Given the public and animal health concerns related to Coxiella burnetii infection (Q fever), the control of this disease is crucial. As ruminants are the main reservoir [Reference Arricau-Bouvery and Rodolakis1], decreasing the exposure of humans and animals to shedder ruminants is key to limiting the spread of the infection.
The vaccination of dairy cattle using a phase I vaccine was shown to be effective in preventing C. burnetii shedding when applied to non-infected animals [Reference Guatteo2]. Previous studies conducted in infected cattle herds reported that most nulliparous heifers were not infected [Reference Guatteo2–Reference Taurel4]. However, the within-herd prevalence (WHP) of infected dairy cows varied widely between herds. Therefore, the WHP of dairy cows needs to be determined prior to vaccination in order to confirm the relevance of the procedure on this category of animal.
Enzyme-linked immunosorbent assay (ELISA) applied to milk or serum [Reference Beaudeau5] currently is recognized as the most suitable serological method to identify ruminants infected by C. burnetii. Under the assumption that the level of antibodies in bulk tank milk (BTM) could increase with the increased prevalence of seropositive cows, as well as the proportion of highly seropositive cows in a herd, ELISA applied to BTM could be an efficient, cost-effective, alternative to exhaustive blood sampling to estimate the WHP of infected dairy cows. The approach has already been demonstrated to be effective for bovine viral diarrhoea virus in cattle [Reference Beaudeau5] and Q fever in dairy sheep [Reference Ruiz-Fons6].
The objective of the present study was to assess the relationship between the level of antibodies in BTM and the WHP of C. burnetii in cows in naturally infected dairy herds.
The herds included in the study: (i) had at least 50% of seropositive animals in a sample of at least six animals and a positive PCR result either on the placenta of an aborted cow or on BTM, and (ii) had not been vaccinated against Q fever in the last 5 years to avoid putative false-positive ELISA results.
The study was conducted between 2008 and 2010 in 55 dairy herds in western France. In each herd, one sample of BTM was collected and blood samples were taken from all of the milking cows. The samples were sent immediately to the Institut Départemental d'Analyses et de Conseil (Nantes, France) to be tested using the Q fever LSI ELISA kit (LSI, France). Results were expressed in an optical density sample/positive control (S/P) ratio. Serum were considered negative when the S/P ratio was ⩽40, low positive if 40 <S/P ratio ⩽100, positive if 100 <S/P ratio ⩽200, high positive if 200 <S/P ratio ⩽300, and very high positive if S/P ratio >300. BTM samples were considered negative if the S/P ratio was ⩽30, low positive if 30 <S/P ratio ⩽100, positive if 100 <S/P ratio ⩽200, and high positive if S/P ratio >200. The lactation number of each sampled cow and the number of milking cows were also recorded. This work was conducted in compliance with the STROBE statement for cross-sectional studies (www.strobe-statement.org).
The statistical unit was the herd. The outcome variable was the WHP of seropositive cows contributing to the BTM (PBTM), defined as the number of positive individual serum samples (S/P ratio >40) divided by the total number of individual blood samples collected. The relationship between the ELISA S/P ratio in BTM (S/PBTM) and the PBTM was first described using the Pearson correlation test and then quantified using a General Linear Model (GLM procedure, SAS Institute Inc., 1999). It is written as follows:
where μ is the overall mean, S/PBTM the ELISA S/P ratio in BTM (in four classes: ⩽30, 30<S/P ratio⩽100, 100<S/P ratio⩽200, >200); Hsize the number of milking cows (in two classes: <46, ⩾46), and PL1 the proportion of primiparous cows contributing to BTM (in two classes: <25%, ⩾25%). The herd size was included as a confounding factor to account for a putative dilutive effect of the number of cows tested on the S/PBTM. The proportion of primiparous cows contributing to BTM was taken into account by assuming that the risk of encountering C. burnetii is lower for primiparous cows than for older cows. The univariate analysis was performed first. Correlation was tested between explanatory variables retained after the first screening (P value <0·25) using a χ2 test. The GLM procedure included all factors retained. The variable with the highest P value was removed, and the model rerun until all variables had a P value <0·05.
The relationship between the number of highly seropositive cows (S/P ratio >200) and the S/PBTM was assessed using a zero-inflated Poisson (ZIP) model [Reference Cameron, Trivedi, Maddala and Rao7], after adjustment for herd size. To assess whether it was relevant to perform the ZIP model, and because many herds showed no highly seropositive cows, the excess of zero was first tested.
Fifty out of the 55 BTM samples tested were considered positive (S/PBTM>30), showing that ELISA applied to BTM to detect infected herds had a sensitivity of 91% (95% CI 85–99). The relationship between the S/P ratio in BTM and PBTM is shown in Figure 1. The Pearson correlation coefficient between the S/P ratio in BTM and PBTM was moderate (r=0·38, P value=0·005); graphically, no linear relationship was observed. We observed that when S/PBTM<100 (negative or low positive), all herds except one had a PBTM <30%, PBTM below 20% for S/PBTM<30. When S/PBTM ⩾100 (positive and high positive results), PBTM had a wide distribution (8–79%).
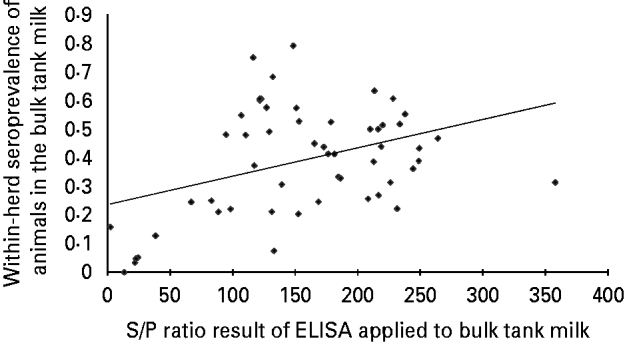
Fig. 1. Relationship between the ELISA S/P ratio of bulk tank milk and the within-herd seroprevalence of C. burnetii infection in milking cows in 55 naturally infected dairy herds.
In the final GLM model (Table 1), the PBTM increased significantly (P<0·05) in herds with higher S/PBTM. However, the mean PBTM did not increase when S/PBTM >100.
Table 1. Variables significantly (P<0·05) associated with within-herd seroprevalence of C. burnetii infection in milking cows of 55 naturally infected dairy herds
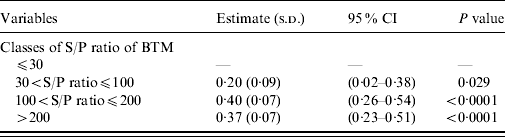
BTM, Bulk tank milk; CI, confidence interval.
Intercept=0·06 (−0·04 to 0·16), R 2=0·43, P value <0·0001.
The distribution of the number of highly seropositive animals confirmed the presence of excess zero values (P=0·0014). The ZIP model showed a positive relationship between the number of highly seropositive animals and the S/PBTM (results not shown). Consequently, a high S/PBTM may result from the contribution of a high number of seropositive animals and/or a few animals with a high level of antibodies.
To our knowledge, our study is the first to aim at assessing the relationship between the level of antibodies in BTM using ELISA and the within-herd seroprevalence of C. burnetii in cows based on an exhaustive sampling of cows carried out concomitantly with BTM collection, and taking into account some confounding factors. Consequently, the WHP reported here is deemed more reliable than those reported by previous studies conducted in cattle [Reference Muskens8] and sheep [Reference Ruiz-Fons6] that were assessed on a sub-sample of animals (30 per herd). Moreover, by taking the BTM and blood samples on the same day, any possible bias that could have been introduced by taking samples on different days (e.g. variation in individual serological status over time, variation in the population contributing to BTM) was avoided [Reference Ruiz-Fons6, Reference Muskens8].
In 5/55 infected herds, ELISA indicated a negative BTM result, leading to a negative predictive value below 100%. We could not assess its positive predictive value because we only investigated infected herds; consequently, we could not determine the specificity of ELISA applied to BTM.
As shown in previous studies [Reference Ruiz-Fons6, Reference Muskens8], the level of antibodies in BTM increased when the WHP of seropositive cows increased. Our findings suggest that in the case of a negative or low positive ELISA result on BTM, a high number of seronegative cows (assumed to be non-infected) can be expected. As vaccination is effective when applied to non-infected animals, the vaccination of dairy cows in addition to nulliparous heifers should thus be considered when the S/P BTM ratio suggests a low WHP. In addition to detecting infected herds [Reference Agger9], ELISA applied to BTM could be used to estimate a proxy of within-herd seroprevalence and create opportunities for epidemiological surveillance. However, as a differentiating infected from vaccinated animals (DIVA) ELISA has not yet been developed, once a herd has been vaccinated, ELISA can no longer be used on BTM to monitor the status of herds over time. Further studies on a larger scale should be performed to identify easy-to-collect information to improve the estimation of within-herd seroprevalence.
ACKNOWLEDGEMENTS
The authors thank all of the farmers, veterinarians and staff involved in this study (especially Jean-Yves Audiart), the Groupements de Défense Sanitaire of Western France and the Direction Générale de l'Alimentation (DGAl) for their financial support.
DECLARATION OF INTEREST
None.




