INTRODUCTION
Avian influenza (H5N1) infection has a high fatality rate compared to H3N2 and H1N1 infections [Reference Abdel-Ghafar1, Reference Oner2]. The last H1N1 epidemic demonstrated the importance of influenza pandemics and the role of human-to-human transmission [3, 4]. In the case of H1N1 infections, human-to-human transmission is widespread but mortality is low, whereas if this type of transmission was seen with avian influenza there could be a large number of deaths worldwide. An outbreak of highly pathogenic H5N1 virus infection caused 12 human infections, including four deaths, in Dogubayazit and Van, two cities in Eastern Turkey between 3 and 15 January 2006. The infections were confirmed by reverse transcriptase–polymerase chain reaction (RT–PCR) performed on nasopharyngeal and oropharyngeal swabs or tissue samples [Reference Oner2]. All cases had a history of close contact with diseased or dead chickens. Among these cases, eight were admitted to the Pediatric Intensive Care Unit (PICU) of the Van 100. Yil University Hospital.
The outbreak was investigated by the Turkish Ministry of Health with the support of academic groups and the World Health Organization. Positive cases were isolated in the hospital. Infected and exposed poultry were culled, and individuals exposed to human cases or infected poultry were screened for respiratory symptoms during the outbreak [Reference Liem and Lim5].
In contrast to previous outbreaks in Southeast Asia, the human outbreak in Turkey occurred in a small geographic area affecting some members of the same families during a short time period [Reference Abdel-Ghafar1, Reference Gambotto6]. Clustering of cases with H5N1 infection in a single family was reported previously from Sumatra Island, Indonesia in 2007 [Reference Yang7]. Of the 12 confirmed cases in Turkey, three were from one family while two cases were from another family.
H5N1 strains are adapted to birds more than humans; the virus has caused widespread outbreaks and deaths in poultry, although a relatively small number of humans have been infected. The receptor-binding sites (RBSs) of these avian viruses do not bind efficiently to receptors located at the surface of most of the human upper respiratory tract target cells [Reference Nicholls8]. The limited human-to-human transmission of H5N1 virus might be a consequence of human-to-human transmission due to adaptive mutations in the RBS of the virus, leading to the binding of H5N1 virus to human cell receptors [Reference Yen9]. H5N1 viruses isolated from Turkish patients infected during the 2006 outbreak were shown to have this mutation [Reference Altiok10].
In the event of the occurrence of undetected subclinical human infections, a silent circulation of H5N1 could facilitate the emergence of a new human-adapted virus. To assess this risk, we conducted a serological survey during and after the H5N1 influenza outbreak in Turkey.
MATERIAL AND METHODS
We analysed the serological response to H5N1 virus in different groups of subjects with different levels of possible exposure to the virus, from siblings and parents of confirmed cases (high-risk group) to unexposed subjects living in the endemic area (low-risk group). A number of confirmed cases were also included in order to understand the rate of serological response to H5N1 virus and provide validity for the serological tests. The rate of subclinical infection, even after direct contact with diseased poultry in Turkey was unknown. For this reason we enrolled culling personnel and individuals that had been in contact with diseased chickens in order to observe the rate of subclinical infection in individuals exposed to infected fowl.
Study population
Following ethical approval by the Central Ethical Committee of the Turkish Ministry of Health, blood samples were collected from six groups of subjects on 4 and 5 February 2006. Group A comprised the surviving cases who were accessible during the study (group A1) and asymptomatic exposed family members that had close contact with the cases during the infectious period (group A2). Family members were identified as exposed if they had close contact with the cases during the infectious period. The infectious period was defined as 2 weeks before and 2 weeks after the onset of symptoms in a particular case. Close contact was defined as having contact with a person who was within at least 3 m of an H5N1-infected case who was in the infectious period. Group B included staff members involved in culling, that had been in contact with infected poultry and had worked for at least 7 days during the epidemic. Group C consisted of asymptomatic individuals living in the area where human cases had occurred and that had been in contact with diseased chickens. Group D included individuals living in the same area, but with no known contact with the diseased chickens. Finally, group E included asymptomatic healthcare workers (HCWs) at Van 100. Yil University Hospital that had been in contact with the human cases. These subjects were chosen from the sample, no randomization was carried out and study subjects consisted of all eligible persons identified. All subjects were asked to complete a questionnaire about their age, sex, type and duration of exposure to poultry or human cases, as well as the presence of respiratory symptoms at the time of blood collection. A single serum sample was collected from all individuals in groups A–C at about 3–4½ weeks after the first possible contact with the H5N1 virus and for group D individuals at 3–4 weeks after the appearance of the first poultry case in their area. In group E, paired serum samples were collected. The first samples were collected from HCWs who cared for the patients and designated group E1. The second set of samples was collected from HCWs 22–23 days after the conclusion of the outbreak and designated group E2. After centrifugation, all serum samples were stored at −20°C until analysis.
Laboratory assays
Serum samples were blindly tested for antibodies by enzyme-linked immunosorbent assay (ELISA) and haemagglutination inhibition (HI) assay. A microneutralization (MN) assay was performed on all samples with antibodies detected by either ELISA or HI, as well as on 25 samples randomly chosen from those that were negative by HI. The first two tests were performed in a biosafety level-3 laboratory. The MN tests were carried out in a biosafety level-4 laboratory. HI and MN assays were performed by using the A/Turkey/13/06 H5N1 strain; a virus isolated during the Turkish epidemic that was similar to the reference strain A/Turkey/Turkey/1/2005 H5N1. All assays, except the ELISA, were performed at the Centre National de Référence des Virus Influenza, Lyon, France. The ELISA assays were performed at the Duzen Group Laboratory, Ankara, Turkey.
ELISA
A commercial ELISA assay (BioAssay Systems, USA) that detected H5 antibodies was used according to the manufacturer's instructions. Briefly, 100 μl of sera were added to pre-coated microtitre plates with H5-specific antigens. After incubation, an anti-IgG antibody linked to a horseradish peroxidase conjugate was used to detect antibodies. The final measurement was performed at 450 nm on a photometer using a reference wavelength ⩾620 nm (ELx808, Bio-Tek, USA). Samples giving an absorbance greater than or equal to the cut-off value were considered to be positive while an absorbance less than the cut-off value was considered negative. No quantitative evaluation was performed.
HI test
HI tests were performed in microtitre plates as described by Palmer et al. in 1975 using chicken red blood cells [Reference Palmer11]. Viral antigen (the wild-type strain A/Turkey/13/06 H5N1) was used at a dilution of 4 HA units per 50 μl. Sera were pre-treated to inactivate non-specific inhibitors of viral haemagglutination [12]. A HI titre ⩾20 was considered to be positive. HI titres of 10 were considered as a partial reaction and <10 as negative.
MN assay
Virus titration
Madin–Darby Canine Kidney (MDCK) cells were grown in Ultra-MDCK medium (Lonza, cat. no. BE12-749Q, Fisher Scientific, USA) supplemented with 200 U/ml penicillin, 200 μg/ml streptomycin, and 2 mml-glutamine. At confluence, the MDCK cells were harvested and subsequently seeded at 1·5×105/ml in 96-well plates in order to reach confluence after 72 h at 37°C under a 5% CO2 atmosphere. Serial dilutions of stock viruses were made to the infection medium [Eagle's minimum essential medium (EMEM)] (Lonza, cat no. BE 12-125F, Fisher Scientific) supplemented with 200 U/ml penicillin, 200 μg/ml streptomycin (Lonza cat. no. DE17-602E, Fisher Scientific), and 2 mml-glutamine (Lonza cat. no. BE17-605E, Fisher Scientific), containing trypsin (Roche, cat. no. 109819, Denmark), at a final concentration of 1 μg/ml. Briefly, 50 μl of tenfold serial dilutions of wild-type A/Turkey/13/06 H5N1 were inoculated into four replicate wells. The 96-well microplates were then incubated at 33°C under a 5% CO2 atmosphere, and the cytopathic effect (CPE) was checked 4 days after inoculation. The virus titre was calculated according to the method of Reed & Muench [Reference Reed and Muench13].
Neutralization assay
MN tests were performed in flat-bottomed microtitre plates (96 wells), 3 days after seeding of the MDCK cells. Twofold serial dilutions of inactivated sera starting at a 1/10 dilution were mixed with an equal volume of wild-type A/Turkey/13/06 H5N1 virus (100 TCID50/50 μl), and incubated for 1 h at 37°C. One hundred microliters of the serum-virus mixture and 100 μl of infection medium were inoculated in each well (4 wells/dilution), and the plates were incubated for 4 days at 33°C in 5% CO2 atmosphere. On day 4, supernatant from each well was tested for haemagglutination-inhibiting activity using chicken erythrocytes. Neutralization titres were expressed as the reciprocal log10 of the final dilution of serum that neutralized 50% of the inoculated wells. Since we observed no CPEs due to serum cytotoxicity at a dilution of 1/10, a MN titre ⩾10 was considered as positive. Standard virus neutralization tests were performed as described previously [Reference Palmer11, Reference Kohler and Milstein14, Reference Russell and Alexander15].
RESULTS
A total of 478 serum samples from 381 subjects were included in the study (Table 1). Of these, five were accessible surviving cases (group A1) and 28 were asymptomatic subjects who were exposed family members (group A2). Ninety-five staff members of culling teams (group B) and 75 asymptomatic individuals with a known contact with diseased chickens (group C) and 81 individuals with no known contact with diseased chickens (group D) were recruited for the other groups. A total of 194 paired serum samples from 97 asymptomatic HCWs were included in group E. Of the study subjects, only the five infected individuals had symptoms suggestive of avian influenza infection (fever, headache, sore throat, running nose, malaise, muscle ache, cough, and diarrhoea).
Table 1. Characteristics of study subjects
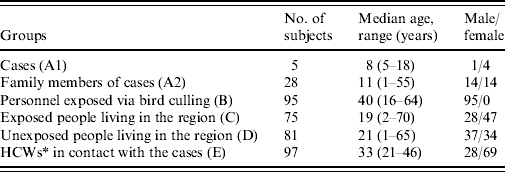
* Healthcare workers.
In total, only four samples were found to be positive by MN. These four sera had been collected from three subjects from group A1 (patients) and one subject from group A2 (family members), and were also positive by HI.
Immune response in five surviving cases (group A1)
Three cases were seropositive both by MN and HI, and only one of them was positive by ELISA. Two cases had no detectable antibodies with any of the techniques used (Table 2).
Table 2. ELISA, HI and MN test results of the subjects
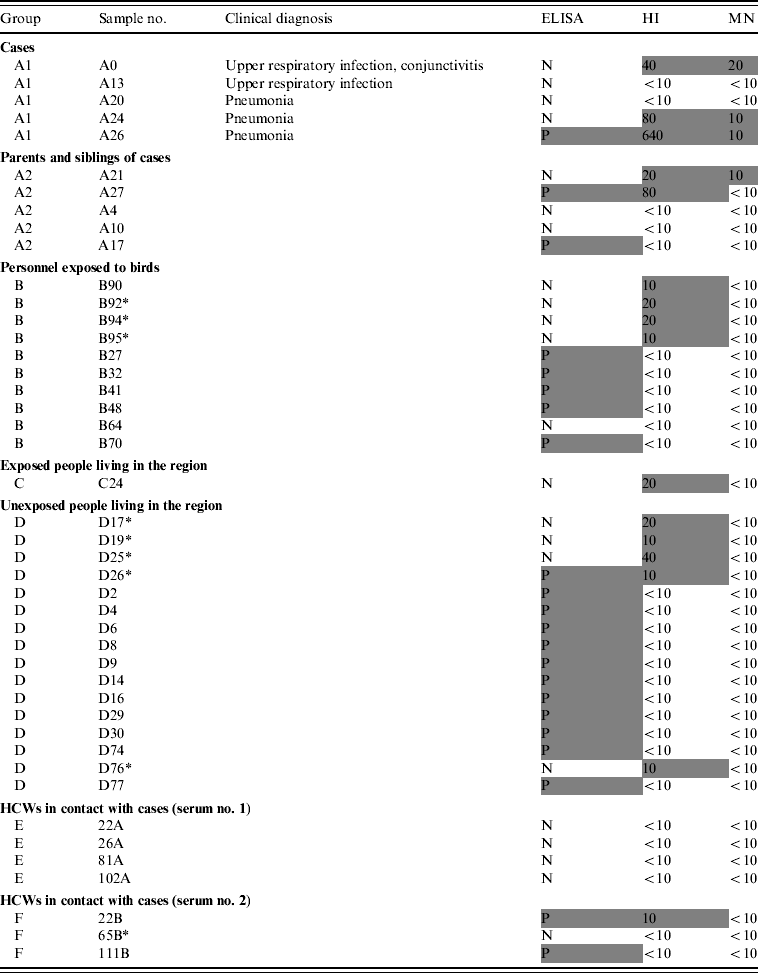
ELISA, Enzyme-linked immunosorbent assay; HI, haemagglutination inhibition; MN, microneutralization; N, negative; P, positive; HCW, healthcare worker.
Positive results are in shaded cells.
Immune response in asymptomatic exposed family members (group A2)
Of the 28 serum samples collected from the family members, only one (3·6%) sample had a detectable MN response (A21). One subject was positive by ELISA and one by HI whereas one showed a positive reaction with both ELISA and HI (Table 2).
Immune response in culling staff (group B)
Five out of 95 (5·3%) subjects were found to be seropositive by ELISA whereas four (4·2%) samples were positive by HI and none by MN (Table 2).
Immune response in asymptomatic individuals living in the area with known contact with diseased chickens (group C)
No samples were positive by ELISA and only one was seropositive by HI. There was insufficient serum remaining for MN testing.
Immune response in asymptomatic individuals living in the area with known contact without any known contact with diseased chickens (group D)
Eleven of 81 (13·6%) subjects were positive by ELISA and four (5·0%) with HI but only one (1·2%) subject had a positive result by both ELISA and HI. None of the subjects tested positive by MN.
Overall, in groups B, C and D, among the samples with an immune response detected by HI or ELISA, none had a detectable MN response. Furthermore, 25 sera selected randomly in the list of subjects in groups B, C and D that were negative by both ELISA and HI were also negative by MN.
Immune response in HCWs (group E, sera E1 and E2)
None of the paired sera collected from 97 HCWs, were positive by ELISA or HI (Table 2). In the second set of sera, two were positive by ELISA, one was also positive by HI (1% of HCWs had a seroconversion according to HI results only). These HI- and/or ELISA-positive sera were found negative by MN.
DISCUSSION
We observed that there was evidence of silent infection in only one of 37 study subjects tested by ELISA, HI and MN. That individual was the mother of one of the cases and had close and continuous household contact with a diseased chicken. One of her children and the chicken had confirmed H5N1 infection and the chicken had been living in the house with family members. Both the child and the H5-antibody positive parent had eaten the infected chicken. Therefore, it is difficult to demonstrate that this was a case of human-to-human transmission.
Previous studies have shown that subclinical infection has occurred during H5N1 outbreaks. The detection of H7 antibodies in 3·8% of poultry workers during the H7N3 outbreak in Italy highlighted that people exposed to avian influenza viruses can undergo silent seroconversion [Reference Puzelli16]. Similarly, a retrospective cohort study conducted in Hong Kong after the H5N1 outbreak of 1997 among HCWs exposed to H5N1-infected patients reported that 8/217 exposed HCWs (3·7%) were H5 antibody positive. Both reports suggested that subclinical infection has occurred in previous H5 outbreaks in humans.
HCWs caring for hospitalized H5N1 patients in Turkey reported inconsistent wearing of personal protective equipment (PPE) during their care of infected patients. Accordingly, HCWs were exposed to H5N1 and might have been infected through human-to-human transmission. However, our study showed there was no subclinical infection in those HCWs.
Another highly exposed group of subjects are the poultry cullers. The culling was performed with protection such as masks, glasses, caps, protective dress and gloves. However, all culling personnel reported at least one exposure to diseased chickens while not wearing PPE. No H5 antibody was detected in blood samples collected from the cullers [Reference Ortiz17].
Detection of H5 antibodies in humans is difficult; therefore, serological studies need to combine different assays [Reference Rowe18]. MN is described as the gold standard for the detection of human H5N1 antibodies in both vaccinees and infected individuals [Reference Katz19, Reference Frank20]. Besides this reference method, HI and H5 antibody-specific indirect ELISAs have also been developed. HI has been shown to be poorly sensitive for the detection of human H5-specific antibodies. To improve its sensitivity, red blood cells from different animals, such as horse red blood cells, have been tested successfully.
ELISA methods have been reported to give inconsistent results compared to other techniques, while good consistency has been observed between HI and MN results [Reference Rowe18, Reference Kayali21]. In some studies, H5-specific Western blot assays have also been used to improve the specificity of both assays [Reference Rowe18]. In our study, we combined ELISA, HI and MN assays. Our results confirmed the poor predictive value of ELISA and HI with an almost complete lack of consistency with MN. Only 4/22 sera positive by ELISA were positive by HI, and 12 additional HI-positive sera were negative by ELISA. Of the 16 HI-positive sera, only four were also positive by MN, despite using a lower threshold level of antibody detection in the MN assay than those previously reported (10 vs. 40 and 80) [Reference Katz19, Reference Frank20]. The difference in our results in MN than previous reports may have been due to the antigenic differences in the Turkish virus strain used compared to the eastern Asian strains used in previous studies. These MN- and HI-positive sera were from three PCR-confirmed cases [Reference Oner2], and one from an individual who was a contact. Two of the H5N1-infected cases had no detectable specific H5 antibodies. This lack of detection has been previously described, either due to inappropriate sampling collection or to mild H5N1 infections [Reference Katz19]. Although the time that elapsed between contact and blood sampling (3–4½ weeks) should have been sufficient to develop and detect a serological response, no antibody was seen. The sera of two seronegative cases were collected 3 weeks after onset, but we did not collect paired sera. These two seronegative cases had mild infections, one of them presented with an upper respiratory tract infection alone.
Of the 28 family control subjects, two might be thought of as having serological evidence for asymptomatic transmission. Both had an HI titre ⩾20, and one had detectable antibodies by MN. These subjects were the mother and the father of the case with the highest HI antibody titre. This antibody response in asymptomatic subjects could reflect a possible subclinical infection. However, it is impossible to determine if this result reflects human-to-human transmission or infection due to exposure to infected poultry, because these subjects had close contact with diseased poultry [Reference Oner2].
Although the study has some limitations, e.g. absence of randomization due to the limited number of subjects, and laboratory methods not being completely standardized, our serological study included people who had a wide range of exposures to patients with confirmed H5N1 infection and poultry infected with H5N1. By testing such diverse groups, this serological survey confirmed the absence of evidence for sustained subclinical H5N1 influenza infections during the outbreak in Turkey in 2006, despite adaptive changes observed in the RBS of the influenza A virus (H5N1) lineages.
ACKNOWLEDGEMENTS
The authors acknowledge Dr Béatrice Barret, Gee Marsh and Dr Tamer Pehlivan for stimulating discussions and Kimberly Shea for her language review. Thanks are especially due to Dr Mark Zuckerman for his helpful contribution regarding the manuscript. This study received financial and logistical support from Sanofi Pasteur.
DECLARATION OF INTEREST
None.




