The long-chain PUFA 20 : 5n-3 and 22 : 6n-3 are known to exert beneficial physiological effects including a reduction in CVD risk, which are at least in part related to the hypotriacylglycerolaemic potential of these fatty acids(Reference Simopoulos1, Reference Williams2). Even though clinical and biomedical studies have provided evidence that low intakes of 20 : 5n-3 and 22 : 6n-3 in the human diet are associated with increased chronic disease risk(3), consumption of long-chain n-3 PUFA within European populations is often suboptimal(Reference Givens and Gibbs4). Ruminant-derived foods are a major source of fat in typical Western diets(Reference Hulshof, van Erp-Baart and Anttolainen5, Reference Givens and Shingfield6), indicating the potential of enriching the 20 : 5n-3 and 22 : 6n-3 content of milk and meat as a nutritional strategy to enhance the n-3 PUFA status of human populations.
Inclusion of fish oil (FO) in the diet of ruminants is known to enhance the concentration of 20 : 5n-3 and 22 : 6n-3 in milk(Reference Donovan, Schingoethe and Baer7, Reference Shingfield, Ahvenjärvi and Toivonen8) and meat(Reference Scollan, Choi and Kurt9, Reference Wistuba, Kegley and Apple10), but enrichment is limited due to the metabolism of long-chain n-3 PUFA in the rumen. While a number of studies have examined the potential of FO to increase post-ruminal 20 : 5n-3 and 22 : 6n-3 supply in lactating and growing ruminants(Reference Shingfield, Ahvenjärvi and Toivonen8, Reference Wachira, Sinclair and Wilkinson11, Reference Scollan, Dhanoa and Choi12), there is evidence to suggest that the extent of ruminal metabolism of n-3 fatty acids varies according to the composition of the basal diet. This comes in part from observations that increases in the ratio of starch to fibre in the diet decrease the rate of lipolysis of dietary lipids in vitro (Reference Gerson, John and King13), that ruminal biohydrogenation of 18 : 3n-3 is reduced on high concentrate starch-rich diets(Reference Kalscheur, Teter and Piperova14, Reference Loor, Ueda and Ferlay15) and that metabolism of 20 : 5n-3 in the rumen is more extensive in cattle fed red clover (Trifolium pratenses) silage compared with grass (Lolium perenne) silage(Reference Lee, Shingfield and Tweed16). Furthermore, 18 : 2n-6 was shown to inhibit metabolism of 20 : 5n-3 and 22 : 6n-3 by mixed rumen microbes in vitro (Reference Wąsowska, Maia and Niedźwiedzka17). These findings suggest that the extent of ruminal metabolism of long-chain n-3 fatty acids is potentially lower in ruminants fed diets containing maize (Zea mays) silage rich in starch and 18 : 2n-6 as the basal forage, but there are no data to substantiate this hypothesis. In the present experiment, the effects of incremental amounts of FO as a source of 20 : 5n-3 and 22 : 6n-3 on ruminal lipid metabolism and the flow of fatty acids at the duodenum in steers fed maize silage-based diets were examined.
Material and methods
Animals and experimental design
All experimental procedures used were licensed, regulated and inspected by the UK Home Office under the Animals (Scientific Procedures) Act of 1986. Four Aberdeen Angus steers of 555 (se 12·0) kg live weight at the start of the experiment fitted with rumen cannula (internal diameter 100 mm) and a simple duodenal cannula located within 50 mm of the pylorus were used in a 4 × 4 Latin square design with 21 d experimental periods. Steers were housed in individual tie stalls and offered daily rations as equal meals at 06.00 and 18.00 hours. Animals had continuous access to water and trace-mineralised salt blocks (Baby Red Rockies, Winsford, Cheshire, UK). Steers were weighed at the beginning of the experiment and at the end of each experimental week at 12.00 hours. At the end of the 84 d experiment, steers weighed 636 (se 10·6) kg.
Experimental diets
Steers were offered total mixed rations based on maize silage (forage:concentrate ratio 60:40 on a DM basis) containing 0, 8, 16 or 24 g/kg DM of refined herring (Clupea spp.) and mackerel (Scomber spp.) oils (Napro Pharma, AS, Brattvaag, Norway; Table 1) fed at a rate of 85 g DM/kg live weight0·75/d equivalent to 95 % of ad libitum intake measured at the start of the experiment. Experimental treatments were designed to be within the range of FO doses evaluated in cattle fed grass silage(Reference Lee, Shingfield and Tweed16), grass silage-based diets(Reference Scollan, Dhanoa and Choi12, Reference Lee, Tweed and Moloney18, Reference Kim, Huws and Lee19) or red clover silage(Reference Lee, Shingfield and Tweed16), allowing inferences to be drawn on the role of forage species on the potential to enhance the supply of 20 : 5n-3 and 22 : 6n-3 for incorporation into meat and milk in ruminants. Diets were fed as total mixed rations at a restricted intake to avoid selection of dietary components and maintain a constant forage:concentrate ratio across treatments. Ration mixes were adjusted weekly for changes in component DM content. Supplements of FO were mixed with concentrate ingredients immediately before the addition of maize silage to optimise oil dispersal in the diet. FO was stored in the dark at 4°C before inclusion in daily rations.
Table 1 Ingredient and chemical composition of experimental diets (g/kg DM)

OM, organic matter; NDF, neutral-detergent fibre; WSC, water-soluble carbohydrate.
* Maize silage contained (g/kg) 12 : 0 (0·06), 14 : 0 (0·07), 16 : 0 (3·92), cis-9 16 : 1 (0·08), 17 : 0 (0·04), 18 : 0 (0·58), cis-9 18 : 1 (5·03), cis-11 18 : 1 (0·21), 18 : 2n-6 (11·18), 18 : 3n-3 (1·47), 20 : 0 (0·30), cis-9 20 : 1 (0·04), cis-11 20 : 1 (0·06), 20 : 2n-6 (0·04), 22 : 0 (0·13), cis-13 22 : 1 (0·02), cis-15 24 : 1 (0·02) and total fatty acids (23·7).
† Solvent-extracted rapeseed meal of low glucosinolate content.
‡ Fish oil contained (g/kg) 12 : 0 (1·26), 14 : 0 (69·0), cis-9 14 : 1 (0·52), 16 : 0 (143), cis-9 16 : 1 (74·5), 16 : 2n-4 (10·9), 16 : 3n-4 (13·5), 16 : 4n-1 (23·5), 16 : 4n-3 (1·49), 17 : 0 (3·85), 18 : 0 (24·7), cis-9 18 : 1 (105), cis-11 18 : 1 (25·7), cis-12 18 : 1 (0·55), 18 : 2n-6 (11·1), 18 : 3n-3 (8·67), 18 : 3n-6 (2·37), 18 : 4n-3 (28·0), 20 : 0 (1·65), cis-9 20 : 1 (1·50), cis-11 20 : 1 (11·8), cis-13 20 : 1 (2·26), 20 : 2n-3 (0·49), 20 : 2n-9 (1·84), 20 : 3n-6 (1·42), 20 : 4n-3 (6·82), 20 : 4n-6 (7·54), 20 : 5n-3 (157), 21 : 5n-3 (6·56), 22 : 0 (0·66), cis-11 22 : 1 (6·88), cis-15 22 : 1 (2·24), 22 : 5n-3 (16·7), 22 : 5n-6 (2·36), 22 : 6n-3 (99·4), 24 : 0 (0·31), cis-15 24 : 1 (4·40) and total fatty acids (950).
§ Regumaize 44 (SvG Intermol Limited, Bootle, Merseyside, UK); declared composition (g/kg DM) crude protein (440), water-soluble carbohydrate (550) and metabolisable energy content (11·8 MJ/kg DM).
∥ Proprietary mineral supplement (Dairy direct, Bury St. Edmonds, UK) declared as containing (g/kg) Ca (270), Mg (60), Na (40), P (40), Zn (5·0), Mn (4·0), Cu (1·5); (mg/kg) iodine (500), Co (50), Se (15), retinyl acetate (150), cholecalciferol (2·50) and dl-α-tocopheryl acetate (500).
Forage maize (cv. Hudson) was harvested using a forage harvester fitted with grain crackers and ensiled directly without additive. Concentrates were formulated(20) to meet the nutrient requirements of growing cattle.
Measurements and sampling
Individual animal intakes were recorded daily, but only measurements collected during the last 5 d of each experimental period were used for statistical analysis. During this period, samples of fresh maize silage, concentrate ingredients and feed refusals were collected daily, and DM content was determined by drying in a forced draught oven at 100°C for 24 h. Feed samples collected daily were added to a composite sample for each experimental period and stored at − 20°C. Frozen samples of maize silage were analysed for volatile fatty acids (VFA), ethanol, lactic acid and ammonia nitrogen using accredited and Parliamentary approved procedures for feedstuff analysis (Statutory Instruments, 1982; 1985) by a commercial laboratory (Natural Resources Management, Bracknell, UK), and used to correct the DM content of maize silage for volatile losses during drying(Reference Juniper, Browne and Fisher21). Organic matter (OM) content of maize silage and concentrates was determined by ashing at 550°C for 16 h. Neutral-detergent fibre (NDF) concentrations in maize silage and concentrates corrected for residual ash were measured in the presence of SDS and α-amylase using an ANKOM Fibre analyser (ANKOM-Technology, Fairport, NY, USA)Reference Juniper, Browne and Fisher21. Feed starch content was measured using the amyloglucosidase technique(Reference MacRae and Armstrong22) followed by the determination of total reducing substances and correction for water-soluble carbohydrates, nitrogen was assessed by the Kjeldahl technique and water-soluble carhohydate content was determined by spectrophotometry according to standard procedures(Reference Juniper, Browne and Fisher21). Samples (40 ml) of rumen fluid (n 9) were collected on day 20 of each period from each steer at 1·5 h intervals starting at 06.00 hours. Following removal, pH was measured (pH meter HI8520; Hanna Instruments Ltd., Leighton Buzzard, UK), and samples were stored at − 20°C. At the end of the experiment, samples of rumen fluid collected at each time point were bulked on an equal volume basis, and daily composite samples were analysed for VFA by a commercial laboratory (Natural Resources Management) using the same procedures applied to feeds.
Digesta flow was determined using LiCoEDTA and Cr-mordanted straw as indigestible markers for liquid and particulate phases, respectively(Reference Ahvenjärvi, Vanhatalo and Shingfield23). Coarsely chopped barley straw was soaked in tap water overnight, rinsed with neutral detergent and labelled with chromium(Reference Udén, Colucci and Van Soest24). Cr-mordanted straw containing 40·5 (se 0·30) mg Cr/g DM was administered (20 g/d) twice daily on top of the rumen contents via the cannula at 12 h intervals starting at 18.00 hours on day 14 of each experimental period. LiCoEDTA (6 g) prepared according to standard procedures(Reference Udén, Colucci and Van Soest24) was dissolved in 3 litres of distilled water and infused at 18.00 hours on day 14 into the rumen at a constant rate (2·1 ml/min). Ruminal infusions were made using polyamide tubing (internal diameter 4 mm) that passed through the rumen fistula and a peristaltic pump (Model 202; Watson-Marlow, High Wycombe, UK). Markers were administered to each animal to provide daily doses of 0·8 and 0·9 g/d of Cr and Co, respectively. At the start of each marker administration, steers were given priming doses of Cr-mordanted straw and LiCoEDTA supplying 1·0 and 1·35 g of Cr and Co, respectively, to facilitate rapid equilibration of the marker concentrations in the rumen.
Spot samples (250 ml) of digesta at the duodenum were collected three times daily at 4 h intervals over the last 4 d of each experimental period starting at 06.00 hours on day 18. Immediately after collection, 2·5 ml of 2,6-di-tert-butyl-4-methoxyphenol in 80 % (v/v) methanol (1 mg/1 ml) were added, and samples were stored under nitrogen at − 20°C. At the end of the study, digesta from each animal was thawed at room temperature, and pooled on an equal volume basis across sampling times to provide a composite sample for each experimental period. Composite digesta samples were stirred vigorously and split into two equal subsamples. One subsample was frozen and lyophilised as whole duodenal digesta, while the remainder was separated into liquid and solid phases by centrifugation at 200 g for 10 min at 4°C. The supernatant was decanted and stored at − 20°C, while the solid phase was frozen immediately, lyophilised and stored at − 20°C. Samples of solid and whole digesta were analysed for DM, OM, N, ammonia N, starch and NDF using the same methods used for feed ingredients. Concentrations of Cr and Co in digesta were measured by atomic absorption spectroscopy (SpectrAA-10 analyser, Varian Limited, Walton-On-Thames, UK) using reference procedures(Reference Williams, David and Riismaa25) and samples of duodenal digesta and faeces collected from one steer before the start of the experiment for calibration purposes.
Whole-tract apparent digestibility coefficients were determined by total faecal collection. Faeces were collected over 120 h starting at 10.00 hours on day 17 of each experimental period. Total faeces excreted were weighed, thoroughly mixed, subsampled (10 %, w/w) and stored at − 20°C until analysed for DM, OM, NDF, starch, N, Cr and Co contents using the same methods used for the analysis of duodenal digesta. Flows of digesta at the duodenum were calculated after mathematical reconstitution of true digesta(Reference Faichney, McDonald and Warner26). Marker administration was based on faecal excretion. Appearance of Co in faeces was not corrected for potential absorption from the gastrointestinal tract.
Lipid analysis
Fatty acid methyl esters (FAME) of lipids in FO and freeze-dried samples of maize silage and concentrates were prepared in a one-step extraction–transesterification procedure using chloroform(Reference Sukhija and Palmquist27) and 2 % (v/v) sulphuric acid in methanol(Reference Shingfield, Ahvenjärvi and Toivonen8). Feed fatty acid content was determined using trinonadecanoin (T-165; Nu-Chek-Prep, Elysian, MN, USA) as an internal standard. Following the addition of 100 μl of internal standard (heneicosanoate in chloroform (15 mg/ml)), lipid in solid and whole digesta samples was extracted in triplicate using a mixture of chloroform–methanol (2:1; v/v). Organic extracts were combined, dried under nitrogen at 50°C, dissolved in hexane and converted to FAME using a base–acid-catalysed transesterification procedure by incubation with freshly prepared 0·5 m-sodium methoxide in methanol at 50°C for 15 min followed by reaction with 5 % (v/v) hydrochloric acid in methanol at 50°C for 60 min(Reference Kramer and Zhou28).
The FAME were separated and quantified using a gas chromatograph (3800 CP, Varian Instruments, Walnut Creek, CA, USA) equipped with a flame ionisation detector, automatic injector, split injection port and a 100 m fused silica capillary column (CP-SIL 88 for FAME; Chrompack, Middelburg, The Netherlands) with helium as the carrier gas and hydrogen as the fuel gas. Total FAME profile in a 1 μl sample at a split ratio of 1:30 was determined using a temperature gradient programme(Reference Lee, Tweed and Moloney18). Peaks were identified by comparison of retention times with authentic FAME standards (ME61, Larodan fine chemicals, Malmo, Sweden; S37, Supelco, Poole, Dorset, UK). Methyl esters in feed ingredients and duodenal digesta not contained in commercially available standards were formally identified by GC-MS analysis of 4,4-dimethyloxazoline fatty acid derivatives prepared from selected samples of FAME by incubation overnight with 2-amino, 2-methyl-1-propanol under a nitrogen atmosphere at 150°C(Reference Shingfield, Reynolds and Hervás29). Impact ionisation spectra of 4,4-dimethyloxazoline fatty acid derivatives were recorded under an ionisation energy of 70 eV using a gas chromatograph (Model 6890; Hewlett-Packard, Wilmington, DE) equipped with a selective quadrupole mass detector (Model 5973N, Agilent Technologies Inc., Wilmington, DE) and a 100 m fused silica capillary column (internal diameter 0·25 mm) coated with 0·2 μm film of cyanopropyl polysiloxane (CP-SIL 88; Chrompack 7489, Middelburg, The Netherlands) using a temperature gradient and helium as the carrier gas(Reference Shingfield, Reynolds and Hervás29). Double bond geometry was determined based on atomic mass unit distances, with an interval of twelve atomic mass units between the most intense peaks of clusters of ions containing n and n-1 carbon atoms being interpreted as cleavage of the double bond between carbon n and n+1 in the fatty acid moiety.
Samples of FAME were evaporated under nitrogen, dissolved in heptane and analysed for conjugated linoleic acid (CLA) methyl ester composition by HPLC using four silver-impregnated silica columns (ChromSpher 5 lipids, 250 × 4·6 mm; 5 μm particle size, Varian Ltd., Walton-on-Thames, UK) coupled in series and 0·1 % (v/v) acetonitrile in heptane as the mobile phase(Reference Shingfield, Ahvenjärvi and Toivonen8). Isomers were identified using an authentic CLA methyl ester standard (O-5632, Sigma-Aldrich) and chemically synthesised trans-9, cis-11 CLA(Reference Shingfield, Reynolds and Lupoli30). Identification was verified by cross-referencing with the elution order reported in the literature(Reference Delmonte, Kataoka and Corl31) using cis-9, trans-11 CLA as a landmark isomer.
Statistical analysis
Experimental data were subjected to ANOVA using the mixed linear model procedure of Statistical Analysis Systems software package version 8.2 (SAS Institute, Cary, NC, USA) with a model that included the random effects of animal and fixed effects of period and treatment. Sums of squares for treatment effects were further separated using orthogonal contrasts into single degree of freedom comparisons to test for the significance of linear, quadratic and cubic components of the response to experimental treatments. Least-square means are reported, and treatment effects were declared significant at P < 0·05. Treatments effects at P < 0·10 were considered as a trend towards significance.
Results
Food composition
Maize silage had the following chemical composition and fermentation characteristics (g/kg DM, unless otherwise stated): DM (g/kg fresh weight), 372 (se 4·7); OM, 936 (se 2·0); N, 14·1 (se 0·13); NDF, 371 (se 24·3); starch, 396 (se 10·7); pH, 3·84 (se 0·024); lactic acid, 27·9 (se 6·11); VFA, 15·5 (se 1·90); ethanol, 2·91 (se 1·186); water-soluble carbohydrate, 3·28 (se 0·744); ammonia N (g/kg total N), 50·0 (se 4·22). The basal concentrate contained (g/kg DM, unless otherwise stated): DM (g/kg fresh weight), 877 (se 3·2); OM, 871 (se 2·5); N, 58·8 (se 0·52); NDF, 192 (se 5·9); starch, 61·8 (se 2·13); water-soluble carbohydrate, 96·5 (se 0·90).
Maize silage contained 23·7 g fatty acids/kg DM with relatively high amounts (g/kg DM) of 16 : 0 (3·92), cis-9 18 : 1 (5·03) and 18 : 2n-6 (11·2). The basal concentrate (total fatty acids 28·2 g/kg DM) was relatively abundant in 16 : 0, cis-9 18 : 1 and 18 : 2n-6 (4·36, 9·02 and 7·93 g/kg DM, respectively), while FO contained (g/kg) 20 : 5n-3 (157), 22 : 5n-3 (16·7) and 22 : 6n-3 (99·4) with a total fatty acid content of 950 g/kg. FO also contained several fatty acids not present in the other feed ingredients including 18 : 4n-3 (28·0), 20 : 4n-3 (6·82), 20 : 4n-6 (7·55) and 21 : 5n-3 (6·56).
Nutrient intake
Incremental inclusion of FO in the diet decreased (P < 0·05) DM, OM, N, NDF, starch and water-soluble carbohydrate intakes in a quadratic manner with the effects being greater at the highest amount of FO (Table 2). Supplementing the diet with FO increased linearly (P < 0·01) the intake of most fatty acids including 14 : 0, 16 : 0, 18 : 0, cis-9 18 : 1, 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3, while the highest FO dose decreased (P = 0·003) 18 : 2n-6 ingestion (Table 2).
Table 2 Effect of incremental amounts of fish oil in the diet on feed and nutrient intake in growing cattle
(Mean values with their standard errors)

OM, organic matter; NDF, neutral-detergent fibre; WSC, water-soluble carbohydrate.
* Significance of linear (L) and quadratic (Q) components of the response to fish oil in the diet. Cubic responses to fish oil in the diet were NS (P>0·05).
† sem for n 16 measurements; error df 6.
Rumen fermentation
Inclusion of FO in the diet had no effect (P>0·05) on rumen pH or ammonia N concentrations, but tended (P = 0·06) to decrease linearly rumen VFA content (Table 3). Supplements of FO increased linearly (P < 0·01) molar proportions of propionate and reduced linearly (P < 0·01) molar butyrate proportions, with a trend (P = 0·08) towards a linear decrease in molar proportions of acetate in rumen VFA (Table 3).
Table 3 Effect of incremental amounts of fish oil in the diet on rumen fermentation characteristics in growing cattle
(Mean values with their standard errors)
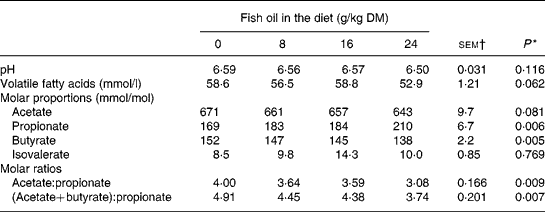
* Significance of linear (L) components of the response to fish oil in the diet. Quadratic and cubic responses to fish oil in the diet were NS (P>0·05), with the exception of a trend (P = 0·097) towards a cubic decrease in rumen volatile fatty acid concentrations to fish oil supplementation.
† sem for n 16 measurements; error df 6.
Nutrient flow at the duodenum
Increasing amounts of FO in the diet decreased linearly (P < 0·05) duodenal DM, OM, N and non-ammonia N flow, and tended to reduce (P = 0·07) post-ruminal NDF flow, but had no effect (P>0·05) on the amount of starch or total fatty acids at the duodenum (Table 4). Supplements of FO altered the composition of fatty acids flowing into the duodenum, changes that were characterised by linear or quadratic increases in 14 : 0, 15 : 0, 16 : 0, 3, 7, 11, 15-tetra-methyl-16 : 0, 16 : 1, 17 : 0, 18 : 1, 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3 and linear reductions in 18 : 0 and 18 : 3n-3 at the duodenum (Table 4). FO enhanced linearly (P < 0·001) the flow of all trans 16 : 1 isomers (Δ6–13), while changes in the amount of 18 : 1 at the duodenum to FO were isomer dependent (Table 5). Duodenal flow of trans-4, -5 and -16 18 : 1 was independent of treatment, whereas FO in the diet increased linearly (P < 0·05) the flow of trans-6 to -15 18 : 1 at the duodenum, with most of the increase being related to elevated levels of trans-11 18 : 1. FO in the diet also altered the profile of 18 : 2 of duodenal digesta, increasing linearly (P < 0·05) trans-11, cis-15 18 : 2, trans-9, trans-12 18 : 2 and trans-8, trans-10 CLA and decreasing linearly (P < 0·05) trans-11, cis-13 CLA flow at the duodenum (Table 6). Supplements of FO also tended (P = 0·07) to reduce the amount of cis-9, cis-12 18 : 2 at the duodenum and altered trans-9, trans-11 CLA flow (Table 6) in a quadratic manner (P < 0·01). Incremental amounts of FO in the diet increased linearly (P < 0·05) the extent of cis-9 18 : 1, 18 : 3n-3, 20 : 5n-3 and 22 : 6n-3 biohydrogenation in the rumen (Table 7).
Table 4 Effect of incremental amounts of fish oil in the diet on the flow of nutrients at the duodenum in growing cattle
(Mean values with their standard errors)

OM, organic matter; NDF, neutral-detergent fibre; N, nitrogen; NAN, non-ammonia nitrogen; CLA, conjugated linoleic acid.
* Significance of linear (L) and quadratic (Q) components of the response to fish oil in the diet. Cubic responses to fish oil in the diet were NS (P>0·05).
† semfor n 16 measurements; error df 6.
‡ Total 18 : 2 excluding isomers of CLA.
§ Phytanic acid, all isomers of 3, 7, 11, 15-tetra-methyl-hexadecanoic acid.
Table 5 Effect of incremental amounts of fish oil in the diet on the flow of 16 : 1 and 18 : 1 isomers at the duodenum in growing cattle
(Mean values with their standard errors)

* Significance of linear (L) and quadratic (Q) components of the response to fish oil in the diet. Cubic responses to fish oil in the diet were NS (P>0·05).
† sem for n 16 measurements; error df 6.
‡ Contains cis-14 18 : 1 as a minor component.
Table 6 Effect of incremental amounts of fish oil in the diet on the flow of 18 : 2 isomers at the duodenum in growing cattle
(Mean values with their standard errors)

CLA, conjugated linoleic acid.
* Significance of linear (L) and quadratic (Q) components of the response to fish oil in the diet. Cubic responses to fish oil in the diet were NS (P>0·05).
† sem for n 16 measurements; error df 6.
Table 7 Effect of incremental amounts of fish oil in the diet on the apparent ruminal biohydrogenation of unsaturated fatty acids in growing cattle
(Mean values with their standard errors)
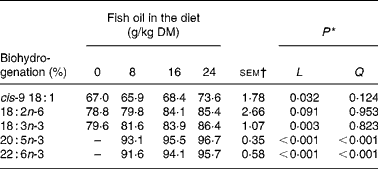
* Significance of linear (L) and quadratic (Q) components of the response to fish oil in the diet. Cubic responses to fish oil in the diet were NS (P>0·05).
† sem for n 16 measurements; error df 6.
Nutrient digestibility
Inclusion of FO in the diet had no effect (P>0·05) on forestomach (defined as rumen, reticulum, omasum and abomasum) or whole-tract nutrient digestibility coefficients, with the exception of a trend (P = 0·06) towards an increase in total-tract DM digestibility (Table 8).
Table 8 Effect of incremental amounts of fish oil in the diet on rumen and whole-tract apparent digestibility coefficients in growing cattle
(Mean values with their standard errors)

OM, organic matter; NDF, neutral-detergent fibre; N, nitrogen.
* Significance of linear (L) and quadratic (Q) components of the response to fish oil in the diet. Cubic responses to fish oil in the diet were NS (P>0·05).
† sem for n 16 measurements; error df 6.
Live weight
Supplementing the diet with FO had no effect (P < 0·05) on mean animal live weight (604, 612, 605 and 602 kg for diets containing 0, 8, 16 and 24 g FO/kg DM, respectively).
Discussion
A number of experiments have examined the potential of FO to increase the supply of 20 : 5n-3 and 22 : 6n-3 available for absorption in growing cattle fed grass silage(Reference Lee, Shingfield and Tweed16), red clover silage(Reference Lee, Shingfield and Tweed16) or grass silage-based diets(Reference Scollan, Dhanoa and Choi12, Reference Lee, Tweed and Moloney18, Reference Kim, Huws and Lee19), but measurements in ruminants fed diets containing maize silage are limited(Reference Doreau and Chilliard32). In the present experiment, the effects of incremental amounts of FO on ruminal lipid metabolism and the flow of fatty acids at the duodenum in growing cattle fed maize silage-based diets were examined. Experimental treatments were designed to be within the range of FO doses evaluated previously in growing cattle(Reference Scollan, Dhanoa and Choi12, Reference Lee, Shingfield and Tweed16, Reference Lee, Tweed and Moloney18, Reference Kim, Huws and Lee19), allowing inferences to be drawn on the possible role of dietary forage type on the potential of FO to enhance 20 : 5n-3 and 22 : 6n-3 supply in ruminants.
Nutrient intake and digestion
Even though steers were fed diets at a restricted intake in the present experiment, inclusion of 24 g/kg DM of FO lowered DM intake, whereas lower rates of supplementation had no effect compared with the control. Previous studies have demonstrated that incremental inclusion of FO in the diet from 0 to 39 g/kg DM had no effect on DM intake in growing cattle fed grass silage diets(Reference Lee, Tweed and Moloney18, Reference Kim, Huws and Lee19, Reference Keady and Mayne33). In contrast, FO in the diet at a rate of 30 g/kg DM was reported to reduce DM intake of steers fed red clover silage, but not grass silage(Reference Lee, Shingfield and Tweed16), while studies in lactating cows have shown that FO in the diet depresses nutrient intake in a dose-dependent manner(Reference Donovan, Schingoethe and Baer7, Reference Keady, Mayne and Fitzpatrick34, Reference Loor, Doreau and Chardigny35). Comparisons of responses to ruminal or duodenal infusions of FO indicate that the effects on DM intake are related to the effects arising from changes in rumen function(Reference Doreau and Chilliard32, Reference Loor, Doreau and Chardigny35), possibly mediated via an increase in the amount of unsaturated fatty acid leaving the rumen(Reference Shingfield, Reynolds and Hervás29). The extent to which FO alters nutrient intake appears to be related to several factors including the overall composition of the diet and level of DM intake, as well as the amount and composition of FO supplements.
Negative effects of lipids rich in PUFA on DM intake have often been attributed to reductions in ruminal digestion and a shift towards more extensive digestion of nutrients in the intestine, as well as to increases in the amount of lipid reaching the small intestine(Reference Jenkins36). Even though the highest rate of FO supplementation in the present experiment lowered DM intake, this was not accompanied by a reduction in ruminal DM, OM or NDF digestion, when expressed in relation to the amounts consumed, with no evidence of a greater proportion of nutrient digestion occurring in the hindgut. In growing cattle fed grass silage or grass silage-based diets, FO has been reported to either decrease(Reference Lee, Shingfield and Tweed16) or increase the extent of OM and NDF digestion in the rumen(Reference Kim, Huws and Lee19), whereas the proportion of these nutrients digested in the forestomach was shown to be independent of FO in the diet in steers fed red clover silage(Reference Lee, Shingfield and Tweed16). In lactating cattle, FO tends to improve ruminal OM digestion due to increases in ruminal N digestibility(Reference Shingfield, Ahvenjärvi and Toivonen8) and increase whole-tract DM, OM and NDF digestibility coefficients(Reference Shingfield, Ahvenjärvi and Toivonen8, Reference Doreau and Chilliard32, Reference Keady, Mayne and Fitzpatrick34). Alterations in the site and extent of nutrient digestion due to FO in the diet probably reflect associated changes in nutrient intake, since reductions in DM intake generally improve all of these parameters(Reference Huhtanen and Kukkonen37).
Rumen fermentation
Incremental inclusion of FO in the diet had no effect on rumen pH, but altered rumen fermentation towards propionate at the expense of butyrate with a trend towards decreased molar proportions of acetate. Lipolysis of ingested TAG liberates glycerol that serves as a substrate for propionate production in the rumen(Reference Varman, Schultz and Nichols38), but this would not in isolation explain the changes in rumen fermentation observed in the present experiment. Earlier studies have shown that FO had no major effect on rumen pH or fermentation characteristics in steers fed grass silage(Reference Lee, Shingfield and Tweed16), red clover silage(Reference Lee, Shingfield and Tweed16) or grass silage-based diets(Reference Scollan, Dhanoa and Choi12, Reference Lee, Tweed and Moloney18, Reference Kim, Huws and Lee19), whereas FO has been reported to enhance the ratio of glucogenic:lipogenic precursors in the rumen of lactating cows fed grass silage(Reference Shingfield, Ahvenjärvi and Toivonen8), lucerne hay(Reference Varman, Schultz and Nichols38) and maize silage-based diets(Reference Doreau and Chilliard32). Furthermore, inclusion of herring and mackerel oils in concentrate supplements for growing cattle fed grass silage(Reference Keady and Mayne33) had no effect on rumen VFA, while the addition of a FO premix of different fatty acid composition decreased the molar proportion of acetate and increased that of propionate. Overall evidence from the literature suggests the impact on rumen fermentation being dependent on several factors including the source and inclusion rate of FO supplements, as well as the intake potential and composition of the diet. It is possible that variation in rumen fermentation patterns between studies is, at least in part, related to FO inducing changes in the relative abundance of specific microbial populations in the rumen. Recent reports on the 16S ribosomal RNA-based denaturing gradient gel electrophoresis profiling of ruminal digesta have provided the first indications that FO alters total ruminal eubacteria and Butyrivibrio populations in growing cattle(Reference Kim, Huws and Lee19).
Rumen lipid metabolism
Comparison of the intake and duodenal flow of fatty acids indicated a net synthesis of fatty acids in the rumen of steers fed maize silage-based diets containing 0, 8 and 16 g/kg DM of FO (151, 150 and 95 g/d, respectively), whereas FO at 24 g/kg DM resulted in comparable intake and flow of fatty acids at the duodenum. Measurements in growing cattle fed grass silage or red clover silage reported a net synthesis of fatty acids to moderate amounts of FO (10 g/kg DM), whereas higher amounts (20–30 g/kg DM) of FO in the diet resulted in a net disappearance(Reference Lee, Shingfield and Tweed16). On typical diets, 75–80 % of ingested fatty acids are recovered at the duodenum, but the balance between post-ruminal fatty acid flow relative to intake can be expected to be negative in ruminants fed diets containing more than 40–50 g lipid/kg DM(Reference Doreau and Ferlay39, Reference Schmidely, Glasser and Doreau40). Data from the present and earlier experiments(Reference Shingfield, Ahvenjärvi and Toivonen8, Reference Lee, Shingfield and Tweed16) indicate that at relatively high rates of supplementation, FO can be expected to decrease the contribution of bacterial lipid to total post-ruminal fatty acid flow.
Supplementing maize silage-based diets with FO enhanced the flow of 20 : 5n-3 (mean recovery 4·57, 3·36 and 2·49 % for diets containing 8, 16 and 24 g/kg DM of FO, respectively) and 22 : 6n-3 (mean recovery 5·85, 4·57 and 3·42 %) at the duodenum, but the increases were marginal relative to the intake of these fatty acids, confirming previous reports(Reference Lee, Shingfield and Tweed16, Reference Lee, Tweed and Moloney18, Reference Kim, Huws and Lee19) that the potential to enhance the flow of long-chain n-3 fatty acids leaving the rumen in growing cattle is limited. Indirect comparisons with reports in the literature provide little support that the extent of ruminal long-chain n-3 fatty acid metabolism is lower in growing cattle fed diets containing maize silage than in those fed diets containing grass silage or red clover silage when comparisons of biohydrogenation are made based on the concentration of 20 : 5n-3 and 22 : 6n-3 in the diet (Fig. 1).

Fig. 1 Relationship between the concentration in the diet and biohydrogenation of 20 : 5n-3 (a) and 22 : 6n-3 (b) in the rumen of growing cattle. Data derived from studies reporting the effects of fish oil in the diet in steers fed red clover silage (○)(Reference Lee, Shingfield and Tweed16), grass silage (●)(Reference Lee, Shingfield and Tweed16), grass silage-based diets (□(Reference Scollan, Dhanoa and Choi12), ▲(Reference Lee, Tweed and Moloney18), △(Reference Kim, Huws and Lee19)) or maize silage-based diets determined in the present experiment (■).
By analogy with the known pathways of C18 unsaturated fatty acid metabolism in the rumen(Reference Harfoot, Hazlewood and Hobson41), complete biohydrogenation of 20 : 5n-3 and 22 : 6n-3 would be expected to yield 20 : 0 and 22 : 0 as final end products, respectively. Measurements in the present experiment indicated that in low amounts FO enhanced the flow of 20 : 0 and 22 : 0 at the duodenum, but at inclusion rates above 8 g FO/kg DM, the magnitude of increase relative to the control diet declined. Since changes in duodenal flow of 20 : 0 and 22 : 0 are not explained by the intake of these fatty acids from the diet, it appears that 20 : 5n-3 and 22 : 6n-3 or other C20 and C22 unsaturated fatty acids in FO can be completely hydrogenated in the rumen, but at higher rates of FO inclusion, the biohydrogenation of long-chain unsaturated fatty acids becomes progressively incomplete.
Recovery of dietary fatty acids at the duodenum for total C20 (141, 82, 37 and 34 %) and C22 (191, 52, 27 and 19 %) fatty acids in diets containing 0, 8, 16 and 24 g FO/kg DM, respectively, could be interpreted as evidence of the removal of long-chain fatty acids from the rumen via β-oxidation or diffusion across the rumen epithelium(Reference Doreau and Ferlay39, Reference Schmidely, Glasser and Doreau40). However, under normal circumstances, losses of fatty acids in the rumen are thought to be of minor importance owing to the association of non-esterfied fatty acids with feed particles(Reference Harfoot, Hazlewood and Hobson41). A low recovery of long-chain fatty acids at high levels of FO in the diet is most probably explained by the formation of C20 and C22 biohydrogenation intermediates that were unable to be identified and quantified in the present experiment. It has been speculated that metabolism of 20 : 5n-3 and 22 : 6n-3 involves the formation of intermediates with five or six double bonds with at least one in the trans configuration(Reference Jenkins, Wallace and Moate42), while two-dimensional GC analysis of milk fat has provided the first indications that ruminal biohydrogenation of 22 : 6n-3 results in the formation of numerous C22 metabolites(Reference Vlaeminck, Harynuk and Fievez43).
Supplements of FO also altered ruminal metabolism of C18 unsaturated fatty acids, resulting in an increase in the flow of trans 18 : 1 and trans 18 : 2 and reduction in 18 : 0 and 18 : 3n-3 at the duodenum. Earlier studies have demonstrated that FO inhibits the complete biohydrogenation of C18 unsaturated fatty acids in the rumen, leading to the accumulation of 18 : 1 and 18 : 2 biohydrogenation intermediates, trans-11 18 : 1 in particular(Reference Shingfield, Ahvenjärvi and Toivonen8, Reference Lee, Shingfield and Tweed16, Reference Lee, Tweed and Moloney18, Reference Kim, Huws and Lee19). In the present experiment, dose-dependent changes in the flow of 18 : 0 and C18 biohydrogenation intermediates were also associated with increases in the extent of ruminal cis-9 18 : 1, 18 : 2n-6 and 18 : 3n-3 metabolism. Previous studies have reported that FO has no effect(Reference Shingfield, Ahvenjärvi and Toivonen8) or increases the extent of C18 unsaturated fatty acid metabolism(Reference Lee, Shingfield and Tweed16,18), or in some circumstances, can decrease ruminal cis-9 18 : 1 biohydrogenation(Reference Kim, Huws and Lee19) in vivo. Differences in the impact of FO or long-chain n-3 fatty acids on the extent of C18 fatty acid biohydrogenation are difficult to reconcile, but they may reflect alterations in the rumen microbial community and the proliferation of rumen bacterium involved in lipolysis and biohydrogenation.
Even though FO altered the flow of CLA at the duodenum and the distribution of specific CLA isomers in a quadratic manner, the magnitude of the changes in cis-9, trans-11 CLA, trans-11, cis-13 CLA and trans-9, trans-11 CLA while significant on diets containing 8 or 16 g FO/kg DM, was relatively minor in absolute terms. In ruminants fed 18 : 3n-3-rich diets, FO enhanced ruminal outflow of trans-7, trans-9 CLA, trans-8, trans-10 CLA and trans-9, trans-11 CLA and decreased cis-12, trans-14 CLA accumulation(Reference Shingfield, Ahvenjärvi and Toivonen8, Reference Lee, Shingfield and Tweed16). Results from the present experiment confirmed that cis-9, trans-11 CLA is the major isomer at the duodenum in cattle fed maize silage-based diets(Reference Piperova, Sampugna and Teter44), and that irrespective of diet composition, trans-7, cis-9 CLA is not produced in the rumen(Reference Shingfield, Ahvenjärvi and Toivonen8, Reference Lee, Shingfield and Tweed16, Reference Piperova, Sampugna and Teter44). Furthermore, there was no clear effect on the flow of trans-10, cis-12 CLA at the duodenum, indicating that the inhibitory effects of FO in the diet on mammary lipogenesis in ruminants must be related to increased formation of other biohydrogenation intermediates and/or other mechanisms(Reference Shingfield and Griinari45).
In addition to the effects on ruminal C18 biohydrogenation, the present experiment also revealed that FO inhibits the metabolism of C16 unsaturated fatty acids in the rumen. Supplements of FO increased the flow of trans (Δ6–13) 16 : 1 isomers at the duodenum, with the changes in trans-11 16 : 1 being quantitatively the most important. It is probable that the increased accumulation and outflow of these isomers in response to FO addition are explained by incomplete metabolism of 16 : 2n-4, 16 : 3n-4, 16 : 4n-1 and 16 : 4 n-3 in the rumen. It has been suggested that the effects of FO on ruminal lipid metabolism are related to a reduction in the number and activity of bacteria capable of metabolising C18 unsaturated fatty acids to 18 : 0 or due to the direct inhibition of reductases which catalyse the penultimate step of biohydrogenation in the rumen(Reference Shingfield, Ahvenjärvi and Toivonen8, Reference Lee, Shingfield and Tweed16–Reference Lee, Tweed and Moloney18). Thus far, the only rumen bacterium known to metabolise 18 : 2n-6 and 18 : 3n-3 to 18 : 0 is Clostridium proteoclasticum (Reference Wallace, Chaudhary and McKain46, Reference Maia, Chaudhary and Figueres47), but data to support that FO alters ruminal lipid metabolism via a direct effect on this rumen bacterium are equivocal(Reference Kim, Huws and Lee19).
Conclusions
Supplementing maize silage diets with FO to high amounts reduced DM intake of growing cattle, and shifted rumen fermentation towards propionate at the expense of acetate and butyrate. Incremental inclusion of FO in the diet enhanced the flow of 20 : 5n-3 and 22 : 6n-3 at the duodenum, but the magnitude of increase was marginal relative to the intake from the diet due to extensive metabolism of these fatty acids in the rumen. Supplementing the diet with FO altered ruminal lipid metabolism leading, to dose-dependent increases in the flow of trans 16 : 1, trans 18 : 1 and trans 18 : 2, and a decrease in 18 : 0 at the duodenum. In conclusion, current data offered no support that ruminal metabolism of long-chain n-3 fatty acids is lower in growing cattle fed diets based on maize silage compared with other forages.
Acknowledgements
The present research was funded by a grant from the Department for Environment, Food and Rural Affairs. All authors have contributed to the preparation of the manuscript and agree with the submitted manuscript content. There are no conflicts of interest. The present research was funded by a grant (Project LS 3511: Producing Low-fat Healthy Ruminant Products) from the Department for Environment, Food and Rural Affairs. The authors gratefully acknowledge the skilled technical assistance of staff of the CEDAR Metabolism Unit for diligent care of experimental animals and assistance during sample collection, and J. K. S. Tweed for assistance with sample lipid analysis.











