INTRODUCTION
Anthrax, primarily a zoonotic disease, has been brought back to the public's attention by its deliberate release in the postal system in the USA in 2001 [Reference Dewan1]. Caused by the Gram-positive spore-forming bacterium Bacillus anthracis, anthrax shows different forms of clinical disease in humans, including inhalation anthrax, which has a high case-fatality rate, and cutaneous anthrax, which is the most frequent form of disease and has low mortality. Transmission is through exposure to spores either through inhalation or handling of infected animals or contaminated animal products such as skins or hairs.
Nowadays anthrax is most often agriculturally related, although it also used to be an industrial disease. At the end of the 19th century, anthrax was known as ‘wool sorter's disease’ [Reference Bell, Fee and Brown2]. Although today wool processing is a rare industry in the Western industrialized world, it was common in the late 19th and first half of the 20th century, particularly in the UK and the USA [Reference Bell, Fee and Brown2, Reference Brachman and Fekety3]. Despite the decline of this industry, outbreaks of anthrax in textile factories were reported in the latter half of the 20th century in the USA and in Switzerland, and contaminated goat hair or wool was the primary vehicle of infection in all outbreaks [Reference Bales4–Reference Pfisterer6]. Exposure to raw goat hair was identified as a risk factor for acquiring anthrax [Reference Brachman and Fekety3, Reference Pfisterer6]. Nowadays, goat hair is still imported from countries where anthrax is endemic, such as Central Asia and the Middle East. Higher incidence of anthrax was reported in persons involved in the early stages of the processing of goat hair, including sorting, carding and combing [Reference Brachman and Fekety3, Reference Metcalfe7]. Since 2006 cases of inhalation and cutaneous anthrax related to drumming and drum making have also been reported in the UK and the USA [8–10].
The level of disease risk associated with a low degree of exposure to aerosols containing B. anthracis is unclear. Evidence of subclinical infection was already shown in the 1950s in a study describing positive serology in exposed asymptomatic persons [Reference Norman11]. In the US Capitol 2001 deliberate anthrax release, no associations were found between clinical disease, exposure to spores and immune response [Reference Doolan12]. Antibody responses were also detected in persons with low-level exposure to anthrax spores. The protection provided by immune response in asymptomatic infection is unclear.
A recent study investigated samples from a Belgian factory that processes wool and goat hair from all over the world [Reference Wattiau13]. In 2006, living anthrax spores were found in goat-hair fibres, air dust collected in the goat-hair processing area, and unprocessed wastewater produced from goat-hair scouring [Reference Wattiau14]. Concentrations of anthrax spores in the air increased during the processing of raw goat hair, to peak at around one spore in 5 litres of air. No confirmed case of anthrax had been recorded in the factory employees.
Anthrax vaccines are not licensed in Belgium and thus not available nor recommended for occupational exposure. No employees had been vaccinated against anthrax, but other protective measures were available at the factory, such as systematic disinfection, air filtering in the wool-processing rooms and protective gear for employees working with raw goat hair. Workers and local physicians were informed about the risk of anthrax, and received guidance on how to recognize and manage anthrax. After findings of anthrax spores in June 2006, the factory reinforced the recommendation to employees working with raw wool and goat hair to wear filter face piece (FFP) masks. In addition, the question as to whether it was feasible to offer anthrax vaccination to workers was raised. As only some of the workers were in contact with raw goat hair, the occupational medicine service aimed to determine who was at risk from anthrax exposure.
As part of the occupational medicine intervention following the findings of anthrax spores in the factory, we conducted a seroprevalence study among the factory personnel. We have described the immunological responses of workers in a recent publication [Reference Wattiau14]. In the current study, we evaluated the risk factors associated with positive serology in order to help advise on protective measures. A secondary objective was to contribute towards the knowledge of antibody response to B. anthracis in a population not vaccinated against anthrax and regularly exposed to low doses of anthrax spores in a natural setting.
METHODS
We conducted a seroprevalence study in two points in time and investigated self-reported risk factors. All persons employed by the wool factory in the period January 2006 to December 2007 were invited to participate in the study. The first survey took place on 11 December 2006 and the follow-up survey on 17 December 2007. All participants signed an informed consent form.
Serological testing
Serological analyses, using a commercial ELISA kit for detection of the presence of specific anti-protective antigen (PA) IgG (Serion, Germany) have been reported previously [Reference Wattiau14]. This commercial kit was developed to assess antibody levels indicating protection after vaccination. Because the purpose of this seroprevalence study was to assess antibody response and not vaccine-induced immune protection, we established a cut-off based on results from presumed anthrax-naive control subjects [Reference Doolan12, Reference Biagini15]. A positive anti-PA IgG result was defined as the mean value plus 3 standard deviations (s.d.) of the optical density of 71 uninfected controls. A borderline case was defined as a result ranging between 2 and 3 s.d. of the mean.
Data collection and analysis
Data on risk factors and clinical symptoms during the past 12 months were collected in face-to-face interviews using standardized questionnaires.
In the factory, warehousemen transport bales of wool or goat hair from the storage area to the processing area. There, people working on machines processing raw wool or goat hair (MPW) load the wool or goat hair onto the machines that pull it into small pieces and remove larger impurities. The fibres are then dropped down into the scouring area, where they are cleaned and disinfected in five successive washing vats. After drying at a very high temperature, wool, but not goat hair, is frequently processed further using chemicals to eliminate any remaining impurities. This is called the ‘carbonization’ process and is performed in the carbonization hall. Carbonization workers often rotate with MPW workers. Wool sorters then sort this highly treated wool. Other wool sorters work predominately at the beginning of the processing stage with raw wool and goat hair. At a final stage, packers take the processed wool or goat hair and pack and wrap it tightly; occasionally they also pack raw material.
Risk factors included type of job performed, use of protective material (masks, gloves), contact with raw goat hair, and underlying medical problems. As an initial study in the factory showed that the presence of anthrax spores was related to the processing of raw goat hair and not of wool [Reference Wattiau13], we also measured the level of exposure to raw goat hair. Factory records on exact numbers of hours a person worked on a given goat-hair load were available for MPW workers. From this, cumulative hours worked with goat hair were calculated. Anyone working >12 days per year processing goat hair on MPW were considered as MPW workers (maximum days worked on MPW with goat hair by anyone was 24·7 days/year). Employees who were never in contact with raw goat hair and worked in other premises (warehousemen, packers, administrative staff) were included in the ‘low exposure’ group, and considered as the reference group. Air samples collected in a control area, where no industrial activity occurred but at the time goat hair was processed on the upper floor, showed a slight increase in air-suspended particles [Reference Wattiau13]. The concentration was, however, much lower than in the goat-hair loading area (maximum 25 000 particles 5 μ/litre of air compared to around 1000/litre in the control area), indicating a substantially lower exposure to B. anthracis.
Factors associated with positive B. anthracis serology were determined based on the first (2006) survey. Prevalence ratios for risk factors were obtained using Poisson regression with robust variance. A priori risk factors and risk factors significant to the 0·25 level at univariate analysis were added into a multivariable model to adjust for confounding factors. Serological results from the follow-up survey were used to identify the subjects who seroconverted during the study period.
All statistical analyses were performed in Stata SE 9.2 (Stata Corp., USA).
RESULTS
In the first survey, 66/67 eligible workers provided serum samples and were interviewed (response rate 98·5%). Study participants were aged from 29 to 62 years (mean 50 years). Twelve (18·2%) persons suffered from chronic underlying disease (chronic bronchitis, asthma, emphysema, diabetes). The majority (52/66) of participants had worked in the factory for more than 20 years (Table 1). Twenty (30·3%) workers, working either on MPW or sorting goat hair, reported regular close contact with goat hair.
Table 1. Distribution of risk factors in study participants and univariate results [prevalence ratio, 95% confidence intervals (CI) and P value] for association with positive B. anthracis serology
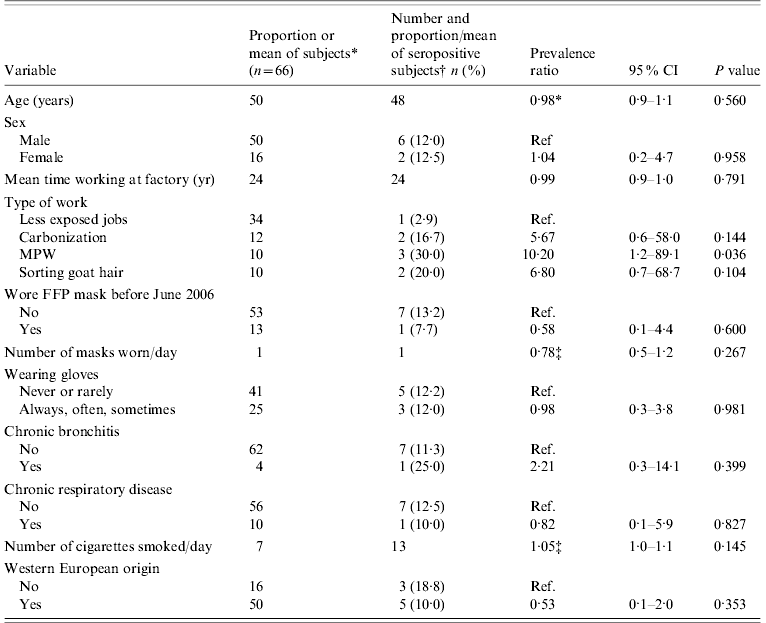
MPW, Machines processing raw wool or goat hair; FFP, filter face piece.
* Distribution of study participants (categorical variables) or mean of study participants (continuous variables).
† Percent for categorical variables and mean for continuous variables.
‡ Increase in prevalence for each unit increase of the continuous variable.
The 71 negative control sera had a mean anti-PA IgG value of 1·02 U/ml with a s.d. of the sample at 1·27 U/ml, yielding a positive cut-off of 4·84 U/ml anti-PA IgG and a cut-off for a borderline case at 3·57 U/ml anti-PA IgG. Eight (12·1%) workers were seropositive in survey 1. No clinical cases of either inhalation or cutaneous anthrax were reported in the last 12 months. However, one person described symptoms that were clinically compatible with cutaneous anthrax, occurring in 2002, although no laboratory confirmation had been performed.
At the univariate level, the type of job was associated with a positive serology. MPW workers, wool sorters and carbonization workers were, respectively, 10, 7 and 6 times more likely to be seropositive compared to those from the low-exposure group, but the associations were only significant for MPW workers (Table 1). Chronic disease was not significantly associated with seropositivity. An increase in the number of cigarettes smoked significantly increased the risk of being seropositive at the 0·25 level.
In multivariable analyses adjusting for mask use, persons working on MPW, sorting raw goat hair and working in the carbonization hall were, respectively, 44, 15 and 43 times more likely to be seropositive compared to the low-exposure group (Table 2). An increase in the number of masks used per day decreased the probability of this outcome significantly. Wearing gloves also decreased the risk but not significantly. The model containing the type of job performed and the number of masks used per day explained only about 25% of the variation in seropositivity between individuals.
Table 2. Multivariable model of risk factors for association with positive B. anthracis serology
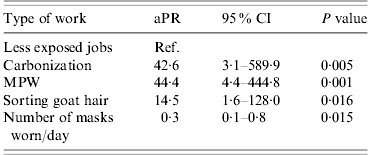
aPR, Adjusted prevalence ratio; CI, confidence interval; MPW, machines processing raw wool or goat hair.
Variables are adjusted for each other in the model.
Cumulative exposure
In total nine goat-hair loads were processed in 2006. Factory data reported that 16 people worked on these goat-hair loads on MPW (including two carbonization hall workers). Four (25%) of these were seropositive. At univariate analysis, the probability of being seropositive significantly increased with the number of days working on MPW without a mask (Table 3). The association was no longer significant when all working days were considered (using a mask or not).
Table 3. Univariate analysis in persons reported working on MPW with raw goat hair in 2006 (n=16), risk factors for association with positive B. anthracis serology

CI, Confidence interval; MPW, machines processing raw wool or goat hair.
* Increase in prevalence for each day worked.
Comparison of serological results over time
In the second survey (2007), 52 persons from the 66 subjects in the first survey participated, along with two persons who had just started working at the factory in 2007 (response rate 79%). Thirteen (24·1%) out of 54 persons were found seropositive. Everyone seropositive in the first study remained seropositive in the second study.
Individual serological results did not vary much on average over the two surveys (Fig. 1). However, five persons seroconverted between the two periods (Table 4), although no factors associated with seroconversion could be identified, due to sparse data. One seroconverted MPW worker reported having chronic bronchitis during the year prior to the second survey, but not in the first survey. A further seroconverted MPW worker worked 9 more days on goat hair in 2007 compared to 2006. These two persons reported using FFP masks frequently. The three remaining seroconverted workers reported less frequent or no mask use.
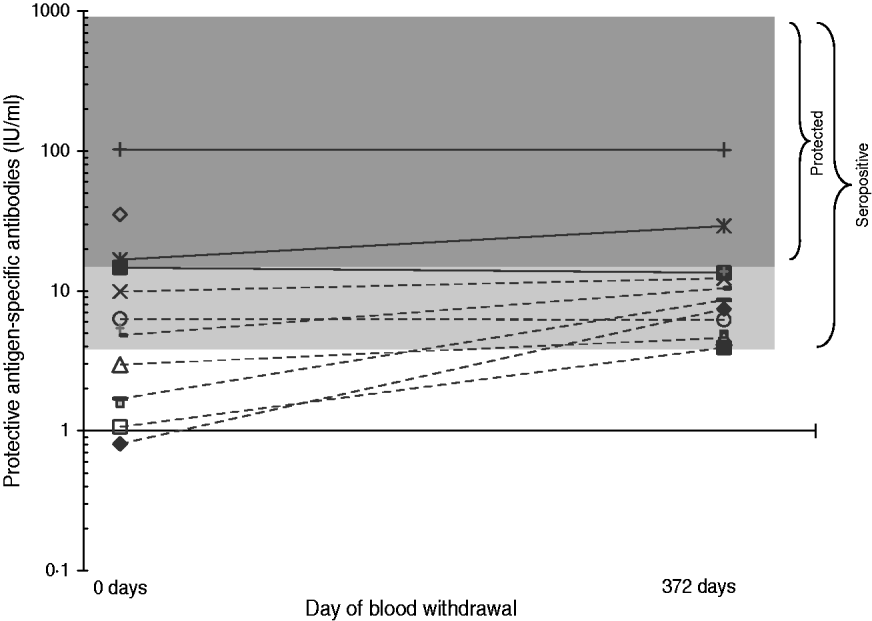
Fig. 1. Protective antigen-specific antibodies of B. anthracis (IU/ml) for persons with positive B. anthracis serology; data from first blood sampling (11 December 2006) and second blood sampling (17 December 2007), logarithmic scale. Continuous lines indicate persons positive or borderline using the ELISA cut-off for vaccine protection in survey 1.
Table 4. Anti-protective antigen IgG level, and serostatus for all workers testing positive in survey 1 (December 2006) or at follow-up (December 2007)
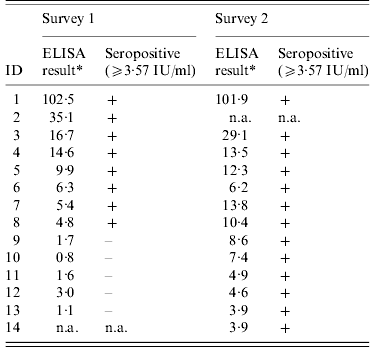
+, Positive result; –, negative result; n.a., did not participate in this study.
* Mean ELISA result given here where more than one test was performed.
DISCUSSION
In this wool-processing factory, a previous investigation demonstrated that workers were repeatedly exposed to low doses of anthrax spores to varying degrees. In 2006, 12% of workers showed positive serology for B. anthracis, in the absence of clinical disease and anthrax vaccination. We showed that working with raw goat hair in the early stages of processing was significantly associated with positive serology, and wearing a mask was protective. Other seroprevalence studies using sensitive assays also showed that sizable percentages of unvaccinated persons highly exposed to anthrax spores had evidence of humoral immune response without history of clinical infection, and these responses also occurred mainly in persons with higher levels of exposure [Reference Bales4, Reference Norman11, Reference Doolan12, Reference Hadler16].
We also found that workers more exposed to the risk of aerosolization of spores, such as those working on wool-processing machines, were more likely to be seropositive than those non-exposed, and masks protecting against inhalation of spores had a protective effect. These findings suggest that seroconversion may have followed inhalation of spores. The concentration of anthrax spores found in the factory's air dust was close to the rates observed in a Manchester goat-hair processing mill in 1957 where an anthrax epidemic was reported [Reference Wattiau13, Reference Dahlgren17]. However, several factors differed in our study and may explain the lack of clinical cases: the factory was equipped with air extractors, workers were wearing protective gear, exposure to spores was transient as spores were only found in air dust when raw goat hair was processed, and the population at risk was much smaller [Reference Wattiau13]. A mathematical model showed that modest increases in room ventilation are likely to decrease risk for anthrax inhalation considerably [Reference Fennelly18]. Appropriate ventilation should be ensured in such settings.
More days worked with goat-hair on wool-processing machines without wearing a mask was associated with a higher seropositivity risk, suggesting a dose-dependent association between exposure and antibody response. Moreover, persons in jobs less exposed to contaminated animal products (including administrative and laboratory staff, warehousemen, packers) were less likely to develop a humoral response to B. anthracis. An exposure–dose trend was also suggested in seroconverted subjects in the US Capitol deliberate anthrax release [Reference Doolan12].
However, other factors than the level of exposure to anthrax spores seem to play a role in seroconversion. While prolonged exposure to raw goat hair was associated with seropositivity in this study, it was not predictive. Many workers with almost identical exposure to raw goat hair, similar use of protective equipment, similar age and length of time working at the factory as those who were seropositive, showed negligible antibody titres. These findings suggest that antibody response varies from person to person even within an exposure group; the job performed and mask use only explained only 25% of the variation between the serological results of individuals. However, the small sample size of our study limited its power to identify other true associations with seropositivity. Past studies in goat-hair processing mills and in the 2001 deliberate anthrax release hypothesized that underlying medical conditions could explain variations in symptomatic disease between individuals [Reference Dewan1, Reference Bales4]. However, no association could be shown between underlying medical condition and positive serology in our study, possibly due to small numbers.
Similarly, some individuals presumed to have low exposure were seropositive, such as a packer and a warehouseman in 2007 who did not work on wool-processing machines or wool sorting. This suggests that low or indirect exposure to anthrax spores may result in (asymptomatic) infection. Similarly in the US Capitol deliberate anthrax release, persons previously deemed unexposed – but working in the Capitol – showed positive serology [Reference Doolan12]. In past anthrax outbreaks in the 1950s, persons not directly exposed to raw goat hair but living within the vicinity contracted anthrax disease, including wives of factory workers and a secretary at a goat-hair processing mill [Reference Bales4, Reference Metcalfe7, 19]. Similarly, samples from the mail distribution centre in Washington, where envelopes containing B. anthracis were processed, showed that spores were widely dispersed into the areas surrounding the processing machines [Reference Sanderson20].
In our study, no factory worker among the eight persons who showed positive serology in 2006 had a laboratory-confirmed history of anthrax. One worker had a history of a lesion clinically compatible with cutaneous anthrax 4 years before the first survey but displayed negative B. anthracis serology. By contrast, he tested positive by lymphocyte proliferation assay, demonstrating cell-mediated immunity, as well as by Western blot and dot blot [Reference Wattiau14]. Cellular immune responses in the absence of clinical disease and antibody response have also been reported in the Capitol anthrax outbreak, in persons who had taken post-exposure prophylaxis with antibiotics [Reference Doolan12, Reference Hadler16]. These authors suggest that asymptomatic infection could be due to antibiotics suppressing spore germination. In the current study, six of the eight seropositive cases reported not taking antibiotics in 2006, suggesting that asymptomatic infection can occur in the absence of antibiotic prophylaxis. But a difference in our study is that workers were repeatedly exposed to anthrax spores and not to a single exposure as in deliberate release events.
In this factory, individual serological titres showed limited variations over the two surveys, and all seropositive workers in the first study remained positive in the second study. Serological titres showed more variation over a similar time period after vaccination with UK human anthrax vaccine as well as in patients with anthrax inhalation [Reference Grunow21, Reference Quinn22]. However, in patients with cutaneous anthrax serological titres were lower and varied less, particularly from 50 to 175–400 days after symptom onset. Data comparing the levels of antibodies in asymptomatic seropositive persons over lengthy time periods are not available from other studies. In our study, the repeated exposure to anthrax spores might play a role in the relative stability of ELISA results from 2006 to 2007. Even the worker who had a very high ELISA titre in 2006 showed a similar result in 2007.
Our study has several limitations. First, the small sample size of our study, due to the small size of the factory, limited the study's power to identify associations with seropositivity, especially when adjusting for confounders in a multivariable model. Only very strong associations became statistically significant. However, the 98·5% response rate indicates that we have a correct picture of the factory workers, but inference to other populations is limited by the small sample size. Second, any misclassification of employees in terms of job performed and mask use could impact strongly on associations given the small sample size of the study. Third, given the cross-sectional design of this seroprevalence study design we could not assess temporal relationships between exposure and seropositivity. Fourth, validation studies on anthrax serology are scarce and results vary across studies. Serological studies performed by CDC in postal workers after the anthrax bioterrorism attacks in 2001 showed no subject with detectable antibody levels, but the use of a more sensitive assay by Doolan et al. [Reference Doolan12] found that 40% of highly exposed but unvaccinated persons had humoral immune responses to B. anthracis PA in a similar bioterrorism setting in the Capitol [Reference Dewan1]. Finally, we only tested antibodies against PA, not against lethal factor (LF), based on the greater immunogenicity of the former [Reference Abboud and Casadevall23]. While PA-specific antibodies have been shown to correlate with protection, antibodies to protective antigen only develop in 68–92% of cases of cutaneous anthrax [Reference Quinn22, Reference Swartz24]. For instance, a case of cutaneous anthrax in a Belgian traveller showed detectable anti-LF antibodies in the absence of detectable anti-PA antibodies [Reference Van den Enden, Van Gompel and Van Esbroeck25]. We may have missed cases with humoral response, by only testing for anti-PA IgG.
This study contributes to the knowledge on immune response of and risk factors for persons exposed to anthrax spores in an industrial setting. Exposure to repeated low doses of anthrax spores can cause an anti-PA IgG response to B. anthracis in the absence of clinical disease, antibiotic prophylaxis and vaccination. Results suggest a dose–response association between exposure and antibody responses but also by the influence of protective gear and individual characteristics. Although the absence of confirmed clinical cases throughout the years suggests that the risk of acquiring anthrax is negligible, protective measures are essential to prevent a potentially fatal disease and should not be limited to workers directly exposed to raw goat hair. Safe anthrax vaccines should be available to protect exposed workers from disease. More research is needed in methods to assess the risk of disease in populations exposed to anthrax spores and to determine which control measures are effective.
ACKNOWLEDGEMENTS
The authors acknowledge the director and employees of the wool and goat-hair processing company for their helpful collaboration, Mieke Van Hessche for technical assistance, the nurses of Provikmo, Belgium, for taking the blood samples and Dimitrios Frangoulidis from the Bundeswehr, Germany, for his support with the ELISA testing. Special thanks to Stefan Brockman, Landesgesundheitsamt Baden-Württemberg, Stuttgart, Germany, for helpful discussions and critical reading of the article and Herman van Oyen, Institute of Public Health, Belgium, for review of the article.
DECLARATION OF INTEREST
None.







