Primary tumours of the spinal cord can be characterized as intradural extramedullary (IDEM) or intramedullary. Intramedullary spinal cord tumours (IMSCTs) are the most destructive and invasive because they originate from tissue within the spinal cord itself. Ependymomas are the most common IMSCT; typically, they are IMSCT because they originate from the ependymal cells that line the inside of the central canal of the spinal cord. However, in very rare cases, they can be IDEM. Reference Das, Hoang and Mesfin1–Reference Landriel, Ajler, Tedesco, Bendersky and Vecchi3 Ependymomas are typically soft, well encapsulated and slow growing tumours centrally located within the spinal cord leading to symmetric focal enlargement. Reference Das, Hoang and Mesfin1 They usually appear as a single lesion that can span multiple spinal levels, more commonly in the lower spinal cord – conus or filum terminale. Reference Das, Hoang and Mesfin1 Radiographically, they are contrast enhancing, appearing hypo- or isointense on T1 and hyperintense on T2. Reference Das, Hoang and Mesfin1,Reference Celano, Salehani, Malcolm, Reinertsen and Hadjipanayis4,Reference Reni, Gatta, Mazza and Vecht5 Unlike astrocytoma, ependymomas have a clear border separating the tumour and healthy spinal cord interface, making gross total resection a practical option. Reference Das, Hoang and Mesfin1,Reference Celano, Salehani, Malcolm, Reinertsen and Hadjipanayis4,Reference Reni, Gatta, Mazza and Vecht5 The rate of recurrence is correlated with the amount of tumour resection; fortunately, gross total resection can be achieved in more than 90% of cases. Reference Das, Hoang and Mesfin1 Several reports have been published providing valuable information on rare cases of ependymoma that don't appear with typical characteristics. In this report, we present an unusual and rare case of a spinal anaplastic ependymoma with contradictory radiographic, intra-operative and pathological findings. The purpose of this report is to provide further information on atypical ependymomas and a brief summary of the relevant literature.
We present a 45-year-old male patient with a 1-week history of sudden onset intermittent bilateral symmetric lower extremity weakness/numbness and back pain. He reported no upper extremity symptoms, history of trauma, saddle numbness, bowel/urinary incontinence or any other neurological symptoms. The examination was most in keeping with an upper motor lesion involving the spinal cord. The physical exam was significant for:
-
1. Bilaterally increased tone and spasticity in the lower extremities
-
2. Hyperreflexia in the upper (3+) and lower extremities (4+) with bilateral sustained clonus
-
3. Decreased sensation to light touch below the T4 spinal level
-
4. Bilateral positive Hoffman’s sign
-
5. Positive pectoralis reflex
-
6. Spastic paretic gait, with inability to complete tandem gait
Multiple enhancing IDEM masses causing severe compression at T4 and moderate compression at C7 and T6–7 were identified on the spinal MRI (see Figure 1). Based on the imaging, the differential diagnosis included hereditary neoplasms (paragangliomas, ependymomas, meningiomas or hemangioblastomas) and less likely lymphoproliferative disease or metastases. The patient underwent surgical resection of these lesions using a C6–T7 bilateral laminoplasty and T8 laminectomy, with intraoperative neurophysiological monitoring. Intraoperatively, the lesions appeared intramedullary but exophytic with no clear plane between the spinal cord and the tumours. Due to its invasive and adherent nature, gross total resection was very difficult. In a piecemeal fashion, the tumours were debulked until there was satisfactory debulking and the resolution of the spinal cord compression (see Figure 2). The histopathological diagnosis was anaplastic ependymoma, CNS WHO Grade 3 (see Figure 3). Follow-up molecular analysis confirmed amplification of the MYC-N oncogene, satisfying WHO criteria for a diagnosis of spinal ependymoma, MYC-N amplified; a clinically aggressive variant of spinal ependymoma. In the immediate post-operative period, the patient had no sensation below T8 and minimal motor strength in the lower extremities; however, symptoms gradually improved. The patient’s prognosis was estimated to be 2–3 years. He required immediate in-patient full spine radiation treatment to limit progression and address the drop metastases in the lumbar spine. Unfortunately, his symptoms returned after about 5 months, at which time repeat imaging revealed recurrence of his tumours. The patient opted for palliative care and died shortly thereafter.
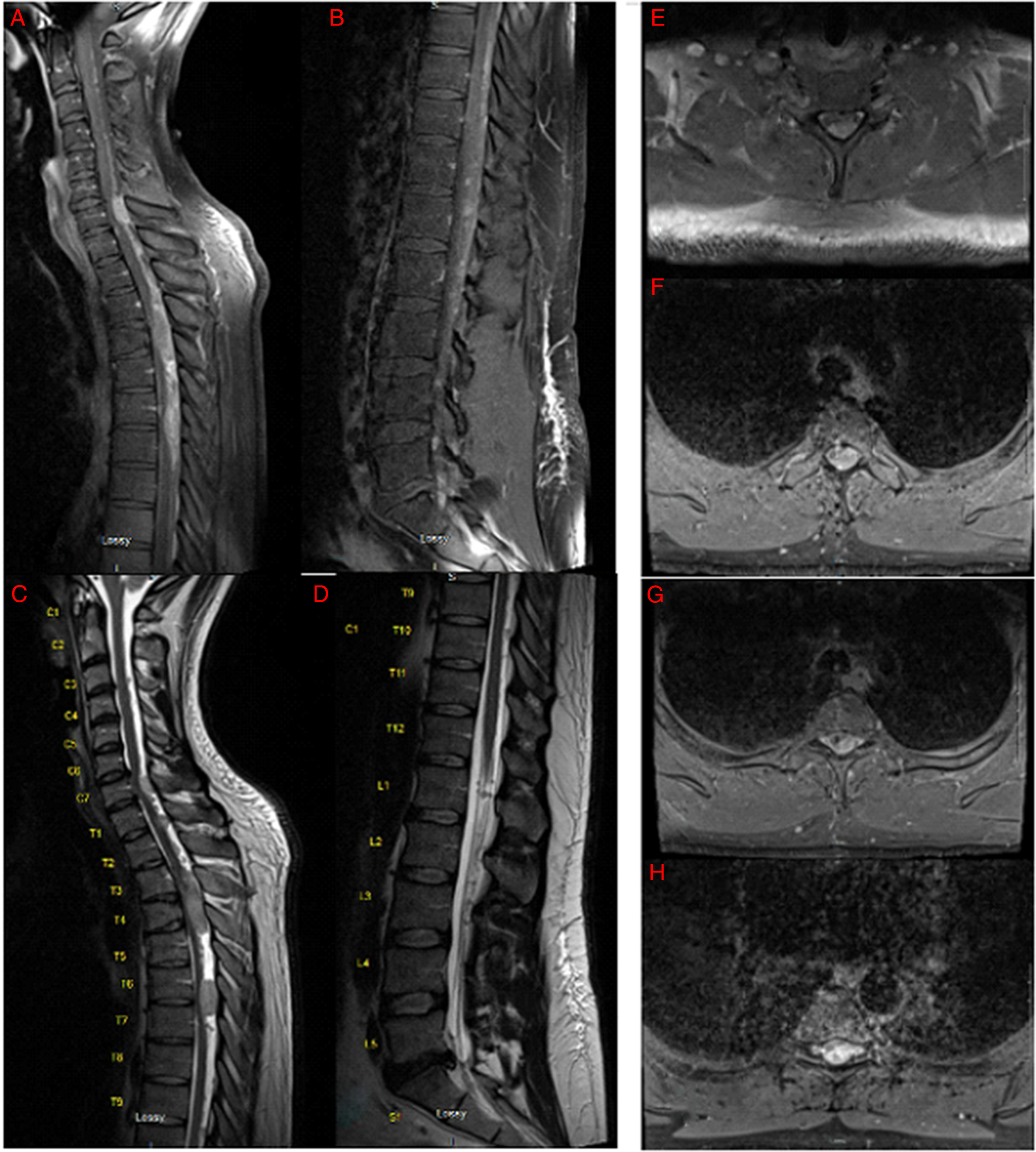
Figure 1: Pre-operative spine MRI demonstrating multiple enhancing posterior intradural, extramedullary masses with severe compression of the spinal cord. (A) Midsagittal T1 MRI with gadolinium of cervical and upper thoracic spine. (B) Midsagittal T2 MRI of lower thoracic and lumbar spine. (C) Midsagittal T1 MRI with gadolinium of cervical and upper thoracic spine. (D) Midsagittal T2 MRI of lower thoracic and lumbar spine. (E–H) Axial T2 MRI of the cervical (E) and thoracic spine (F–H).

Figure 2: Post-operative spine MRI demonstrating decompression of the spinal cord. (A) Midsagittal T2 MRI of cervical and upper thoracic spine. (B) Midsagittal T1 MRI of cervical and upper thoracic spine.
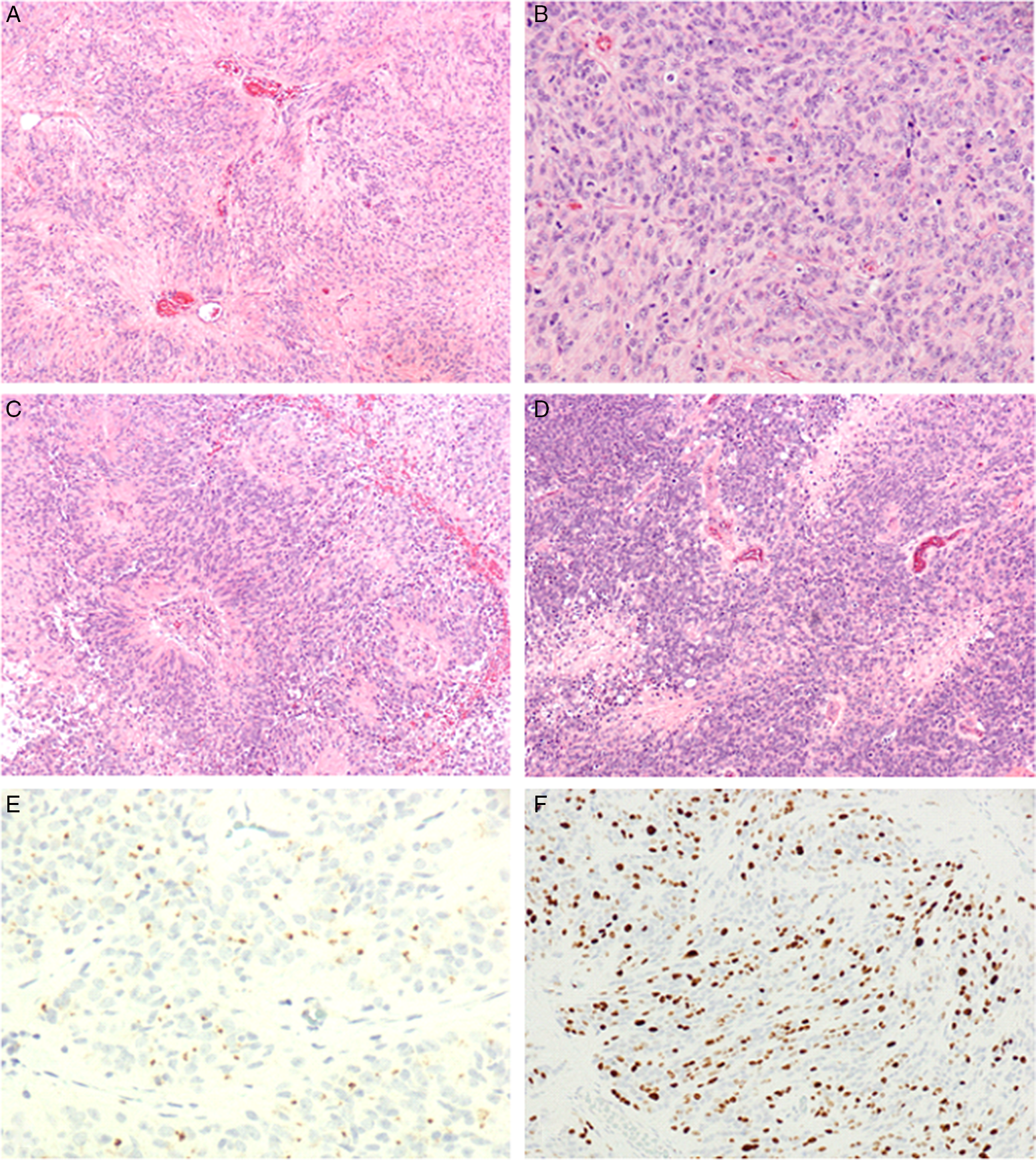
Figure 3: (A) Focal area showing lower grade features with prominent perivascular pseudorosettes (×10). (B) High-grade region showing increased cellularity with abundant mitotic figures (×20). (C) High-grade features: examples of microvascular proliferation at the center of perivascular pseudorosettes (×10). (D) High-grade features: multiple small foci of necrosis (×10). (E) Immunohistochemistry for epithelial membrane antigen shows characteristic juxtanuclear dot-like staining (×40). (F) Immunohistocjemistry for Ki67 reveals a high proliferation index (×20).
This case is unique as the radiographic, intra-operative and pathology findings were in contradiction. Radiographically, the lesions appeared to be IDEM, which is an uncommon localization for spinal ependymomas. Reference Das, Hoang and Mesfin1,Reference Landriel, Ajler, Tedesco, Bendersky and Vecchi3 In addition, spinal ependymomas are usually a single lesion and located in the lower spinal cord Reference Das, Hoang and Mesfin1,Reference Celano, Salehani, Malcolm, Reinertsen and Hadjipanayis4 ; however, this patient had multiple lesions in the cervical and thoracic region. When the tumours were surgically resected, they were found to be intramedullary and not well encapsulated, which was in direct contradiction to the radiographic findings. Typically, invasive intramedullary tumours that are difficult to resect are more in keeping with spinal astrocytoma Reference Das, Hoang and Mesfin1 ; however, the final pathology for this tumour was anaplastic ependymoma with grade 3 features. Consistent with the aggressive clinical, radiographic and histopathological features, molecular analysis confirmed MYC-N amplification, warranting a diagnosis of spinal ependymoma, MYC-N amplified. A few case reports have described multisegmented IDEM ependymoma, Reference Landriel, Ajler, Tedesco, Bendersky and Vecchi3,Reference Trivedi and Trivedi6 yet to our knowledge, no report has described spinal lesions clearly appearing as IDEM on imagining but intramedullary intra-operatively. It is important to recognize that regardless of the radiographic or pathological findings, the surgical management would not change. It is well accepted that indication for surgery and extent of resection should be dictated by clinical presentation and intraoperative judgement, respectively.
The pathological classification of ependymomas has evolved substantially over the past decade with the incorporation of molecular genetics. Specifically, the 2021 WHO Classification of CNS Tumours defines nine subtypes of ependymoma which are distinguished on the basis of their anatomical localization, histological features and, in some cases, molecular genetic profiles. Spinal ependymal tumours comprise subependymomas (WHO Grade 1), myxopapillary ependymomas (WHO Grade 2) and spinal ependymomas (usually WHO Grade 2 and associated with NF2 mutations). Spinal ependymoma, MYC-N amplified is a relatively new subtype. It is rare and is characterized by high grade histology, rapid clinical progression and a dismal prognosis. Reference Reni, Gatta, Mazza and Vecht5,Reference Kim, Kim, Kwak and Choi7 The histologic appearance of these tumours is defined by hypercellularity and a high nuclear-to-cytoplasmic ratio, few perivascular pseudorosettes, necrosis, microvascular proliferation and brisk mitotic activity. Reference Celano, Salehani, Malcolm, Reinertsen and Hadjipanayis4 The lesions are exceeding difficult to safely resect, and often require adjuvant radiotherapy after surgery. Reference Celano, Salehani, Malcolm, Reinertsen and Hadjipanayis4,Reference Kim, Kim, Kwak and Choi7,Reference Schuurmans, Vanneste, Verstegen and van Furth8 The role of adjuvant chemotherapy has not been well studied in the literature and not common practice at this time. Reference Celano, Salehani, Malcolm, Reinertsen and Hadjipanayis4 Malignant spinal ependymomas also have the potential to recur or metastasize to the brain, requiring full neuroimaging screening. Reference Celano, Salehani, Malcolm, Reinertsen and Hadjipanayis4,Reference Schuurmans, Vanneste, Verstegen and van Furth8
Anaplastic ependymomas of the spine are exceedingly rare tumours; nevertheless, it is important for neurosurgeons to recognize that they can present atypically. This report demonstrates that spinal anaplastic ependymomas can present as multisegmented lesions in the cervical and thoracic spine, which appear IDEM on imaging yet invasive and intramedullary intraoperatively. These cases are surgically complex because the invasive nature of the lesions limits the ability for safe total resection. Therefore, it is paramount that the surgical team prepare for an invasive tumour regardless of its appearance on imaging. In addition, anaplastic ependymomas require adjuvant radiation, with full neuroimaging screening for recurrence or metastasis. Though these tumours have a poor prognosis, early detection and intervention can provide patients with improved quality of life and extended time of survival.
Statement of Authorship
Conception of idea by CT, AMA, and BD; data acquisition and interpretation: CT, AMA and JW.
Drafting the manuscript drafting by CT and AMA; revising the manuscript critically for important intellectual content: JW and BD.
Approval of the version of the manuscript to be published: CT, AMA, JW and BD.
Disclosures
All authors certify this work and have no conflicts of interests to disclose.





