaints such as headaches, back pain and gastrointestinal ailments. Sample items include ‘I suffer from a lot of pain,’ ‘I frequently have diarrhea’ and ‘Much of the time I don’t feel well.’ For each of the eight items, the respondent was instructed to rate with a 4-point scale (0 = not at all true to 3 = very true). The ratings were summed over the eight items to obtain a total score of the somatization scale. Thus, higher scores indicate more severe symptoms. Cronbach’s alpha of the eight items in the current sample was .76. Similar to other psychiatric screening measures, the somatization scale was positively skewed, with a skewness of .82. We performed square root transformation of the raw score, which resulted in a skewness of .17.
HB symptoms scale
The HB symptoms scale consists of 15 self-report items concerning emotional problems such as feelings of unfairness, anger, depression and anxiety, and typical physical symptoms of HB such as heat sensation, chest tightness, digestion problems, fatigue and tremor of hands. Sample items include ‘When my anger is rising, my hands are shaking,’ ‘I really feel tight,’ ‘My face is flushed with anger’ and ‘I am often disappointed at myself.’ Psychometric properties of the HB symptom scale have been shown to be acceptable (Kwon et al., Reference Kwon, Kim, Park, Lee, Min and Kwon2008). Twins were instructed to rate themselves on a 5-point Likert-type scale from not true (0) to certainly true (4) for each of the 15 items. The ratings were summed to obtain a total score of HB so that higher scores represent more severe HB symptoms. The total score of the HB symptoms scale was positively skewed, with a skewness index of .65. We performed square root transformation of the raw score, which resulted in a skewness of −.14. Cronbach’s alpha of the 15 items was .92 in the present sample, which was close to that found in the normative sample (Kwon et al., Reference Kwon, Kim, Park, Lee, Min and Kwon2008).
Statistical Analysis
A general sex-limitation model (Neale & Cardon, Reference Neale and Cardon1992) was used to estimate additive genetic and shared and individual-specific environmental variance components of somatization. Additive genetic variance (A) represents the sum of the average effect of all alleles that influence a trait (r mz = 1.0, r dz = 0.5). Shared environmental variance (C) refers to those environmental factors shared between the two members of a twin-pair (r mz = 1.0, r dz = 1.0). Individual-specific environmental variance (E) represents those environmental factors unique to each member of a twin-pair and measurement error (r mz = 0, r dz = 0). To determine sex differences in genetic and environmental influences on somatization, we allowed the magnitudes of A, C and E to differ across sexes in the full model. In addition, to test sex-specific genetic effects, the genetic correlation for opposite-sex DZ twins was set to be different to 0.5. Age was treated as a covariate in the general sex-limitation model.
A bivariate Cholesky model was used to decompose the phenotypic correlation between somatization and HB into additive genetic and shared and individual-specific environmental variances and covariances. Additive genetic and shared and individual-specific environmental correlations between somatization and HB were also derived from these variances and covariances. Additive genetic correlation indicates the extent to which the same additive genetic factors affect the two symptom scales. For example, if the additive genetic correlation is estimated at unity, this would indicate that HB and somatization share all of their genetic factors. On the other hand, if the additive genetic correlation is estimated at zero, this would suggest that HB and somatization are genetically independent. The same logic applies to shared and individual-specific environmental correlations. In the Cholesky model, we placed HB prior to somatization because we were interested in genetic and environmental factors unique to somatization as well as those factors common to the two symptoms.
We used the maximum likelihood raw data option in Mx (Neale et al., Reference Neale, Boker, Xie and Maes2003) that calculates twice the negative log-likelihood (-2LL) of the data. To determine the best-fitting, most parsimonious model, parameters in the full model were constrained sequentially, and the resulting changes in -2LL were evaluated. As the difference in -2LL is chi-square distributed, when models were nested to each other, the likelihood ratio test (LRT) was used to compare alternative models. A significant change in chi-square would indicate that the reduction of the parameters in the nested model causes a significant decrease in model fit, whereas a non-significant change would suggest that constraining parameters in the nested model is acceptable. When alternative models were not nested to each other, Akaike’s information criterion (AIC = -2LL – 2df) for alternative models was compared to evaluate superiority among competing models. Models having lower AIC are considered more parsimonious, and thus preferred (Akaike, Reference Akaike1987).
Results
Descriptive Statistics and Twin Correlations
We previously reported means (SDs) and age-corrected maximum likelihood twin correlations for HB in detail (Hur et al., Reference Hur, Choi, Kim, Jin and Lee2018). Briefly, although sex difference was not found significant in the variance of HB, the mean was significantly higher in females than in males (t = 4.7, p <.001). The correlation of HB with age attained statistical significance. However, the size was very small (r = .07). Maximum likelihood correlations for HB were .31 (95% CI [.16, .45]) for MZM, .19 (95% CI [−.05, .41]) for DZM, .50 (95% CI [.41, .58]) for MZF, .28 (95% CI [.11, .44]) for DZF and .23 (95% CI [.05, .40]) for OSDZ twins. These patterns suggested the presence of genetic effects in HB in both sexes as MZ twin correlations were consistently greater than DZ twin correlations. Although both MZ and DZ twin correlations were higher in females than in males, sex differences in correlations were not statistically significant.
Table 1 presents descriptive statistics and age-corrected maximum likelihood twin correlations for the somatization scale. Both mean and variance were significantly greater in female than in male twins in somatization (t = 6.08, p < .001; F = 17.88, p < .001). Age was modestly but significantly positively associated with somatization in both sexes (r = .11, p < .01 for males,r = .20, p < .01 for females), suggesting that somatization increases with age in both sexes in adolescents and young adults.
Table 1. Descriptive statistics and age-corrected maximum likelihood twin correlations for the somatization scale

Note: MZM = monozygotic male, DZM = dizygotic male, MZF = monozygotic female, DZF = dizygotic female and OSDZ = opposite-sex dizygotic twins. 95% CIs are in square brackets.
MZ twin correlations were consistently greater than DZ twin correlations for somatization in both sexes, indicating the presence of genetic influences. The patterns of twin correlations suggested that shared environmental influences were negligible. These informal observations were tested using model-fitting analysis described below.
Univariate Model Fitting for Somatization
The results of the general sex-limitation, model-fitting analysis for the somatization scale showed that there were no significant sex-specific genetic effects (Δχ1 2 = 0.42, p < .52), and that the magnitudes of additive genetic and shared environmental influences were not significantly different between the two sexes (Δχ4 2 = 3.85, p < .52). Furthermore, while additive genetic effects were significant (Δχ5 2 = 23.3, p < .00), shared environmental influences were not significant in any of the two sex groups (Δχ 5 2 = 3.85, p < .57). The best-fitting univariate model for the somatization scale yielded additive genetic effects of .44 (95% CI [.37, .51]) and individual-specific environmental effects of .56 (95% CI [.50, .63]) for both sexes.
Bivariate Model Fitting
As there were no significant sex differences in additive genetic and individual-specific environmental effects in HB (Hur et al., Reference Hur, Choi, Kim, Jin and Lee2018) or somatization prior to bivariate model-fitting analysis, we combined males and females and corrected the data for the effects of sex, age, age2 and sex × age by using a regression procedure (McGue & Bouchard, Reference McGue and Bouchard1984). Age- and sex-corrected phenotypic correlation between somatization and HB was .53 (p < .001). Age- and sex-corrected cross-twin, cross-trait correlations were .30 (p < .01) for MZ twins and .08 (ns) for DZ twins, indicating significant genetic influences on the phenotypic relationship between somatization and HB.
Table 2 presents the results of bivariate model-fitting analysis. Comparison of -2LL between models 2 and 3 indicated that while additive genetic variances and covariance between somatization and HB were significant, corresponding shared environmental variances and covariance were not. Removing additive genetic and individual-specific environmental covariances from model 3 worsened model fit (models 4 and 5), which suggested that both additive genetic and non-shared environmental covariances should be retained in the model. From these model comparisons using LRT, model 3 was chosen as the best fit. In agreement with the results from LRT, AIC suggested that model 3 was the best because it showed the lowest value.
Table 2. Bivariate model-fitting results for Hwabyung symptoms and somatization
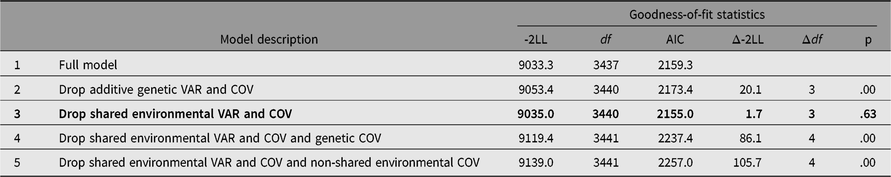
Note: Best-fitting model is indicated in bold. LL = log-Likelihood. VAR = variance, COV = covariance. 95% CIs are in parenthesis.
Figure 1 shows standardized path coefficients, and Table 3 summarizes additive genetic and individual-specific environmental variance estimates and correlations derived from the best-fitting bivariate model. Additive genetic and environmental effects were, respectively, .43 (95% CI [.36, .50]) and .57 (95% CI [.50, .64]) for somatization and .44 (95% CI [.37, .51]) and .56 (95% CI [.49, .63]) for HB. These estimates were very close to the results of univariate model-fitting analyses. Of 43% of the additive genetic variance of somatization, 23% were explained by additive genetic variance unique to somatization, and the remaining 20% were those shared with HB. Of 57% of the individual-specific environmental variance of somatization, 48% were those unique to somatization, whereas only 9% were those shared with HB.

Fig. 1. Path coefficients in the best-fitting bivariate model.
Table 3. Phenotypic correlation between somatization and Hwabyung and a summary of parameter estimates derived from the best-fitting bivariate model
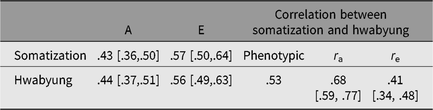
Note: 95% CIs are in square brackets. A = additive genetic factor, E = individual-specific environmental factor including measurement error.
The best-fitting bivariate model also yielded .68 (95% CI [.59, .77]) for genetic and .41 (95% CI [.34, .48]) for individual-specific environmental correlations between somatization and HB. These correlations suggested that 57% (
![]() ${{\left( {\surd .43{\,\,\rm{*}\,\,}\surd .44{\,\,\rm{*}\,\,}.68} \right)} \over {\left( {\surd .43{\,\,\rm{*}\,\,}\surd .44{\,\,\rm{*}\,\,}.68} \right) + \left( {\surd .56{\,\,\rm{*}\,\,}\surd .57{\,\,\rm{*}\,\,}.41} \right)}}$
) of the phenotypic correlation between somatization and HB (r = .53) were associated with shared genes, with the remainder being due to shared individual-specific environment between somatization and HB, including correlated measurement error.
${{\left( {\surd .43{\,\,\rm{*}\,\,}\surd .44{\,\,\rm{*}\,\,}.68} \right)} \over {\left( {\surd .43{\,\,\rm{*}\,\,}\surd .44{\,\,\rm{*}\,\,}.68} \right) + \left( {\surd .56{\,\,\rm{*}\,\,}\surd .57{\,\,\rm{*}\,\,}.41} \right)}}$
) of the phenotypic correlation between somatization and HB (r = .53) were associated with shared genes, with the remainder being due to shared individual-specific environment between somatization and HB, including correlated measurement error.
Discussion
To the best of our knowledge, this is the first twin study of somatization symptoms in East Asians. Our means of somatization in Table 1 were higher than those reported in the US standardization sample of the PAI (Morey, Reference Morey2007), replicating prevailing findings that somatization symptoms are higher in Asians than in Western populations (Dere et al., Reference Dere, Sun, Zhao, Persson, Zhu, Yao and Ryder2013). The magnitude of heritability of somatization found in the present study was at the higher end of the heritability estimates reported from Western twin samples (Bartels et al., Reference Bartels, van de Aa, van Beijsterveldt, Middeldorp and Boomsma2011; Gillespie et al., Reference Gillespie, Zhu, Heath, Hickie and Martin2000; Hansell et al., Reference Hansell, Wright, Medland, Davenport, Wray, Martin and Hickie2012; Kendler et al., Reference Kendler, Walters, Truett, Heath, Neale, Martin and Eaves1995; Vassend et al., Reference Vassend, Roysamb and Nielsen2012). Our finding of no significant shared environmental influences on somatization was consistent with those found in Western twin samples. If Asian cultures that increase the mean level of somatization influence Koreans at the family or neighborhood level, then this would increase shared environmental estimate in our model. However, the near-zero-shared environmental estimate found in our study suggests that this may not be the case. Our findings of significant genetic and individual-specific environment variance components suggest that Asian cultures may exert their influences by interacting with genes and/or individual environment for somatization. Rao et al. (Reference Rao, Young and Raguram2007) showed that more Westernized psychiatric patients tended to present more psychological than somatic symptoms in India, suggesting that the degree of Westernization influences presentation of somatic symptoms. Although these results need to be reconciled with the findings from studies of Asian immigrants in Western countries who showed higher level of somatization than did their European American counterparts (Hsu & Folstein, Reference Hsu and Folstein1997), it would be of interest for future studies to examine how exposures to Western cultures moderate genetic and environmental influences on somatization to better understand the role of cultures in the development of somatization.
Interestingly, sex differences in heritability of somatization often found in Western samples were not observed in our sample. One could argue that our study was underpowered to detect sex differences in genetic influences as model-fitting analysis requires a large sample (Neale & Cardon, Reference Neale and Cardon1992). However, the patterns of MZ and DZ twin correlations were very similar in both sexes (see Table 1), suggesting that the same magnitude of genetic influences on somatization in males and females may be real in South Korean adolescents and young adults. In descriptive analyses, we found that somatization increased with age in both sexes, although the increase was more pronounced in girls than in boys. However, prior studies based on Western twin samples of similar ages found very different patterns. For example, Hansell et al. (Reference Hansell, Wright, Medland, Davenport, Wray, Martin and Hickie2012) showed that somatic symptoms increased with age in girls, but the symptoms either decreased or showed no significant change in boys. Taken together, our results suggest that the sex-specific developmental course of somatization may differ across South Korean and Western adolescents and young adults. Crosscultural twin studies may be needed in the future to resolve the issue of sex differences in genetic and environmental influences on somatization.
To date, numerous candidate genes were speculated to be involved in the presentation of somatic symptoms (Yu et al., Reference Yu, Gwinn, Clyne, Yesupriya and Khoury2008), but genome wide association studies (GWAS) for somatization have not been very successful. As far as we understand, GWAS for HB have not been published. Using multiple study cohorts (n = 32,528 for the discovery sample, n = 6813 for the replication sample), Demirkan et al. (Reference Demirkan, Lahti, Direk, Viktorin, Lunetta, Terracciano and Räikkönen2016) conducted a meta analysis of GWAS of somatic complaints measured by the Center for Epidemiological Studies Depression (CES-D) scale and found evidence for one single-nucleotide polymorphism (SNP) near the brain-expressed melatonin receptor (MTNR1A) gene associated with somatic complaints (p = 3.82 × 10−8) in the discovery sample. However, the SNP was not consistently replicated in the replication sample. Somatizers with the same physical symptoms may share enhanced genetic susceptibilities to experience certain physical pain. Thus, future GWAS researchers may consider searching genes specific to physical symptoms to reduce heterogeneity in the phenotype of somatization. Additionally, as only individuals of European descent were included in the study by Demirkan et al., future GWAS should determine whether MTNR1A is associated with somatic complaints of individuals of Asian ancestry.
We found a significant phenotypic correlation between HB and somatization in the present sample (r = .53), confirming the co-occurrence of HB and somatization. A single gastrointestinal problem item was similar in both measures, although the item was worded somewhat differently in the two measures. We computed the phenotypic correlation between HB and somatization after removing this item, which only slightly reduced the size of the phenotypic correlation (r = .50).
Genetic correlation of .68 found in our study suggested that genes associated with anger may be responsible for somatization as well. Prior studies have shown that patients of severe somatoform disorders had elevated scores in anger dimensions such as trait anger, state anger, angry temperament and a strong tendency to express anger toward other persons or objects (e.g., Kämpfer et al., Reference Kämpfer, Staufenbiel, Wegener, Rambau, Urbach, Mücke and Conrad2016). Common genetic factors in somatization and HB found in the present sample may also include genetic influences on stigma-avoidance coping skills because coping style has been shown to be heritable (Hur et al., Reference Hur, MacGregor, Cherkas, Williams and Spector2012).
Individual-specific environmental correlation between HB and somatization (r = .41) was significant but lower than the genetic correlation (r = .68). Of individual-specific environment factors of 57% that influence somatization, only 9% were those shared with HB, suggesting that environmental factors that affect somatization may be largely independent from those impacting HB. Specific environmental factors that have been studied for the development of somatization include insecure attachment in childhood (Ciechanowski et al., Reference Ciechanowski, Walker, Katon and Russo2002), experience of sexual abuse and other trauma during childhood (Brown et al., Reference Brown, Schrag and Trimble2005; Imbierowicz & Egle, Reference Imbierowicz and Egle2003), parental criticism (Horwitz et al., Reference Horwitz, Marceau, Narusyte, Ganiban, Spotts, Reiss and Neiderhiser2015; Repetti et al., Reference Repetti, Taylor and Seeman2002) and excessive concern and preoccupation with the child’s symptoms (Shulte & Petermann, Reference Schulte and Petermann2011). In contrast, family conflicts, serious family financial problems and interpersonal relationship problems have been shown to be leading risk factors for the onset of HB (Kim et al., Reference Kim, Chung, Suh, Jung, Lee, Kim and Kim2010). One should note that some of these specific environmental factors may be correlated and/or interact with genes for somatization and HB. Thus, future investigations using G × E modeling and extended twin designs will be need for deeper understanding of the developmental origins of HB and somatization.
Our research has some limitations. First, our data were obtained on only one occasion. Thus, temporal order of the occurrence of HB and somatization symptoms is unclear. Second, our assessment was based on self-reports without clinical diagnoses, and our sample was drawn from the general population. Thus, our results may not translate into clinical samples suffering from severe forms of HB and somatization. Third, as we did not assess medical conditions of our twins, some of our twins may have genuine physical illnesses, although this is less likely because our twins are relatively young. Fourth, our sample consisted of adolescents and young adults. Given that somatization increased with age in our sample, the conclusions of the present study should be limited to the age group we studied. Finally, our sample only included South Korean twins. Given the cultural variation in somatization and HB, future studies should explore the generalizability of our findings to other ethnic groups.
Acknowledgment
This work was supported by the ‘Development of Health Prediction Technology based on Big Data’ (K18092) funded by the Ministry of Science and ICT(MSIT) of Korea, given to the Korea Institute of Oriental Medicine (KIOM).






