The incidence of immune-mediated inflammatory diseases increased worldwide in recent decades, and these have become a leading cause of chronic illness in children and young people(Reference Garcia-Larsen, Ierodiakonou and Jarrold1). A range of evidence shows that early dietary exposures may influence the development of these diseases(Reference Garcia-Larsen, Ierodiakonou and Jarrold1). A number of studies have also linked maternal obesity during pregnancy with an increased risk of obesity in the offspring, which persists across the lifespan(Reference Lawlor2). While epidemiological studies in humans are limited in their ability to separate the effects of maternal obesity from those of the maternal diet on fetal development, some evidence suggests that a maternal high-fat diet (HFD) increases the likelihood of obesity in the offspring irrespective of maternal weight(Reference Parlee and MacDougald3). Animal models using maternal HFD have shown a range of effects on the offspring such as altered lung development and function(Reference Heyob, Mieth and Sugar4), metabolic disorders(Reference Barbosa, Figueiredo and Barbosa5) and adipo-immunological ageing(Reference Imai, Fujimoto and Tamura6). We have previously shown that maternal obesity leads to long-term changes in vascular function in mice(Reference Torrens, Ethirajan and Bruce7) but whether maternal exposure to a HFD alters immune regulation in the offspring through fatty acid dysregulation remains unknown.
PUFA can modulate the immune system in part through regulation of lipid mediator synthesis. In general, eicosanoids derived from the n-6 PUFA arachidonic acid (ARA) are pro-inflammatory and immunoactive, whereas eicosanoids and docosanoids derived from the n-3 PUFA EPA and DHA have anti-inflammatory and inflammation resolving functions(Reference Calder8). ARA, EPA and DHA are formed from precursor PUFA through a pathway involving desaturation and elongation. The rate-limiting step in the pathway is catalysed by Δ6 desaturase, encoded by the fatty acid desaturase 2 (FADS2) gene. Elongation of very long-chain fatty acids (ELOVL) 5 converts the products of Δ6 desaturase into fatty acids that are two carbons longer. This is followed by a further desaturation catalysed by Δ5 desaturase (encoded by fatty acid desaturase 1 (FADS1)) to generate ARA and EPA(Reference Moon, Hammer and Horton9). In a rat model, maternal fat intake was demonstrated to inversely correlate with the proportions of ARA and DHA in offspring liver and with epigenetic regulation of FADS2 transcription in offspring liver(Reference Hoile, Irvine and Kelsall10). A recent systematic review of diet during pregnancy and infancy and risk of allergic or autoimmune disease concluded that maternal fish oil supplementation may reduce risk of eczema and allergic sensitisation to food in children(Reference Garcia-Larsen, Ierodiakonou and Jarrold1). Fish oil is a source of EPA and DHA. In addition, a recent trial demonstrated that maternal fish oil supplementation altered the child’s metabolome at age 6 months, with lower levels of n-6 PUFA pathway-related metabolites and decreased risk of the child developing asthma at age 5 years(Reference Rago, Rasmussen and Lee-Sarwar11,Reference Bisgaard, Stokholm and Chawes12) . We have previously observed that gestational oily fish intake epigenetically modifies genes encoding key enzymes of PUFA synthesis and alters allergic risk in early childhood(Reference Losol, Rezwan and Patil13).
In humans, a recent study linked minor allele carriers of several FADS alleles associated with lower blood ARA, with reduced risk of atopic eczema at age of 13 years(Reference Barman, Nilsson and Torinsson Naluai14), while a higher proportion of n-6 PUFA was associated with higher risk of allergy in children(Reference Mikkelsen, Galli and Eiben15). In addition, n-3 PUFA supplementation has been shown to decrease plasma levels of n-6 PUFA, increase plasma n-3 PUFA levels and influence desaturase and elongase activities(Reference Cormier, Rudkowska and Lemieux16). In this study, the authors identified that minor alleles of FADS variants are associated with higher Δ6 desaturase activity and with lower delta-5-desaturase activity, while minor alleles of ELOVL variants were associated with higher elongase activity following n-3 PUFA supplementation. Also, genetic variants of FADS1 and ELOVL5 modified the effect of breast-feeding on cognition, suggesting functional importance of such variants to early development(Reference Morales, Bustamante and Gonzalez17). These findings support the hypothesis that gene–diet interactions modulate the activities of desaturases and elongases and that n-3 and n-6 PUFA are competitive towards these enzymes resulting in altered levels of precursors of eicosanoids and docosanoids. Changes in the fatty acid composition of inflammatory cells affect production of both lipid and peptide mediators of inflammation, and as a result, ARA, EPA and DHA may play an important role in the development of allergy(Reference Calder18).
Obesity increases the risk of common conditions including atherosclerosis, severe asthma and airway inflammation through neutrophil accumulation, generation of inflammatory cytokines (IL-1β, TNF-α and IL-6), an altered gut microbiome and vitamin D deficiency(Reference Tashiro and Shore19). Previous studies reported that maternal exposure to a HFD causes changes in lung morphology(Reference Smoothy, Larcombe and Chivers20) which leads to innate airway hyper-responsiveness(Reference MacDonald, Moran and Scherman21), persistent metabolic abnormality and chronic airway inflammation in offspring(Reference Song, Yu and Wang22). The perivascular adipose tissue (PVAT) is important for the regulation of vascular/endothelial function, and dysfunction of PVAT is characterised by its inflammatory character, oxidative stress and increased production of paracrine factors such as resistin, leptin, cytokines (TNF-α and IL-6) and chemokines (C-C motif chemokine ligands 5 and 2 CCL5 and CCL2)(Reference Nosalski and Guzik23).
The aim of this study was to investigate the effect of maternal and post-natal HFD on PUFA status, on the genes encoding enzymes of fatty acid biosynthesis, on inflammatory gene expression in PVAT and on lung function in the offspring.
Methods
Ethics statement
All animal procedures were performed under the regulations of the British Home Office Animals (Scientific Procedures) Act 1986 and were conducted under Home Office License number 70-6457. The study received Institutional approval from the University of Southampton Biomedical Research Facility Research Ethics Committee.
Animals and diets
The animal diets were from Special Diet Services UK as previously described(Reference Torrens, Ethirajan and Bruce7). Weaned female C57BL/6J mice (n 32) housed in autoclaved cages with autoclaved bedding were fed either standard chow (C, RM1A, fat as 20·4 energy %) or a high-fat (fat as 39·9 energy %) diet (HFD) for 4–6 weeks prior to conception and throughout gestation and lactation (Fig. 1). Dietary macronutrients and energy are shown in Table 1. At 21 d of age, offspring were weaned onto either the HFD or C, generating four experimental groups: C/C, C/HF, HF/C and HF/HF (N 7–10/group). Following weaning of the offspring, dams were killed by cervical dislocation. Two sets of dams were used to generate the 15- and 30-week-old offspring to avoid maternal bias. At 15 weeks of age, a subgroup of offspring was killed by cervical dislocation and liver (left lobe) and perivascular fat from around mesenteric arteries was dissected and snap-frozen in liquid N2 and stored at −80°C until further analyses. Blood was collected by cardiac puncture into tubes containing lithium heparin, and the plasma separated, frozen in liquid N2 and stored at −80°C. At 30 weeks of age, offspring were killed by cervical dislocation and lungs dissected and frozen in liquid N2. In a subgroup at 30 weeks, lung function was assessed.

Fig. 1. Dietary model and study design. C, chow; HF, high-fat diet.
Table 1. Macronutrient composition and energy content of the standard laboratory chow and high-fat diet

Measurement of phosphatidylcholine fatty acid composition
Fatty acid compositions of liver and plasma phosphatidylcholine (PC) were measured by GC as described elsewhere(Reference Fisk, West and Childs24,Reference Kelsall, Hoile and Irvine25) . Each sample (100 mg and 100 µl) was powdered under liquid N2, and lipids were extracted using chloroform:methanol (2:1, v/v containing 50 mg/l butylated hydroxytoluene). PC was isolated by solid-phase extraction. Fatty acids within PC were converted to fatty acid methyl esters by an incubation with methanolic sulphuric acid (2 % H2SO4, v/v). Fatty acid methyl esters were recovered by extraction with hexane. Fatty acid methyl esters were separated by GC using a BXP70-fused silica capillary column (30 m × 0·25 mm × 0·25 µm) on an Agilent 6890 GC system (Agilent) equipped with flame ionisation detection. Running conditions were as described previously(Reference Fisk, West and Childs24). Fatty acid methyl esters were identified by their retention times relative to standards, and area under the peak was quantified using ChemStation software (Agilent).
Gene expression analysis
Total RNA was isolated from livers of 15-week-old offspring, PVAT of 15-week-old offspring and lung tissue of 30-week-old offspring using Tri Reagent (Sigma) according to the manufacturer’s instructions. RNA concentration and purity were assessed using a Nanodrop Fluorospectrometer (Nanodrop 3300, NanoDrop Technologies) after extraction and purification. Fluorescence ratios (260/280 nm and 260/230 nm) were observed as an indication of purity. All samples underwent purification using an ethanol precipitation protocol before being used in reverse-transcription PCR. Complementary DNA was synthesised from total RNA by poly-A reverse-transcription (Primerdesign). cDNA template was amplified by a real-time PCR (Roche LightCycler® 480) under following conditions: 95°C for 10 min, then 15 s at 5°C and 1 min at 60°C for thirty-five cycles. The primers and probes (Eurogentec) are shown in Table 2. The expression of FADS1, FADS2 and ELOVL5 was assessed using Sybr Green (QIAGEN). Each sample was assayed in triplicate, expression of the individual transcripts was normalised to the relevant housekeeper in a single ninety-six-well plate and a mean copy number was calculated. The housekeeper used for liver was YWHAZ and for PVAT and lung it was glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Table 2. Primer and probe sequences used

FADS, fatty acid desaturase; ELOVL5, elongation of very long-chain fatty acid 5; CCL2, C-C motif chemokine ligand 2.
Pulmonary function test
A change in respiratory system resistance in response to increasing concentrations of inhaled methacholine was assessed in vivo. The trachea of 30-week-old offspring under deep anaesthesia was exposed through a neck incision with an eighteen-gauge catheter inserted in a small cut below the cricoid cartilage(Reference Piedimonte, Walton and Samsell26). The catheter was connected via a nebuliser to a Flexivent system (SCIREQ). Resistance was measured at baseline after nebulisation of PBS and then after nebulisation of methacholine at cumulative doses of 0, 25, 50 and 100 mg/ml. On completion of the lung function assessment, mice were killed by cervical dislocation and lungs dissected and frozen in liquid N2.
Statistical methods
The effects of maternal diet upon offspring plasma and liver PC fatty acid composition were assessed by two-way ANOVA with multiple comparison and sex adjustment (SPSS version 26). Relative mRNA expression levels among dietary groups were evaluated by two-way ANOVA, and the comparison between two groups was tested by one-way ANOVA. Proportion of ARA and DHA in the offspring liver and plasma PC was tested among study groups by independent t test to evaluate the effect of pre- and post-natal HFD. Maximum resistance to methacholine was analysed by two-way ANOVA among groups (GraphPad Prism version 8). Results are expressed as means and standard errors of the mean, and sample size in each group was n ≥ 5. Differences were considered to be statistically significant if the associated P value was <0·05 at two-sided significance levels.
Results
Phosphatidylcholine fatty acid composition of mice fed chow and high-fat diet
The proportions of ARA (20 : 4n-6) and DHA (22 : 6n-3) in offspring liver and plasma PC were affected by diet (Tables 3 and 4). Specifically, the proportion of ARA in offspring was significantly higher in the HF/HF group compared with the other (C/C, HF/C, C/HF) dietary groups in plasma PC, and compared with the C/C and HF/C groups in liver PC (P < 0·05; Tables 3 and 4, respectively). The proportion of plasma PC DHA tended to be higher in the HF/HF group, but this did not reach statistical significance. However, DHA in liver PC in offspring in the C/HF and HF/HF groups was significantly higher than that in the C/C and HF/C groups (P < 0·05, respectively; Table 4).
Table 3. PUFA composition (%) of plasma phosphatidylcholine among dietary groups
(Mean values with their standard errors)
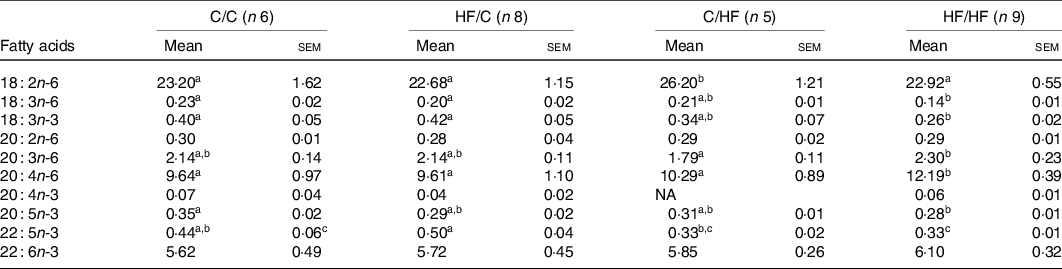
C, chow; HF, high-fat diet.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Table 4. PUFA composition (%) of liver phosphatidylcholine among dietary groups
(Mean values with their standard errors)
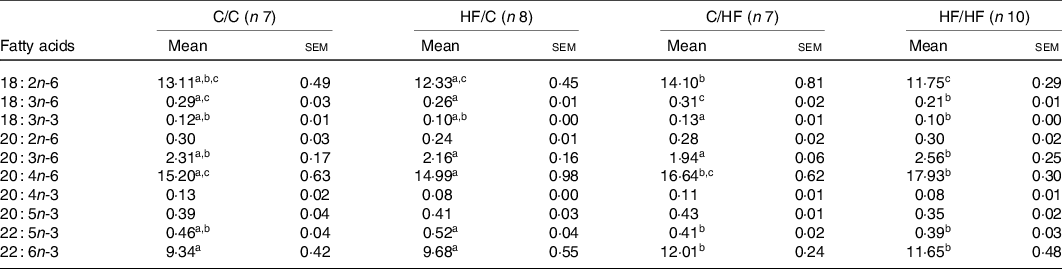
C, chow; HF, high-fat diet.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
To determine the effects of pre- and post-weaning HFD on composition of fatty acids, the study groups were combined as follows: C/C and HF/C considered as pre-natal diet group, and C/HF and HF/HF considered as post-natal diet group. The proportion of ARA was significantly higher in offspring plasma and liver PC in post-natal HF diet group (P = 0·039 and P = 0·001, respectively; Fig. 2(a) and (c)). Also, the proportion of DHA was significantly higher in offspring liver PC in the post-natal HF diet group (P < 0·001, Fig. 2(d)). Post-natal diet did not change the proportion of plasma PC DHA in offspring (Fig. 2(b)).

Fig. 2. Proportion of arachidonic acid (ARA) and DHA in the offspring plasma (a, b) and liver (c, d). Values are means with their standard errors. (![]() ), Chow/chow (C/C) + high-fat diet/chow (HF/C); (
), Chow/chow (C/C) + high-fat diet/chow (HF/C); (![]() ), chow/high-fat diet (C/HF) + high-fat diet/high-fat diet (HF/HF). * P < 0·05, ** P ≤ 0·001.
), chow/high-fat diet (C/HF) + high-fat diet/high-fat diet (HF/HF). * P < 0·05, ** P ≤ 0·001.
Gene expression of PUFA biosynthetic enzymes and pro-inflammatory cytokines and chemokines
Next, hepatic expression of genes encoding enzymes of PUFA metabolism was examined to identify the potential effect of high-fat feeding. FADS1 mRNA expression in the liver of the offspring was higher in females than males, significantly so in HF/C and HF/HF groups (P = 0·001 and P = 0·003, respectively; Fig. 3(a)), but there was no effect of diet. There were significant effects of offspring sex and maternal diet on FADS2 mRNA expression (Fig. 3(b)). FADS2 expression was significantly higher in female than male offspring in the HF/C group (P = 0·001) and tended to be higher in the HF/HF group (P = 0·056), and it was expressed more highly in HF/HF group than in the HF/C group (P < 0·05, respectively; Fig. 3(b)). ELOVL5 mRNA expression was higher in HF/HF males than in HF/C and C/HF males (P = 0·038 and P = 0·009, respectively; Fig. 3(c)).
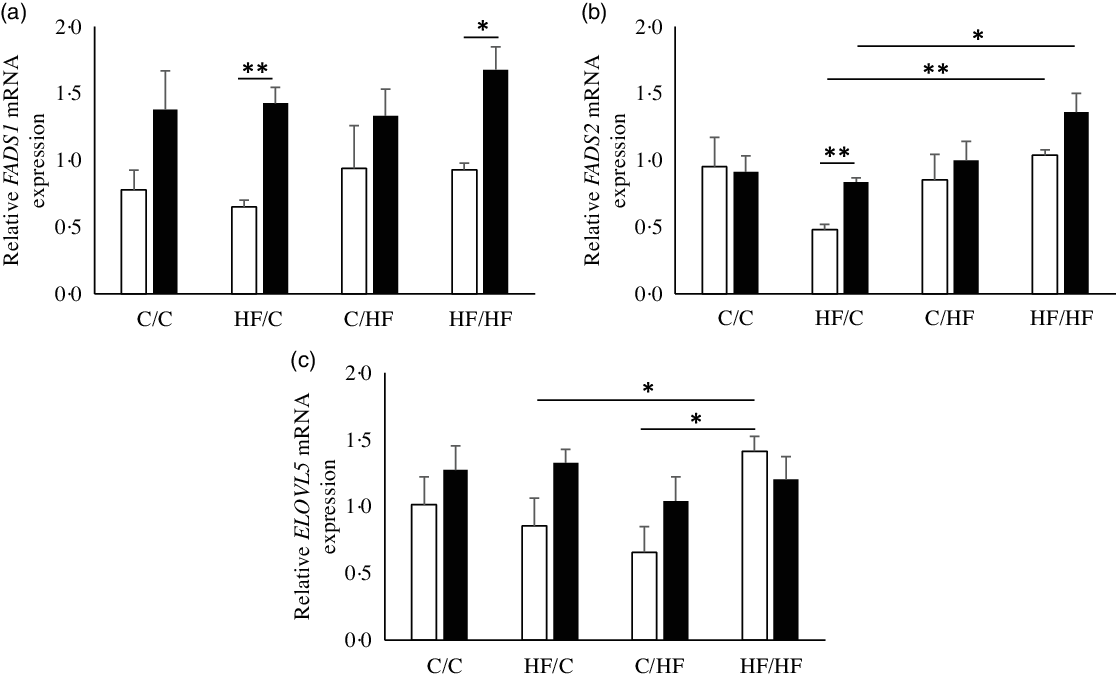
Fig. 3. mRNA levels relative to YWHAZ of fatty acid desaturase 1 (FADS1) (a), FADS2 (b) and elongation of very long-chain fatty acid 5 (ELOVL5) (c) in offspring liver. Values are means with their standard errors. (![]() ), Male; (
), Male; (![]() ), female; C, chow; HF, high-fat diet. * P ≤ 0·05, ** P ≤ 0·001.
), female; C, chow; HF, high-fat diet. * P ≤ 0·05, ** P ≤ 0·001.
Post-natal HFD (C/HF, HF/HF) was associated with higher expression of inflammatory markers IL-6 and CCL2 in PVAT than seen in C/C and HF/C groups (P < 0·001 and P = 0·01, respectively; Fig. 4(a) and (b)). In addition, post-natal HFD (C/HF) significantly increased expression of pro-inflammatory cytokine IL-6 in offspring lung compared with C/C offspring (P = 0·042, online Supplementary Fig. S1).

Fig. 4. mRNA levels relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) of IL-6 (a) and C-C motif chemokine ligand 2 (CCL2) (b) in perivascular adipose tissue from around mesenteric arteries. Values are means with their standard errors. C, chow; HF, high-fat diet. * P < 0·05 pre- v. post-natal fat feeding, *** P < 0·001 pre- v. post-natal fat feeding.
Lung function of offspring
In both male and female mice from pre-natal HFD groups (HF/C and HF/HF), methacholine generated significantly less airway resistance compared with mice in the pre-natal chow groups (C/C and C/HF, P < 0·01, Fig. 5(a) and (b)). In male offspring, there was a significant interaction between the pre- and post-natal diets but no such interaction was seen in the female offspring (HF/C v. CC and HF/HF v. C/HF, P < 0·05, respectively, Fig. 5(c) and (d)).
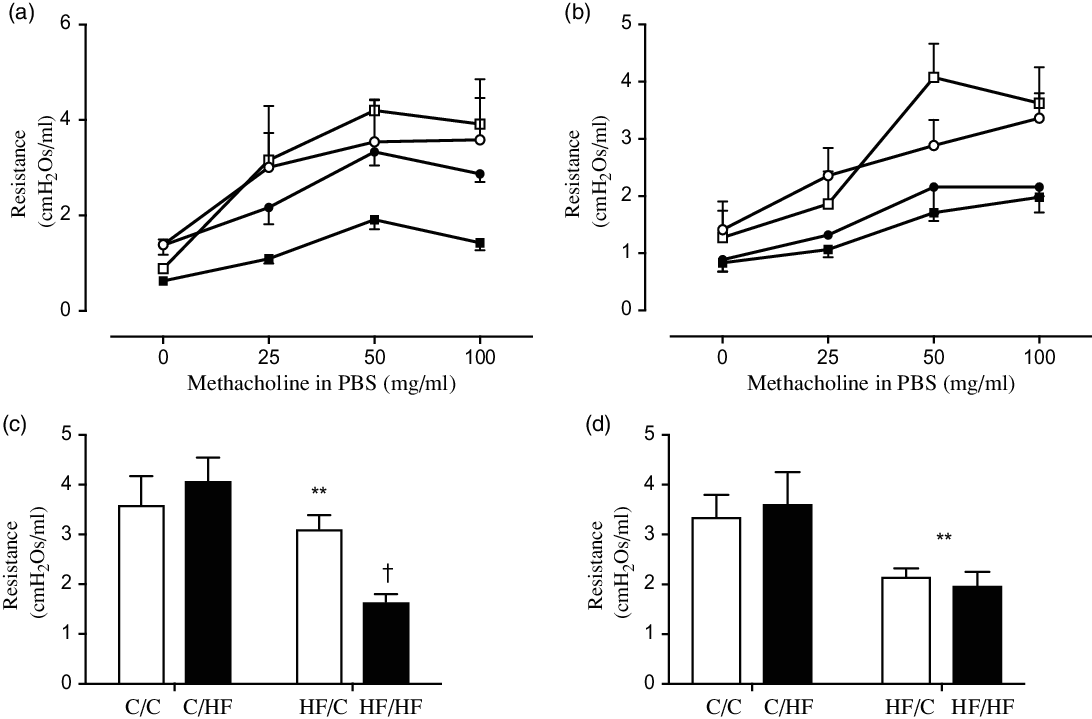
Fig. 5. Change in lung resistance in response to increasing doses of methacholine in 30-week male (a) and female (b) offspring fed either a chow diet (C) or high-fat diet (HF). (a and b) ![]() , C/C;
, C/C; ![]() , C/HF;
, C/HF; ![]() , HF/C;
, HF/C; ![]() , HF/HF. Lung resistance in maximum response to methacholine in male (c) and female (d) offspring. (
, HF/HF. Lung resistance in maximum response to methacholine in male (c) and female (d) offspring. (![]() ), C/C and HF/C; (
), C/C and HF/C; (![]() ), C/HF and HF/HF. ** P < 0·01 pre- v. post-natal fat feeding. † P < 0·05 interaction.
), C/HF and HF/HF. ** P < 0·01 pre- v. post-natal fat feeding. † P < 0·05 interaction.
Discussion
In this study, we have demonstrated a clear effect of a maternal HFD on ARA and DHA status in offspring plasma and liver phospholipids. Maternal HFD exposure also led to altered offspring responses to post-natal HFD with higher expression of genes encoding hepatic long-chain PUFA synthesising enzymes, FADS2 and ELOVL5 (in male offspring only), and impaired lung function. Post-natal HFD was also associated with an increased expression of genes encoding pro-inflammatory (IL-6) and vascular inflammatory (CCL2) markers in offspring PVAT independent of maternal HFD. These observations suggest that exposure to a HFD during development programmes the lipidomic, metabolic and immunological profiles of the offspring which were linked to respiratory dysfunction in early life.
In this model, C57BL/6J offspring were exposed to a HF maternal diet for 21 d in utero and via suckling until 3 weeks of life, before being weaned to either a control diet or a continued HFD. Physiological properties of individual PC are heavily dependent on their FA composition. In general, PC are crucial for maintaining membrane fluidity and for cellular differentiation and proliferation(Reference Gundermann, Kuenker and Kuntz27). PC can also have an anti-inflammatory effect(Reference Treede, Braun and Sparla28). In previous studies in mice, dietary DHA/EPA administration as phospholipids prevented weight gain and reduced plasma lipid concentrations(Reference Rossmeisl, Jilkova and Kuda29) and n-3 PUFA supplementation in addition to a HFD reduced inflammation and size of adipocytes(Reference Awada, Meynier and Soulage30). In the present study, increased ARA and DHA levels in PC were observed in offspring following maternal HFD. Further, post-natal HFD additionally affected the proportions of hepatic and plasma ARA and hepatic DHA, shifting the profile to one with more ARA. Plasma samples from offspring fed the HFD showed no difference in DHA containing PC, in line with previous studies that showed no difference in DHA containing PC in the plasma following high n-3 PUFA diet and/or alteration by dietary supplements of DHA(Reference Balogun, Albert and Ford31,Reference Browning, Walker and Mander32) . Conceivably, DHA containing PC might rapidly convert to lysophosphatidylcholine for delivery to extrahepatic tissues, with clearance from the circulation.
It has been shown that liver lipid accumulation, FA composition and inflammation are all modulated by the dietary composition of lipids and that elongase and desaturase enzymes are also regulated by diet composition and their activities can lead to alterations in cell lipid composition(Reference da Silva-Santi, Antunes and Caparroz-Assef33). Although Su et al. identified low expression of both FADS1 and FADS2 associated with human minor haplotypes, in mice, dietary PUFA showed stronger effects than genotype on PUFA composition in liver(Reference Su, Zhou and Pan34). Also, PUFA composition in erythrocytes was similar to that in liver phospholipids(Reference Su, Zhou and Pan34). In our study, maternal HFD groups had lowered FADS1 and FADS2 expression in male offspring, while FADS2 expression was elevated by post-natal HFD. We also observed increased ELOVL5 expression by pre- and post-natal HFD among male offspring, suggesting that male mice are more sensitive to the effects of a HFD in comparison with females.
DHA and EPA have both metabolic and anti-inflammatory actions, for example, lowering hepatic lipogenesis, increasing adipose tissue insulin sensitivity and decreasing pro-inflammatory cytokines such as TNF-α and IL-6 in adipose tissue(Reference Hames, Morgan-Bathke and Harteneck35). Several investigators have reported decreased expression of genes encoding proteins involved in pro-inflammatory pathways in human, animal and cellular models in response to EPA and/or DHA. For example, a recent study revealed beneficial effects of n-3 PUFA in IL-17A-related inflammatory pathologies through down-regulation of intercellular adhesion molecule-1 expression in monocytes and adipose-derived stem cells(Reference Chehimi, Ward and Pestel36). In contrast, adipocyte-derived PGE2, a derivative of ARA, mediates recruitment of adipose tissue macrophages contributing to inflammation(Reference Hu, Cifarelli and Sun37). Therefore, the imbalance between n-6 and n-3 PUFA has effects on inflammatory processes, and overconsumption of one results in a significant decrease in products of the other(Reference Liou, King and Zibrik38). In line with these observations, we observed that a post-natal obesogenic diet (but not maternal HFD) up-regulated pro-inflammatory gene expression in PVAT and lung tissue. This up-regulation of IL-6 and CCL2 in the post-natal HF groups agrees with previous findings that obesity leads to a pro-inflammatory phenotype(Reference Nosalski and Guzik23). Taken together, the coordination of diet-induced DHA and ARA synthesis seems to be important for the regulation of inflammatory markers in adipose tissue as they share a common synthetic pathway.
Our observations of the effect of maternal HFD on offspring lung function are supported by the results of McDonald et al. (Reference MacDonald, Moran and Scherman21) who reported increased airway hyper-responsiveness to methacholine and levels of inflammatory cytokines in bronchial alveolar lavage fluid following maternal HFD, suggesting that HFD causes lung dysfunction and increased susceptibility to inflammation at an early age. Although we assessed fatty acid and inflammatory markers at week 15, which occur prior to functional changes in the lung measured at week 30, further detailed investigation will be required to determine the longevity of these effects of maternal HFD during pregnancy on offspring development and whether they are associated with functional outcomes of clinical significance.
In conclusion, a maternal HFD during pregnancy leads to altered hepatic PUFA status in offspring through regulation of gene expression of enzymes that are involved in fatty acid metabolism, increased inflammation and respiratory dysfunction in early life (Fig. 6). This suggests that composition of maternal diet rather than maternal obesity per se may be important in increasing risk of asthma in offspring. This hypothesis needs to be examined in suitably designed human studies.
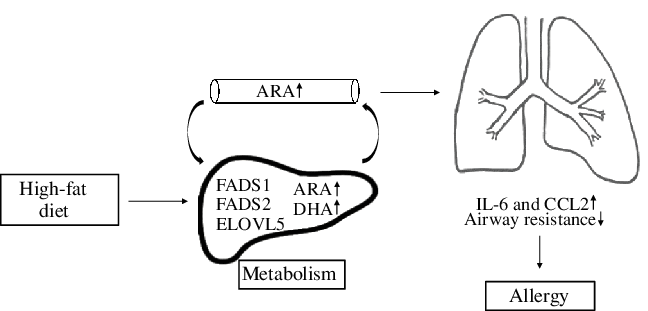
Fig. 6. This diagram provides a schematic representation of the effects that maternal high-fat-rich diet alters plasma and hepatic fatty acid (FA) composition in offspring through regulation of gene expression of FA enzymes. This leads to induced secretion of inflammatory markers and modifies lung development which may further increase risk of allergy in offspring. ARA, arachidonic acid; FADS, fatty acid desaturase; ELOVL, elongation of very long-chain fatty acids; CCL2, C-C motif chemokine ligand 2.
Acknowledgements
This work was supported by the Gerald Kerkut Charitable Trust. P. L. was the recipient of a long-term research fellowship from the European Respiratory Society (2015).
Authors’ contributions were: conceptualisation, J. W. H. and C. T.; formal analysis, P. L., L. P. M., H. L. F., P. C. C., J. W. H. and C. T.; funding acquisition, P. L., J. W. H. and C. T.; investigation, P. L., L. P. M. and H. L. F.; methodology, J. W. H. and C. T.; project administration, J. W. H. and C. T.; resources, P. C. C., J. W. H. and C. T.; supervision, P. C. C., J. W. H. and C. T.; visualisation, P. L., L. P. M. and C. T.; writing, P. L., P. C. C., J. W. H. and C.T.; all authors reviewed and approved the final version of the manuscript.
The authors declare that they have no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520004742













