Introduction
The clinical research professional (CRP) community of practice supports the clinical and translational research (CTR) endeavors of academic medical centers (AMC). This practice group includes a variety of specialization areas: clinical research nurses, clinical research coordinator professionals; data management and informatics professionals; regulatory affairs professionals; and other supervisory, administrative, financial, and management professionals dedicated to sustaining the research work. Ancillary to these are medical center professionals working in laboratories, imaging, medical records, and other healthcare areas. Principal investigators (PIs), faculty and junior faculty working in CTR rely on CRPs to process, conduct, maintain, and sustain upcoming and ongoing clinical research studies. Together, this matrix of interrelated and overlapping faculty and staff roles are part of the AMC landscape of practice for CTR. Clinical research has grown increasingly complex whereby study protocols have a growing number of visit-related assessments and complex operations; Phase II and III studies may involve over 250 procedures per participant and nearly 20 endpoints [Reference Getz and Campo1,2]. Study populations are also more complex as research seeks to solve unmet needs across multiple acute, chronic, and rare disease populations [2]. Current gestalt about CTR team science includes a recognition of the wide spectrum of CRPs engaged in the common aims across studies, tackling complex medical problems, social determinants of health, environmental health issues, and increasing collaborative research, including decentralized approaches for study recruitment and management [Reference McCormack and Strekalova3].
Careers in CTR operations showed an expected 17% growth rate in 2017; however, those projections under-report the current workforce needs for the ever-changing CTR enterprise, especially in the post-COVID-19 era [Reference Roberson4]. The U.S. Bureau of Labor Statistics reported over 4 million Americans resigned from their positions in September 2021 [Reference Kiersz and Kaplan5]. Notably, resignations are highest among the healthcare industry where there has been a reported 3.6% increase in health care employees leaving their positions within the past year [Reference Cook6]. Likewise, AMCs have suffered high CRP turnover rates due to uncertainty of job expectations and vague professional development opportunities, including a lack of role progression pathways, and disappointment with training [7,Reference Henderson8]. Some leave positions for higher paid opportunities with Contract Research Organizations and pharmaceutical sponsors, who also report larger than average job openings. COVID-19 impacted CRP staffing as well; pre-pandemic shortages were reported as being at 15% and subsequently rose to 29% post-pandemic, with AMCs anticipating widespread shortages, significant staffing shifts to hybrid (on-site and remote) work settings, with both productivity and burn-out increasing simultaneously [9,10]. A recent survey found that dated institutional practices were major contributors to AMC clinical research staffing issues, demonstrating the little investment AMCs have made in CRPs, such as lack of uniform job descriptions, inadequate salaries, and ill-defined progression pathways [Reference Buchanan, Goldstein, Pfalzer, Lin, Kang and Claassen11]. The survey also found that positive impacts on job satisfaction and retention were PI-related, such as PI engagement and relationship with CRPs [Reference Buchanan, Goldstein, Pfalzer, Lin, Kang and Claassen11].
Many AMCs and PIs have underappreciated the professional nature of CRP roles and the need to focus attention on current best practices for job titles, job descriptions, career planning, and retention. Institutional and cross Clinical Translational Science Award (CTSA) hub CRP networks can help improve professional identity; however, it is difficult for many institutions to identify the individuals working as CRPs; which can hinder communication, career progression, and training [Reference Baedorf Kassis, Winkler, Gianforti and Needler12]. The current urgency in addressing CRP recruitment and retention is garnering national (and international) attention with multiple institutions reporting staff shortages. Staff shortages can have negative impacts on AMCs that may result in delaying or halting of research activities, adversely affecting compliance and quality in the research, jeopardizing patient safety, or leading to further staff dissatisfaction, causing more turnover in a vicious cycle [Reference Buchanan, Goldstein, Pfalzer, Lin, Kang and Claassen11]. As a benchmark example of proactively addressing CRP staffing issues, Duke University invested resources into establishing multiple CTR job descriptions and clearly defining job titles and levels of progression through a competency-based, tiered approach [Reference Brouwer, Hannah, Deeter, Hames and Snyder13,Reference Deeter, Hannah and Reyes14]. Such innovations decreased the overall turnover rate among CRPs locally. The initial financial investment was recouped through improved retention and reduced training costs [Reference Stroo, Asfaw and Deeter15,Reference Brouwer, Deeter and Hannah16]. Nevertheless, for those working in AMC clinical research and those considering future careers in clinical research, there remains a general lack of knowledge regarding the career pathways and the use of individual career development planning mechanisms for CRPs working at AMCs [Reference Buchanan, Goldstein, Pfalzer, Lin, Kang and Claassen11].
Attracting new hires is challenging. Novices to clinical research find it difficult to get their foot in the door for an initial position; citing obstacles such as the requirement for prior CTR experience in many job postings [Reference Kesling, Jones, Fritter and Neidecker17]. Therefore, describing recruitment and retention facilitators and barriers within the clinical research profession at AMCs will highlight the key issues and present common solutions to improving these stages of hiring and maintenance of the CRP workforce. This paper will discuss factors related to the current state of AMC CRP workforce needs related to recruitment, job titles, retention and diversity, as identified through a qualitative analysis of specific workgroup breakout sessions during the CRP Collaborative Conversations Un-Meeting series [Reference Jones, Lane, Shah, Carter, Lackey and Kolb18]. Our study aims to provide opportunities for National Center for Advancing Translational Science (NCATS) and AMC policy considerations and guidance regarding institutional improvements to sustainability of the CRP workforce for quality CTR operations.
Materials and Methods
Participants and Study Setting
In 2019, several individuals at different CTSA hubs developed a collaborative to explore and address key issues in workforce development of CRPs. The hubs included representatives from the University of Washington, University of Florida, The Ohio State University, and the University of Rochester, with administrative support from the Center for Leading Innovation and Collaboration (CLIC). Additionally, the Association of Clinical Research Professionals (ACRP) was included in the collaborative. The collaborative designed a series of Un-Meetings called “Collaborative Conversations: The Critical Need for Professional Workforce Development at Academic Medical Centers” devoted to discussion of CRP workforce development. The Un-Meeting series was promoted through a variety of email listservs and websites, geared primarily toward individuals involved in CRP workforce development, both at CTSA institutions and private clinical research organizations. Participants registered for Un-Meetings all at once, but attendance was tracked for each Un-Meeting. This study was reviewed by the Institutional Review Board and determined not to be human subjects’ research (IRB #2020E0066).
The Un-Meeting series was composed of six virtual meetings held via Zoom collaborative videoconferencing (Zoom Video Communications Inc., 2016). Table 1 provides a summary of Un-Meeting dates and topics. Each virtual Un-Meeting was 2 hours in length and consisted of a brief presentation to introduce the topic and agenda, followed by interactive activities and brainstorming.
Table 1. Un-Meeting series dates and topics
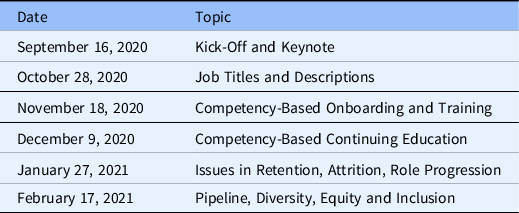
This study’s emphasis was on recruitment and retention issues within the CRP workforce and so the study team focused only on the Un-Meetings that occurred on October 28, 2020, January 27, 2021, and February 17, 2021.
Data Collection
Each Un-Meeting was recorded in Zoom, including all breakout sessions. The introductory session was brief, typically between 15 and 30 minutes, followed by approximately 45 minutes of moderated discussion in small group breakout rooms. In addition to the main session and breakout recordings, each breakout room had a scribe who took notes on the small group discussion and reported back to the full group upon conclusion of the breakouts. Finally, an electronic survey was distributed at each Un-Meeting as a mechanism for brainstorming on the topics in the remote format. The surveys were conducted via Qualtrics by placing the survey URL in the Zoom chat, and participants were given time to complete the survey. These surveys were composed of open-ended brainstorming questions designed to generate discussion of issues and possible solutions related to the topics of interest for each Un-Meeting. We captured and shared these brainstorming responses in the breakout rooms to help guide and augment small group discussions. This study utilized the qualitative data generated by the Un-Meeting Zoom recordings, any available chats and scribe notes from the main session and breakout rooms, and Qualtrics survey responses.
Data Analysis
A research team of 12 coders used an interpretive, inductive approach to analyze the qualitative data generated at the October, January, and February Un-Meetings. We identified one team member as the qualitative lead. This individual was trained in qualitative methods at the doctoral level and guided the group through each step of the analysis process, collating notes and moderating each analysis discussion. The team met for three months from August to October 2021. Each coder analyzed the data independently at first, and the team met bi-monthly to refine interpretations and reach intercoder agreement. We identified two volunteers to serve as lead analysts prior to each team meeting. The lead analysts were responsible for bringing their codes with themes to the meeting to which the rest of the team could respond to and discuss. The lead analysts rotated for each meeting so no individual led an analysis discussion more than once. The research team reviewed all qualitative data for one Un-Meeting at a time, whereby the research team maintained a flexible timeline of analyzing the October 2020 Un-Meeting data in August 2021, the January 2021 data were analyzed in September 2021, and the February 2021 data were analyzed in October 2021.
Results
Participants and Study Setting
In total, 130 participants attended these three Un-Meetings, representing 45 clinical research institutions (35 CTSA hubs; 4 IdEA Clinical Trial Centers; and 6 other institutions), and a diverse spectrum of professional appointments within the CRP workforce development domain. Table 2 provides details on study participants.
Table 2. Participant demographics
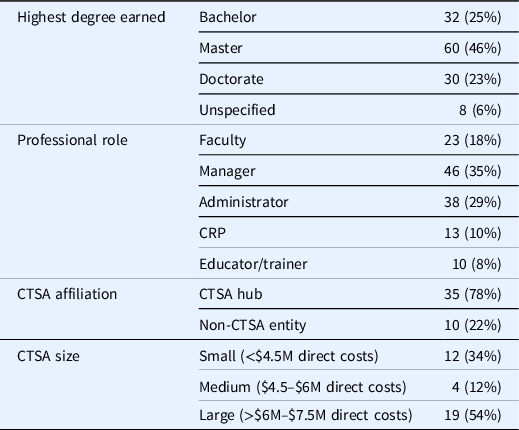
Note. CTSA = Clinical Translational Science Award; CRP = clinical research professional; M = million.
Data Collection
This study analyzed the qualitative data generated by the Un-Meeting Zoom recordings, any available chats and scribe notes from the main session and breakout rooms, and Qualtrics survey brainstorming responses. In total, the data set included approximately 1035 minutes of audiovisual data, or 17.25 hours, 18 conversations captured by Zoom chat, 16 summary note documents recorded by breakout room scribes, and 127 brainstorming responses to Qualtrics surveys. Table 3 summarizes the survey questions, data sources, and data details for each Un-Meeting included in the data set.
Table 3. Participants, survey questions, and data sources/details for each Un-Meeting
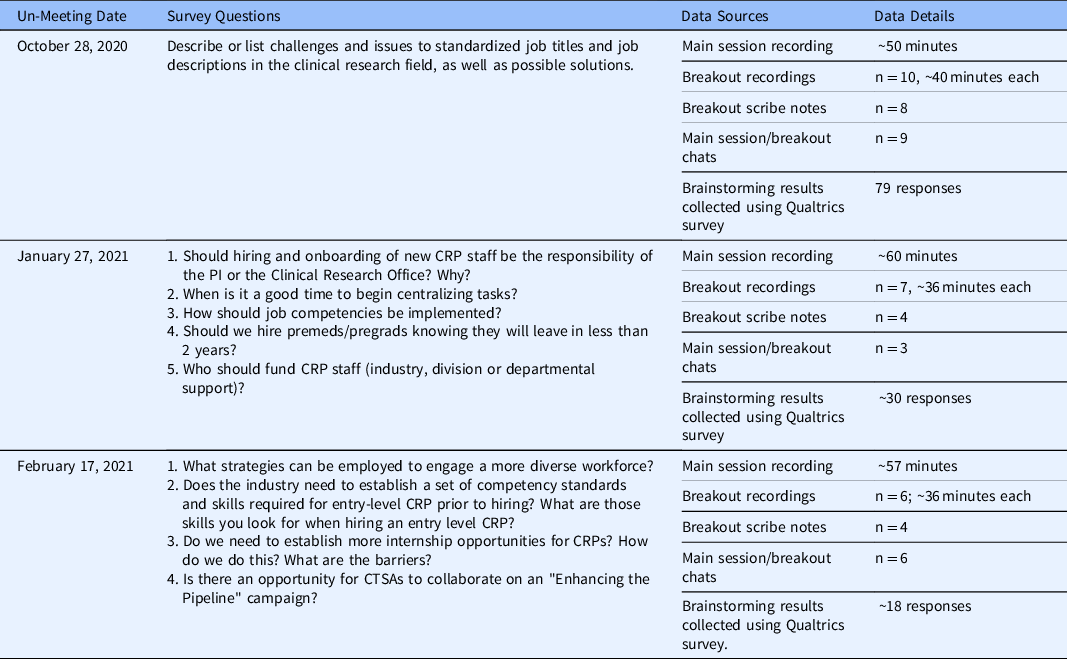
Note. CRP = clinical research professional; CTSA = Clinical Translational Science Award; PI = principal investigator; premeds = pre-medical students; pregrads = pre-graduate students.
Data Analysis
Qualitative analysis of Un-Meeting data led to identification of several barriers to the effective recruitment and retention of CRPs, but also laid the foundation for strategies to address these barriers. Seven key barriers that emerged from the data were 1) disconnected communication/collaboration with institutional human resource departments; 2) resource challenges; 3) organizational challenges; 4) lack of principal investigator (PI) engagement with CRP workforce; 5) lack of standardized training and professional development; 6) lack of career entry pathways (particularly as it relates to increasing diversity, equity, and inclusion in the CRP workforce); and, 7) challenges related to the COVID-19 pandemic (Fig. 1). Potential solutions emerging from the data could be divided into three main categories: 1) the need to garner support for CRP recruitment and retention on multiple institutional levels, 2) the need for national models or initiatives shared and replicated in other AMCs locally; and 3) the need to improve institutional climate through evolved policies to facilitate CRP professionalism, hiring, and job progression.
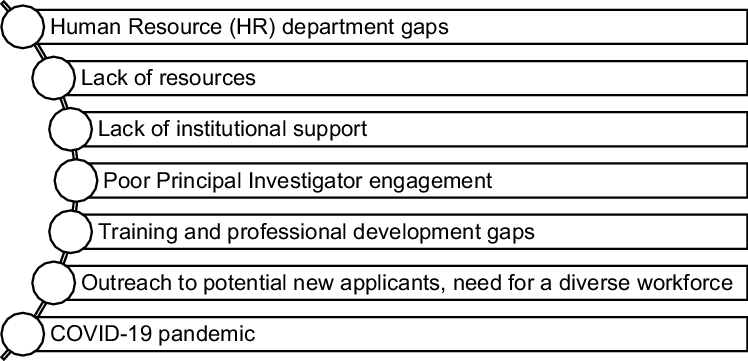
Fig. 1. Key barriers.
Barriers to Effective CRP Recruitment and Retention
Participants at the Un-Meetings shared experiences reflecting the difficulties they faced hiring and retaining well-qualified CRPs. The first of these was collaboration with human resources (HR). Within this theme, participants described that HR departments are unaware of CTR/CRP job variability and scope. This included the lack of (and challenge of creating) standardized job titles and descriptions. This deficit contributed to difficulties in both CRP recruitment and role progression. Moreover, we found that the lack of standardization resulted in inconsistency of promotion of CRP staff across organizational units. Finally, the absence of a standardized job classification system hindered the use of HR data to evaluate important metrics such as identifying CRP staff for education and compliance and tracking hiring and turnover metrics, including exit interview data. Without a standardized approach, there was increased difficulty in tying annual evaluations to competency assessment and other study-related outcomes.
Study participants reported a second barrier to the recruitment and retention of CRPs related to the challenge of securing and retaining resources, primarily financial resources. The codes describing this theme include the implementation of a CRP job ladder being associated with an initial investment (higher resource expenditure or costs). In addition, many participants pointed out that AMC salary ranges are lower and not competitive with clinical research organizations or the biotech industry. To offer comparable salaries, the complexity of funding mechanisms and cycles for CRPs via internal (department or divisional) and external (industry or government) resources and the decentralized nature of CTR at AMCs limit consistent pay scales across organizational units. One resource limitation participants shared was not specifically financial resources, but instead personnel resources: senior CRPs are spread too thin and asked to do too much in terms of hiring, training, and mentoring other CRPs, running studies, and contributing to grant development and manuscript writing.
The third barrier participants described was related to the organizational structure of CTR within AMCs. Participants reported CTR is generally decentralized at their home institutions, which leads to a lack of standardized hiring and CTR administrative processes, including variability of training, job duties, and CTR budgeting practices. Participants also described organizational challenges when working across multiple institutions, which is particularly common in the CTSA consortium where universities and hospital systems collaborate. Recruitment and retention of CRPs with nursing backgrounds compared to non-nursing backgrounds posed a unique challenge. Clinical research nurses are often employed within the healthcare system and therefore maintain clinical competency, whereas non-nurse CRPs do not have those requirements. Legal restrictions imposed on CRP hiring, job descriptions, and pay scales by either state law or employee unions was another organizational challenge unique to CTR occurring at AMCs. Finally, AMCs tend to be hierarchical in nature, with a culture of each department operating as an independent business unit. Participants reported this structural organization and culture inhibited centralization of CRP hiring and retention.
Participants described PI engagement as a challenge contributing to the difficulty of hiring and promoting CRPs appropriately. The codes within this theme included: most PIs do not have the time or skills to manage CTR staff and PIs often do not understand CRP job titles, duties, or the need for a career ladder. In the participants’ experience, these issues lead PIs to view CRPs as “cogs in the wheel,” and a perception that PIs do not support CRP career development and professionalization. Participants emphasized PIs shape the CRP work environment. Therefore, PI training is needed on how to create an inclusive environment that values CRPs as members of the CTR team. Participants expressed some PIs need education on the importance of high-quality research staff and the resulting positive impact on regulatory compliance, data quality, and publication quality. Additionally, participants reported an inconsistency in the promotion of CRPs, where PIs will move study staff between different employment tracks (e.g., clinical research coordinator to research assistant) because it is simpler or less costly.
Training and professional development was another crucial challenge that emerged from the data. Participants underscored the necessity of providing high quality, consistent onboarding training to new CRPs, offering centralized training required for CRPs on an ongoing basis depending on job titles, and formalizing mentorship programs for CRPs that match senior staff with junior staff. Another code within this theme was the need for more formal educational courses and programs that prepare CRPs for the career; participants stated too many CRPs “fall into” the career without adequate preparation, and so the development of formalized coursework that introduces students to the field and profession earlier in their education would be welcome. Another important code within this theme was the challenge of measuring competency in order to provide needed training, and then tying competency to performance evaluations and promotions. We further explored the issue of CRP onboarding and continuing education in a separate qualitative analyses.
The sixth theme identified in the Un-Meeting data set was the need to develop a stronger pool from which to recruit well-trained CRPs to the CTR workforce. One challenge associated with this theme was the difficulty of identifying appropriate educational backgrounds: participants discussed the variety of degrees CRPs obtain before being recruited and the unique access AMCs have to students in related fields such as public health and pharmaceutical sciences. However, though advantageous, a common practice of hiring individuals who stay for 1–2 years before beginning medical or graduate school creates a constant pool of novice staff. Given the variability of educational backgrounds CRPs bring to their work, another related challenge was the identification of the characteristics or qualities, aside from education and experience, that are important to job competency. Participants also described a need for shared nomenclature to describe job and career possibilities within CTR in order to attract potential employees. Internship programs were discussed as one possibility to build a cohort of pretrained staff, although most recognized the concurrent need for funding and infrastructure to support such an initiative if it will not be institutionally supported. Another code within this theme tied to the code within training and professional development, which includes the need to introduce CTR as a career field to a wider student population. Participants felt there was too much messaging to the CTR community pathways about becoming a PI and not enough about the CRP pathway or career track. Participants felt overcoming the pathways development challenge had the potential to greatly improve recruitment a more diverse workforce, which would have the added benefit of helping to meet the inclusion and equity goals of CTR studies.
The Un-Meetings included in this study’s data set occurred at the height of the COVID-19 pandemic (October, 2020 and January–February, 2021), and so barriers introduced or exacerbated by COVID-19 were another theme from participant data. Hiring problems went in both directions: first, many AMCs instituted hiring freezes due to budget uncertainty, and second, when those freezes were lifted, the CTR field saw huge numbers of job openings and not enough applicants. Because clinical research is a rapidly growing field, the COVID-19 pause exacerbated the need. Some CRPs moved onto other related jobs when furloughed; however, many were recruited actively by contract research organizations and the biotechnology industry, to address their huge numbers of openings. This diluted the number of experienced CRPs within AMCs, shifting to a less experienced pool. Another code within this theme was the difficulty of onboarding and training new CRPs when most CRPs, PIs, and other key personnel within CTR studies were working remotely or when the existing staff were not experienced enough to mentor new staff. Finally, the availability of well-trained CRPs who could quickly pivot to COVID-19 research studies was a huge problem for many participants in these collaborative conversations. The pause button was pushed on vital research studies to respond to the plethora of COVID-19 research, vaccination, and testing needs of AMCs.
Strategies to Improve CRP Recruitment and Retention
In their discussions of the barriers to CRP recruitment and retention, participants also touched upon strategies to overcome these barriers. Three themes emerged from the data set as important facilitators: garnering institutional support for effective CRP recruitment and retention, disseminating and replicating benchmarking models, and improving local institutional policies and processes (Fig. 2).

Fig. 2. Strategies leading to solutions.
The first thematic strategy to improve CRP career outcomes was garnering support from key stakeholders from institutional, departmental, and PI groups. This theme was primarily articulated by participants from institutions where change had occurred as a result of having champions who understood the issues and held leadership positions to leverage change. Participants who did not come from such institutions also recognized the important role champions play in implementing positive improvements for CRP recruitment and retention, and they discussed methods for making a case to gain buy-in from key stakeholders. Participants stated that change implementation requires both financial and personnel resources secured only by champions of the change, not by CRPs themselves.
The second theme identified in the domain of strategic facilitators was national models to help guide local discussions of effective CRP recruitment and retention. Participants discussed several recently published models that were of interest, including recommended pay scales to help inform and facilitate collaboration with HR, shared standards of competency and associated curricula implemented and tailored locally as needed and academic programs to prepare CRPs for the career, including professional certification and education at the undergraduate and graduate level. This theme described the need for a network to establish norms and scaffolding at the national level to guide initiatives at the local level more efficiently.
The final theme in the discussion of facilitators to improve CRP recruitment and retention was the need to develop and reform local institutional policies and processes. This theme of strategy, of course, dovetails with several themes in the barriers section tied to policies and processes. As participants discussed barriers, they also shared instances of ways they have worked within their institutional settings to make policies and processes more supportive of effective recruitment and retention of CRPs. Examples of this included regular equity reviews, market analysis adjustments and job analyses for title changes, progression checklists aligned with career ladders, partnering with HR to improve familiarity with CTR and CRP job duties, and creating a comprehensive institutional training program with an accompanying database to track CRP training and progression so such data can be incorporated into annual reviews. Other processes participants suggested that would be helpful included centralization of clinical research hiring so one office hires CRPs and provides general training, with PIs or individual units providing specialized training aligned with specialty requirements (e.g., oncology, cardiology). Many participants stated such a system could effectively maintain a “pool” of CRPs departments can draw from and fund as needed. Finally, participants recognized the need for institutional policies and processes formalizing mentoring for CRPs across units. This would require mentoring training and sustaining a well-trained, experienced staff who could serve as mentors. All of these would greatly contribute to overcoming the barriers identified in the first section of themes.
Discussion
This is a first attempt to collectively discuss and address issues related to the recruitment and retention of AMC CRPs at the national level. The strength of these data is the breadth of contributions across 35 CTSA hubs who participated in the Collaborative Conversations Un-Meeting sessions between September 2020 and February 2021 [Reference Jones, Lane, Shah, Carter, Lackey and Kolb18]. Qualitative research using a cloud-based collaborative platform such as Zoom has been reported as an effective method [Reference Archibald, Ambagtsheer, Casey and Lawless19]. Through a series of three Zoom sessions addressing workforce recruitment, retention, and progression, over 1000 minutes of collaborative discussions were analyzed.
Since the publication of the Joint Task Force Clinical Trial Competency Framework [Reference Sonstein, Seltzer, Li, Jones, Silva and Daemen20], the Association of Clinical Research Professionals (ACRP) and Society of Clinical Research Associates (SoCRA) have adopted the framework, modifying their certification exams [21,22]. Recognizing the need for a more diverse CRP workforce, ACRP launched their CRP career and diversity initiative, “Find your Element” [23]. However, most ACRP career development initiatives lack a specific focus on the AMC setting, where a large majority of government-funded CTR takes place. Elements for professionalization of the CRP workforce, including a blending of competency and performance outcomes; however, recent broad shortages of CRP staff in AMC settings can either blunt movement toward professionalization, maintaining a negative status quo, or can be a new opportunity for institutional culture change [Reference Stevens and Wool24].
The qualitative nature of these data provide key insights across CRP workforce recruitment and retention gaps and suggest potential pathways to solutions. Included in these findings is a need for advancement pathways to grow a sustained, experienced CRP workforce. With the sustained trend of CRP turnover, the CRP workforce remains primarily novice, which does not sufficiently support the current complex research need. Solutions should extend beyond simple revision of leveled job titles and descriptions and should instead emphasize career identity and professionalization across CRP roles. Outreach beyond AMC walls should begin promoting early CRP career identity to attract individuals with a focused educational and career intentionality seeking to work at AMC study sites. Moreover, early career pathways should be developed to streamline hiring of CRP novices and mentor them through individualized development plans, not unlike their investigator trainee counterparts. Institutional commitment to this important workforce should promote dedicated local task forces to benchmark initiatives to innovate recruitment and retention of CRPs [Reference Buchanan, Goldstein, Pfalzer, Lin, Kang and Claassen11]. We further identified a need for a diverse CRP workforce for which intentional outreach to students at all phases of their educational pursuits (graduate, undergraduate, community college, and high school) to promote the CRP workforce could help to broaden the applicant pool. A recent study by the Tufts Center for the Study of Drug Development showed a correlation in the recruitment of a more diverse clinical research population when there is a more diverse workforce [25]. Excellence in CTR performance should include a diverse, growing and sustained, highly trained professional CRP workforce. Addressing barriers identified in Fig. 1 and solution-finding (Fig. 2) will require an intentional approach. Such an approach provides a mechanism to track CRP workforce metrics and contributes to improved research compliance and quality reporting. Only in this way can AMCs be equipped to respond to new challenges currently evolving in the complex landscape of clinical research.
Duke University Office of Clinical Research shares a benchmarking approach to transforming the institutional approach to standardized, competency-based job titles and tiered role progression pathways [Reference Brouwer, Hannah, Deeter, Hames and Snyder13,Reference Stroo, Asfaw and Deeter15,Reference Brouwer, Deeter and Hannah16,Reference Snyder and Freel26]. The initial institutional investments in making these transformative changes were short term, with benefits recouping expenditures significantly. The five-step approach is suggested and further described in Table 4.
Table 4. Competency implementation checklist
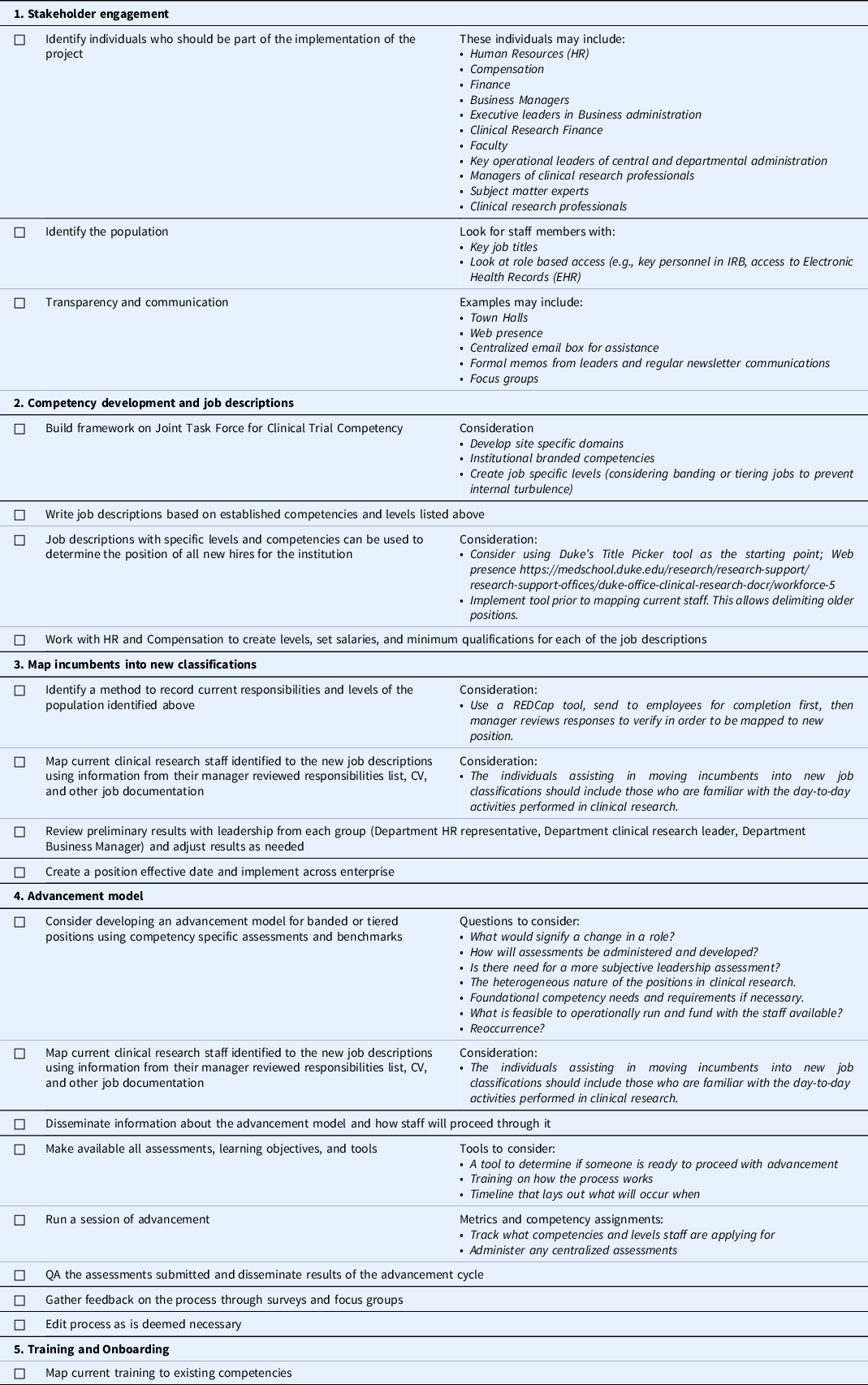
Note. HR = human resources; IRB = Institutional Review Board; CV = Curriculum Vitae.
Competency-based assessment tools that include a spectrum of evaluation mechanisms ranging from knowledge (quizzes or tests), self-perceived self-efficacy ratings, case-study demonstrations, central quality assurance checks, and observational assessments have been made publically available and can be accessed at https://medschool.duke.edu/research/research-support/research-support-offices/duke-office-clinical-research-docr/workforce-2.
Limitations
One limitation of this study is it includes a subset (approximately 68%) of CTSA hub sites and may lack additional input from those not participating. Our pivot to a Zoom platform because of COVID-19 travel restrictions and job demands may have hindered some of the brainstorming and discussion content; however, we did collect over 1000 minutes of collaborative conversations. The challenges and solutions presented here were major issues shared across represented institutions. Individual institutions or non-AMCs may find other unique challenges or solutions.
Conclusion
We aimed to explore factors negatively influencing CRP workforce recruitment, retention, and progression through a series of zoom Un-Meetings targeting this topic. We found there has been limited progress in standardizing job titles and descriptions nationally; however, model programs may provide a benchmark for such standardization. We also found robust institutional support is central to progress, including PI appreciation of CRP roles. Dedicated innovation and policy champions, coupled with financial support for CRP workforce initiatives, would maximize progress. Additional research should be undertaken to more fully explore career identity formation, factors influencing work satisfaction, and mechanisms to address diversity, equity and inclusion issues for the CRP workforce.
Acknowledgments
Special acknowledgement to Melissa D. Vaught, PHD, Director of Research Development; Aric H. Lane, MPA, Research Education Specialist; Arti M. Shaw, MPH, CHES, Director of Education; Russell Lackey, MS, Director of Education, Pavel Kruchek, Director Clinical Trials Office at the Institute of Translational Health Sciences, University of Washington, Seattle and Cherese Pullum, Director, Research Nursing and Clinical Research Support Cores at Seattle Children’s, Tina Allen Research Manager at Seattle Children’s for their generous support of time and resources, for which without this work, it would not have been possible.
The authors also wish to acknowledge the participants of the Collaborative Conversations Un-Meeting series who contributed these important data through their open discussions and sharing of best practices. Participant institutions/organizations included: Association of Clinical Research Professionals; Appalachian Translational Research Network (ATRN)/University of Kentucky; Boston Medical Center; Case Western Reserve University; Children’s Hospital of Philadelphia (U.Penn); Columbia University; Cornell University; Duke University; Georgetown-Howard Universities; Icahn School of Medicine at Mount Sinai; Johns Hopkins Medicine; Mayo Clinic; Medical University of South Carolina; Montana State University; National Institutes of Health; Northwestern University; Ohio State University/Nationwide Children’s Hospital; Oregon Health Sciences University; Orlando Health; Penn State University; Rutgers University; Saint Alphonsus Health System- Idaho; Southcentral Foundation- ALASKA; Stanford University; Tufts University; University of California, Davis; University of Vermont; University of Alabama at Birmingham; University of California at San Francisco; University of Cincinnati/Cincinnati Children’s Hospital; University of Colorado Denver; University of Florida; University of Illinois Chicago; University of Kansas Medical Center; University of Massachusetts Medical School, Worcester; University of Miami School of Medicine; University of Michigan; University of New Mexico; University of North Carolina; University of Rochester; University of Texas Health Science Center, Houston; University of Utah; University of Washington; Vanderbilt University; Virginia Commonwealth University; Washington University; Yale University
This work was supported in part by the following CTSA hub grants from the National Center for Advancing Clinical Translational Science (NCATS): UL1TR002733 (Ohio State University), UL1TR001427 (University of Florida), 2UL1TR001425-05A1 (University of Cincinnati); UL1TR002553 (Duke University), UL1TR003096 (University of Alabama at Birmingham); UL1 TR002319 (University of Washington/Seattle Children’s).
This work was also funded in part by the University of Rochester CLIC, under Grant U24TR002260 and was selected as a Synergy Paper Initiative with the Center for Leading Innovation and Collaboration (CLIC) - Grant Number: #2110001b. CLIC is the coordinating center for the CTSA Program, funded by the NCATS at the National Institutes of Health (NIH). This work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosures
The authors have no conflicts of interest to declare.
Author Biographies
Jaqueline Knapke, PhD is Assistant Professor, Department of Family and Community Medicine Research Division and Associate Director, Center for Clinical and Translational Science and Training, University of Cincinnati, College of Medicine.
Denise Snyder, MS is the Associate Dean of Clinical Research at the Duke University School of Medicine and Operational Director of the Participant and Clinical Interactions Core, Duke University Clinical Translational Science Institute.
Karen Carter is Assistant Director of Education, OSU Center for Clinical Translational Science.
Meredith B. Fitz-Gerald, BSN, MSN, RN, Certificate in Clinical Research Management, is the Director of the Center for Clinical and Translational Science (CCTS) Clinical Research Support Program (CRSP) at the University of Alabama at Birmingham.
Jessica Fritter, MACPR, ACRP-CP is Clinical Research Administration Manager at Nationwide Children’s Hospital, Columbus, OH.
H. Robert Kolb, RN, MS, CCRP is Director of the Clinical Research Professional Development program with the University of Florida Clinical Translational Science Institute’s (UF CTSI) Workforce Development Directorate and Assistant Director of UF CTSI’s Regulatory Knowledge and Support services.
Mark Marchant, MPH, MBA, CCRP is the Director of the Clinical Trials Administrative Office at the University of Alabama at Birmingham.
Angela Mendell, MS, CCRP is Program Manager for the Center for Improvement Science (CIS) with the Center for Clinical and Translational Science and Training (CCTST) at the University of Cincinnati.
Megan Petty, MBA is the Communications Specialist at the Center for Leading Innovation & Collaboration (CLIC).
Cherese Pullum, MS, RN is Director, Research Nursing and Clinical Research Support Cores, Seattle Children’s Hospital, Research Integration Hub.
Carolynn T. Jones, DNP, MSPH, CRN-BC, RN, FAAN is Professor of Clinical Nursing, OSU College of Nursing, and Co-Director of Workforce Development, OSU Center for Clinical Translational Science.








