I. INTRODUCTION
Everyone is responsible for handling the present and future fuel feed and related environmental issues in the 21st century. Chemists are considered in duty bound and competent to overcome corresponding technological challenges. As is well known, carbon-based and nonrenewable fossil fuels, including coal, crude oil, and natural gas, still dominate the whole world energy supply nowadays. As a result, the huge consumption of limited fossil fuels will cause energy depletion and bring about serious environmental contamination and climate change, which threatens human health, ecological sustainability, and social progress. Thus, we should cut the use of fossil fuels and exploit new energy, which must be abundant, low cost, pollution free and inexhaustible. To satisfy these requirements, sunlight may be the optimal choice. Photosynthesis is widespread in nature. During this process, chlorophyll collects solar energy to convert water and carbon dioxide into organic compounds and release oxygen. In a word, sunlight energy is converted into chemical energy through photosynthesis and the obtained chemical energy is stored for valuable uses. In detail, the chemical reaction starts with the photoreaction converting water into free protons and electrons and liberating oxygen. Inspired by the scenery of fuel generation powered by solar energy, scientists have paid close attention to the research field of artificial photosynthesis. However, artificial photosynthesis is difficult to achieve due to the lack of an effective catalyst. In recent years, artificial photosynthesis has drawn public attention to handle the environmental pollution and energy crisis due to the excessive use of traditional fossil fuels. Reference Zhang, Lai and Cao1–Reference Liu, Zhao, Wang, Dou, Yan, Liu, Xia and Wang6 Hydrogen, a sustainable, clean, eco friendly, and economic energy with a superior gravimetric energy density, is known as a potential candidate for new energy resources. Reference Tachibana, Vayssieres and Durrant7–Reference An, Huang, Zhou, Yin, Liu and Xi13 It is mentionable that the sole combustion exhaust of hydrogen is pollution-free water. Moreover, clear molecular hydrogen can be obtained from abundant water resource easily. The whole process shows a perfect circulation without any pollutant discharged, which is completely in conformity with the new energy resource standard. Water splitting, including hydrogen evolution reaction and oxygen evolution reaction (OER), is an attractive way to generate hydrogen efficiently. Reference Chen, Qi, Zhang and Cao14–Reference Zhang, Xie, Zhou, Wang and Pan21 However, OER is the bottleneck in the whole water splitting process because it’s a multistep proton-coupled electron transfer (PCET) process. PCET plays significant roles in many chemical and biological reactions related to energy conversion. The relationship between PCET and the energy conversion reaction has been studied in a review from Meyer and coworkers, in which many underlying principles about PCET have been summarized. Reference Gagliardi, Westlake, Kent, Paul, Papanikolas and Meyer22 PCET plays a crucial part during OER whether thermodynamically or kinetically. In thermodynamics, PCET can effectively avert the charge build up effect and successfully accelerate catalyst activation. In dynamics, PCET can avoid the formation of high-energy intermediates through the simultaneous transfer of electrons and protons to a degree. It is hard to accomplish OER due to the high-energy barrier and slow-reaction dynamics. Reference Chen, Wu, Han, Lin, Sun, Zhang and Cao23–Reference Cook, Dogutan, Reece, Surendranath, Teets and Nocera26 In detail, two water molecules are required to take part in the oxidation reaction to produce one oxygen molecule during the OER process according to the law of the conservation of mass. At the same time, two proton-electron couples must leave from each water molecule to meet the requirement of charge conservation. In other words, generating an oxygen molecule requires four proton-electron couples lost from two water molecules in total, which is an intricate process. In other words, the process of O–H bond breaking and attendant O–O bond formation is difficult. Thus, the researchers worldwide are searching for highly active catalysts to accelerate the reaction and reduce the overpotential of OER. Generally, an excellent OER catalyst should meet three essential criterions. First, high activity with considerable current density is required at low overpotentials. Second, superior durability in mild conditions is also a requisite. OER catalysts with both high activity and superior durability can be meaningful and used for large-scale applications. Third, simple and safe synthetic method is also needed. To our knowledge, the most effective catalysts for water oxidation in both acidic and alkaline conditions are noble metal-based RuO2 and IrO2. Reference Lee, Suntivich, May, Perry and Shao-Horn27 However, the low abundance and high cost largely hinder their large-scale applications. Thus, it is still a challenge to develop inexpensive, easily accessible, highly efficient, and stable catalysts as alternative candidates.
Many efficient OER catalysts with nonprecious metal elements have been reported. Reference Yan, Li, Huo, Chen, Dai and Wang28 Among the various catalysts, the first–row transition metal-based (Mn, Fe, Co, Ni, and Cu etc.) materials including their oxides, Reference Chen and Qiao29–Reference Han, Liu, Hu, Dong, Li, Shang, Chai, Liu and Liu39 (oxy)hydroxides, Reference Zhang, Qi, Liu and Cao40–Reference Friebel, Louie, Bajdich, Sanwald, Cai, Wise, Cheng, Sokaras, Weng, Alonso-Mori, Davis, Bargar, Norskov, Nilsson and Bell45 layered double hydroxides, Reference Liu, Yang, Zhang and Yang46–Reference Liu, Wang, Liu, Zou and Wang51 sulfides, Reference Zhang, Wang, Pohl, Rellinghaus, Dong, Liu, Zhuang and Feng52–Reference Zhou, Wu, Cao, Huang, Tan, Tian, Liu, Wang and Zhang55 selenides, Reference Gao, Qi, Chen, Zhang and Cao56–Reference Tang, Cheng, Pu, Xing and Sun61 borides, Reference Esswein, Surendranath, Reece and Nocera62,Reference Masa, Weide, Peeters, Sinev, Xia, Sun, Somsen, Muhler and Schuhmann63 carbides, Reference Tang, Liu, Huang, Wang, Dong, Li and Lan64 nitrides, Reference Chen, Xu, Fang, Tong, Wu, Lu, Peng, Ding, Wu and Xie65,Reference Xu, Chen, Li, Tong, Ding, Wu, Chu, Peng, Wu and Xie66 and phosphate Reference Li, Baydoun, Kulikowski and Brock67–Reference Ledendecker, Krick Calderon, Papp, Steinruck, Antonietti and Shalom76 have been extensively studied as highly efficient OER catalysts. Cobalt is considered as an attractive nonnoble metal for its better catalytic ability toward OER with many merits, such as abundant reserves, nonpoisonous, various valences, and good electrical conductivity. Extensive study efforts have been devoted to proving that Co-based homogenous molecular catalysts are widely considered as promising candidates for OER. Reference Lai, Cao, Dong, Shaik, Yao and Chen77,Reference Artero, Chavarot-Kerlidou and Fontecave78 However, the actual application of these homogenous catalysts is often limited by their weak durability, inferior activity, and large overpotential. Additionally, homogenous molecular OER catalysts should be equipped with excellent water solubility, which is difficult during the design and synthesis of the molecules. Therefore, considerable current investigations have been focused on exploiting Co-based inorganic materials as heterogeneous OER catalysts possessing excellent OER activity and super stability. Especially, Co-based nanometer materials with small sizes are popular. Recently, we and other groups have studied a number of Co-based materials exhibiting prominent OER performance with innovative morphologies. Reference Wan, Qi, Zhang, Wang, Zhang, Liu, Zheng, Sun, Wang and Cao79–Reference Dou, Dong, Hu, Huang, Chen, Tao, Yan, Chen, Shen, Chou and Wang82 Noteworthy, the three-dimensional Co(OH)F microspheres, which are structured by low-dimensional building units step by step, have shown a promising OER performance. Particularly, α-Co(OH)2 owns good electrical conductivity, unique layered structure, and larger surface area, which is beneficial for electrocatalytic reaction. Furthermore, it is inexpensive and eco friendly. Thus, α-Co(OH)2 catches the attention of researchers as a potential electrode material, especially in the application of supercapacitors. Reference Liu, Jiang, Ma, Ni, Sun, Liu, Chen and Liu83,Reference Wang, Dong, Wang, Zhang and Jin84 However, there has been much less investigation on α-Co(OH)2 as water oxidation catalysts. Reference Jiang, Li, Wang and Wang85,Reference Liu, Yang, Zheng, Zhang and Yang86 On the other hand, the morphologies and phases of nanomaterials play a vital role in their properties and applications. In particular, three-dimensional (3D) flower-like nanostructures possessing relatively open spaces may be beneficial for the rapid electron transfer process and fast diffusion of ions toward the outstanding electrocatalytic activity. Till today, synthetic methods of flower-like architecture still need to be improved due to long time, high-cost, and poor efficiency of the present means. Additionally, the amorphous α-Co(OH)2 can offer more catalytic active sites due to the unordered structure, which open the door to employ amorphous α-Co(OH)2 as the superior OER catalyst. In general, Co(OH)2 was fabricated by hydro/solvo-thermal methods. It usually requires a relatively high temperature and a long time for the costly synthesis. Normally, amorphous Co(OH)2 was obtained through the electrochemistry techniques, which are invalid for large-scale commercial applications due to expensive production costs. To address these limitations, we adopted a facile template-based route to synthesize 3D α-Co(OH)2 hollow nanoflowers comprising of ultrathin nanoflakes at room temperature in this work. Furthermore, the 3D α-Co(OH)2 material is amorphous in this work, which is pivotal to the whole OER process. It has been reported that amorphous Co(OH)2 nanostructures possess superior OER activity than that of the corresponding crystalline materials. Reference Sayeed, Herd and O’Mullane87 We are encouraged by such research results to search for simple and reliable methods of generating amorphous Co(OH)2 nanometer materials for OER. In detail, Cu2O nanospheres, the sacrificial templates, were synthesized via a previously reported method with little modification. Then, α-Co(OH)2 hollow nanoflowers (denoted as α-Co(OH)2/HNFs) were fabricated in a Na2S2O3 solution. During this process, Na2S2O3 plays two significant roles. It was first used as a coordinating ligand to interact with Cu2O, and it is incapable of reacting with Co2+, which is forecasted by classical hard and soft acids and bases principle. Second, S2O3 2− can hydrolyze to generate OH−, which will combine with the dissociative Co2+ to lead to the precipitation of α-Co(OH)2 on the surface of the Cu2O template. Noteworthy, no other etching agents were used to remove the Cu2O template, which is superior to the traditional methods of templated synthesis. In our work, the alkali source S2O3 2− is considered as the etching agent at the same time, which can avoid the introduction of impurity effectively. Both of above characteristics facilitate the fabrication of α-Co(OH)2/HNFs. The as-prepared Co(OH)2 exhibited high activity for electrocatalytic water oxidation and is better than α-Co(OH)2 and β-Co(OH)2 nanoplates. In addition, the resulting catalyst demonstrated excellent stability for 20 h. This facile template-based strategy to synthesize amorphous metal hydroxides or oxides has potential in electrocatalytic areas. We also synthesized α-Co(OH)2 nanosheets [denoted as α-Co(OH)2/NSs] and β-Co(OH)2 nanosheets [denoted as β-Co(OH)2/NSs] as contrast samples.
II. MATERIALS AND METHODS
A. General materials
All chemicals, including copper(II) acetate monohydrate (99%, Energy Chemical, Shanghai, China), polyvinylpyrrolidone (PVP; average Mw 40,000, Energy Chemical, Shanghai, China), polyvinylpyrrolidone (average Mw 24,000, Xiya Reagent, Shandong, China), hexamethylenetetramine (99.5%, Energy Chemical, Shanghai, China), sodium borohydride (98%, Energy Chemical, Shanghai, China), cobalt chloride hexahydrate (95%, Heowns Biochem LLC, Tianjin, China), sodium thiosulfate pentahydrate (98%, Sinopharm Chemicals, Shanghai, China), potassium hydroxide (98%, Sinopharm Chemicals), absolute ethanol (Sinopharm Chemicals, Shanghai, China), and N,N-dimethylformamide (DMF; 99%, Sinopharm Chemicals, Shanghai, China) were obtained from commercial suppliers and used without further purification unless otherwise noted. Milli-Q water of 18 MΩ cm was used in all experiments.
B. Synthesis of Cu2O nanospheres
The Cu2O nanospheres were synthesized by the method reported in the literature with little modification. Reference Zhang, Liu, Peng, Wang and Li88 In this reaction, NaBH4 is used as a strong reductive agent to react with Cu(CH3COO)2·H2O for producing the Cu2O precipitate, which was completed with the help of a trace amount of H2O from DMF. DMF is considered as the reaction solvent with an inferior reaction temperature. PVP is introduced to regulate and control the morphology of Cu2O nanospheres. In a typical experiment, 0.8 g Cu(CH3COO)2·H2O and 0.1 g PVP (MW: 40,000) were dispersed in 40 mL DMF by stirring for 10 min. 75.6 mg NaBH4 was added under stirring for another 10 min. Then, the above mixture was heated to 90 °C until the color changed from brownish black to earthy yellow. Finally, the mixture was cooled to room temperature and collected by centrifugation, washed with deionized water and absolute ethanol separately for three times, and dried at 60 °C for overnight.
C. Synthesis of Co(OH)2/HNFs
Co(OH)2 hollow nanoflowers were prepared by a simple method at room temperature. Reference Nai, Tian, Guan and Guo89 In a typical procedure, 20 mg Cu2O was uniformly dispersed in 10 mL H2O and 10 mL ethanol-mixed solutions. After stirring for 5 min at room temperature, 600 mg PVP (MW: 24,000) was added. The solution was stirred for another 10 min, then, 0.5 mL CoCl2·6H2O (26 mg) solution was added as the cobalt source. After that, 2 mL of 2 M Na2S2O3 solution was added dropwise as both the alkali source and etching agent. At last, the solution was stirred for 5 min. The color of the above mixture was gradually changing from orange red to light green. As a matter of fact, orange Cu2O templates faded away and light green Co(OH)2 gradually generated. The experimental phenomenon manifests that complete etching of the inner Cu2O templates and successful synthesis of Co(OH)2 with a short reaction time required. Finally, the products were collected through certification and then washed with deionized water and absolute ethanol for three times at least and then dried at 60 °C for overnight.
D. Synthesis of α-Co(OH)2/NSs
α-Co(OH)2 nanosheets were synthesized as a contrast sample. In a typical procedure, 237.9 mg CoCl2·6H2O and 70.1 mg C6H12N4 were dispersed in 20 mL deionized water with magnetic stirring for 5 min. The above solution was transferred into a 50 mL of Teflon-lined autoclave and then kept at 90 °C for 5 h. The collected precipitate was washed with deionized water and dried at 60 °C for overnight.
E. Synthesis of β-Co(OH)2/NSs
β-Co(OH)2 nanosheets were synthesized as a contrast sample. Briefly, 237.9 mg CoCl2·6H2O was dissolved in 20 mL deionized water and then 2 mL 2 M NaOH solution was gradually added. The above mixture was continuously stirred for 10 min. Finally, the precipitate was collected by centrifugation, washed with deionized water, and dried at 60 °C overnight.
F. Preparation of electrode
The working electrode was prepared by a drop-casting method as the following procedure. Typically, 4 mg of the corresponding catalyst was dispersed into 1 mL of water–ethanol solution (which was prepared by mixing H2O and ethanol at a volume ratio of 2:1) with 30 μL of Nafion solution (5 wt%, DuPont) added. Then, the obtained mixture was sonicated for 30 min to form a homogeneous ink dispersion. Subsequently, 5 μL of the ink was drop-casted equably onto the polished GC (0.07 cm2) electrode, which was dried naturally.
G. Materials characterization
X-ray diffraction (XRD) patterns were acquired with the 2θ range from 5 to 80° on a Bruker D8 Discover X-ray diffractometer (Bruker, Billerica, Massachusetts) with Cu Kα radiation (λ = 1.5406 Å). The morphology and microstructure of the samples were investigated by a field emission scanning electron microscope (FESEM, SU8020, Hitachi, Tokyo, Japan) at an accelerating voltage of 10 kV. The transmission electron microscopy (TEM) images were determined by a TEM (JEM-2100, JEOL Ltd., Beijing, China) with an accelerating voltage of 200 kV. Energy dispersive X-ray (EDX) analysis was carried out on an AMETEK Materials Analysis (Ametek, Shanghai, China). The X-ray photoelectron spectroscopy (XPS) was performed on a Kratos AXIS ULTRA XPS analyzer (Kratos Analytical Ltd., Hadano, Japan) with monochromatized Al Kα (hν = 1486.6 eV) X-ray source. The XPS binding energies were corrected using the C 1s peak at 284.6 eV.
H. Electrocatalytic measurements
All the electrochemical measurements were performed on the CHI 660E electrochemical workstation equipped with a traditional three-electrode system at room temperature using glass carbon (GC, 0.07 cm2) as the working electrode if not specified, Pt wire as a counter electrode and saturated Ag/AgCl (KCl solution) as the reference electrode. Cyclicvoltammograms (CVs) were measured in 1 M KOH solution at a rate of 50 mV/s with iR-compensation. In these measurements, the working electrode was cycled several times to get a steady response before data recording. All the potentials in this article were calibrated to the reversible hydrogen electrode (RHE) according to the equation: E RHE = E Ag/AgCl + (0.197 + 0.059 pH) V. The electrochemically active surface area (ECSA) was obtained by CV at a non-Faradaic potential window from 1.18 to 1.28 V versus RHE at different scan rates: 20, 40, 60, 80, 100, and 120 mV/s. The charging current densities at the middle potential were plotted versus the scan rate, giving a linear plot whose slope identified the double-layer capacitance. The electrochemical impedance spectroscopy (EIS) was acquired at 1.65 V versus RHE over a frequency range from 106 to 0.1 Hz, with 5 mV as the amplitude potential. Tafel plots were obtained by linear sweep voltammetry tests. Chronopotentiometry and chronoamperometry were carried out in a 1 M KOH aqueous solution to test the stability without iR-compensation with indium-tin oxide (ITO) working electrodes.
III. RESULTS AND DISCUSSION
A. Morphology and structure
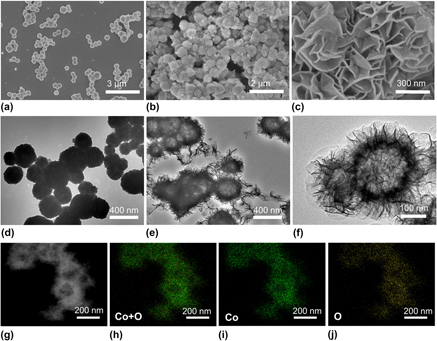
The morphologies of as-prepared Cu2O templates and α-Co(OH)2 were investigated by SEM and TEM. Cu2O shows a uniform nanosphere architecture with a diameter of about 300 nm with the assistance of PVP [Figs. 1(a) and 1(d)]. The as-obtained α-Co(OH)2 has a three-dimensional nanoflower structure with uniform surface nanoflakes as shown in Figs. 1(b) and 1(c). The thickness of the ultrathin flakes is approximately 10 nm, and the ultrathin flakes stand vertically on the surface to offer open spaces. The numerous surface nanoflakes with a 3D architecture will expose abundant surface active sites, which are favorable for the catalytic process. Further, structure information can be obtained from TEM characterization. TEM images [Figs. 1(e) and 1(f)] indicate that the structure of α-Co(OH)2 is hollow and flower-like. By comparing the crust with hollow, the internal cavity leaps to our eyes through the TEM images. A representative image was displayed in Fig. 1(f), in which the transparent areas are in sharp contrast to the dark areas. The formation of dark areas or stripy lines is due to wrinkling, overlapping, or winding of the surface nanoflakes. Therefore, the thickness of the stripy lines identifies the nanoflake thickness. The nearly lucid electron beam in some places can be representative of the unilaminar nanoflake. Such an experimental result testified the thin characteristic of the surface nanoflakes, which will expose more active sites. It should be emphasized that the hollow structural feature and porous shell of α-Co(OH)2 may create a superior specific surface area, which leads to a large exchange in current density. Generally, hollow architectures show outstanding electrochemical performance, owing to clear-cut interior cavities and the pivotal shell architecture that can accelerate mass transfer and enhance the electrocatalytic activity. Reference Zhu, Ma, Jaroniec and Qiao90 And hollow architectures are also connected with large specific surface area. Moreover, the surface of the hollow nanoflower was rough due to the vertical nanoflake, which causes more active sites exposed than those of the smooth-faced hollow structure. Thus, the special hollow structure of α-Co(OH)2 hollow nanoflowers is responsible for their high OER activity. For comparison, SEM images of α-Co(OH)2/NSs and β-Co(OH)2/NSs were displayed in Fig. S1. α-Co(OH)2/NSs has an accumulative nanosheet architecture and β-Co(OH)2/NSs shows the hexagonal nanoplate morphology. The average thickness of the nanoplates is determined to be 30 and 40 nm, respectively. In addition, the scanning transmission electron microscopy (STEM) and EDX element mapping were carried out to determine the hollow structure and element distribution of α-Co(OH)2/HNFs. Figures 1(g)–1(j) display the highly uniform dispersion of Co and O in α-Co(OH)2/HNFs and further emphasize the hollow structure with a weak electronic signal of Co and O in the inner cavity.

FIG. 1. SEM images of the as-prepared Cu2O templates (a) and α-Co(OH)2/HNFs (b, c); TEM images of Cu2O (d) and α-Co(OH)2/HNFs (e, f); STEM image (g) and the corresponding elemental mappings (h–j) of the α-Co(OH)2/HNFs.
The unique structure of the α-Co(OH)2 hollow flower-like is derived from the elaborate design and synthesis. This work adopted a simple templated synthesis of α-Co(OH)2/HNFs using the uniform Cu2O nanosphere as the sacrificial template. Surfactant PVP is significant to assist the formation of high-quality α-Co(OH)2/HNFs. On the one hand, PVP maintain the sphere structure of Cu2O by surface interaction. On the other hand, PVP controls the speed of the etching reaction through restricting the movement of S2O3 2−. The Cu2O nanosphere is introduced as sacrificial templates due to the following several advantages. In the first place, essential properties of spherical Cu2O can be easily adjusted through argument changes including size, crystallinity, and surface construction. Then, spherical Cu2O is synthesized by a simpler method than traditional spherical templates such as silica, carbon colloids, and polymer colloids. Generally, the above-mentioned conventional spherical templates are more difficult for either synthesis preparation or removal. The last but most important is that the Cu2O spherical template is favorable to generate the desired α-Co(OH)2/HNFs based on the hard and soft acids and bases principle. Na2S2O3 is introduced as the alkali source for generating hydroxide ions and as the coordinating reactant for the removal of Cu2O at the same time. Noteworthy, the choice of alkali source in our work is well founded. First, a strong base is impracticable as the alkali source in this work because Co2+ will precipitate quickly due to the abundant hydroxyl ions resulting from strong bases. As a result, irregular Co(OH)2 precipitation will be obtained instead of the perfect hollow structure. Na2S2O3 can offer moderate alkaline environment for this reaction system, which is favorable for the formation of a hollow structure. Then, hard and soft acids and bases principle offer the theoretical basis for this work. According to this principle, Lewis bases prefer to combine with Lewis acids of the same kind and vice versa. Therefore, hard acids tend to exert better affinity for hard bases but interact unwillingly or infirmly with soft bases. On the other hand, soft acids are more likely to react with soft acids. In other words, a soft-hard combine is impracticable in theory. In this system, the Cu+ of Cu2O template is a soft acid and Co2+ is a hard acid, so a soft base is more suitable and capable than a hard base to coordinate with Cu+ instead of Co2+. Thus, a soft base S2O3 2− was introduced in this work. The whole removal of the template and formation processes of α-Co(OH)2/HNFs take place as the following procedure. First, surfactant PVP was used to passivate the surface of Cu2O particles and stabilize the shape by PVP adsorption on the surface of the Cu2O sphere, which is significant for the morphology control of precipitated α-Co(OH)2/HNFs subsequently. Second, two separate reactions take place. On the one hand, soft-base S2O3 2− combines with soft-acid Cu+ to accomplish etching of the Cu2O template with the soluble complex formed. At the same time, hydroxide ion releases due to the etching of Cu2O, and the hydrolysis of S2O3 2− also makes a contribution to the generation of the hydroxide ion. On the other hand, Co(OH)2 is formed on the surface of Cu2O due to the high concentration of the hydroxide ion close to Cu2O. These two reactions occur at the same time, and both take place on the surface of Cu2O. Thus, the obtained Co(OH)2 precipitate inherits the morphology of the Cu2O templates. Simultaneously, an inner hollow structure is realized. It must be stated that the size of α-Co(OH)2/HNFs in thickness will grow with the above-mentioned reactions. But if the number of Co2+ reduces to a vital value that is insufficient to satisfy the demand of precipitation, the thickness of the α-Co(OH)2/HNFs will no longer increase. At the same time, unbroken corrosion of Cu2O can take place even in closed shells, which results in a perfect hollow architecture. In brief, the superior relationship of soft–soft bonding between the etching agent and the template maintains even with a low temperature, which guides the formation of α-Co(OH)2/HNFs step by step. As we all know, the conventional template-removal methods always require the introduction of a special etching agent, such as oxidative, reductive, and acidic agents. In our system, S2O3 2− is considered as the only etching agent and plays other crucial roles at the same time. For example, the formation of the Co(OH)2 precipitate can be accelerated by the hydroxide ion in high concentration, which results from the hydrolysis of S2O3 2− and etching of the template.
In a word, the whole material synthesis is based on the classical theoretical basis and presents numerous advantages. The choice of the reaction solvent is also of great importance for the formation of a perfect α-Co(OH)2 hollow nanosphere. In our system, we choose a water–ethanol solution (which was prepared by mixing H2O and ethanol at volume ratio of 1:1) as the optimal reaction solvent. As mentioned above, the whole formation process of α-Co(OH)2/HNFs includes two essential reactions: removal of the template and precipitation of α-Co(OH)2. If we select water as the only reaction solvent, the hydrolysis of S2O3 2− will be accelerated without a doubt. As a result, the whole reaction solution shows a high concentration of hydroxide ion. Thus, dissociative Co2+ has more chances to react with the hydroxide ion far away from the surface of the template, which leads to the formation of distorted α-Co(OH)2 spheres or other irregular particles. What is worse is that the sphere architecture will collapse due to the quick mass transfer. At the same time, the concentration of S2O3 2− will reduce due to the vast hydrolysis, which slows down the reaction speed of template removal with more reaction times. With these factors considered, a certain amount of ethanol is used to limit the hydrolysis of S2O3 2−. But too much ethanol will not work due to the low ion strength. Of course, the concentration of S2O3 2− also plays an important role for the formation of α-Co(OH)2/HNFs.
In addition to the morphology and structure observation, the phase analysis of resulting samples was determined by XRD. Figure 2(a) shows the XRD pattern of Cu2O templates with the characteristic diffraction peaks at 29.6°, 36.5°, 42.4°, 61.5°, 73.7°, and 77.6°, which can be well indexed to the (110), (111), (200), (220), (311), and (222) facets (JCPDS: 75-1531), and no other impure signal can be detected. Figure 2(b) demonstrates that the as-synthesized α-Co(OH)2/HNFs sample has four peaks at 9.07°, 19.81°, 33.27°, and 59.44°, which are indexed to the (003), (006), (100), (110) planes. Reference Jiang, Li, Wang and Wang85 The absence of other peaks indicates a pure phase of a-Co(OH)2 and a complete removal of the Cu2O template. Furthermore, the broad peaks indicate the poor crystallinity of the α-Co(OH)2 sample, which is beneficial for electrocatalytic processes. The amorphous materials may show superior activity over the corresponding crystalline forms for electrochemical applications due to the disordered structure. It has been reported that the amorphous Co(OH)2 nanostructures exhibit outstanding capacitive performance and OER activity. Reference Sayeed, Herd and O’Mullane87,Reference Li, Yu, Lu, Liu, Liang, Xiao, Tong and Yang91 Furthermore, it has been reported that the virtual electrocatalyst for OER is the surface catalytically active layer composed of amorphous CoO x (OH) y instead of the inner crystalline Co3O4 in a recent work. Reference Bergmann, Martinez-Moreno, Teschner, Chernev, Gliech, de Araujo, Reier, Dau and Strasser92 Thus, we consider that the amorphous characteristic of α-Co(OH)2 can be responsible for the excellent OER performance in this work, which is due to defects, unordered structures, and anisotropy of amorphous materials. As can be seen from the magnified plot in Fig. 2(b), the peaks at 33.27° and 59.44° can be observed, which are consistent with α-Co(OH)2. All the diffraction peaks of another contrast sample are highly consistent with β-Co(OH)2 (JCPDS: 74-1057). Reference Liu, Ma, Osada, Takada and Sasaki93
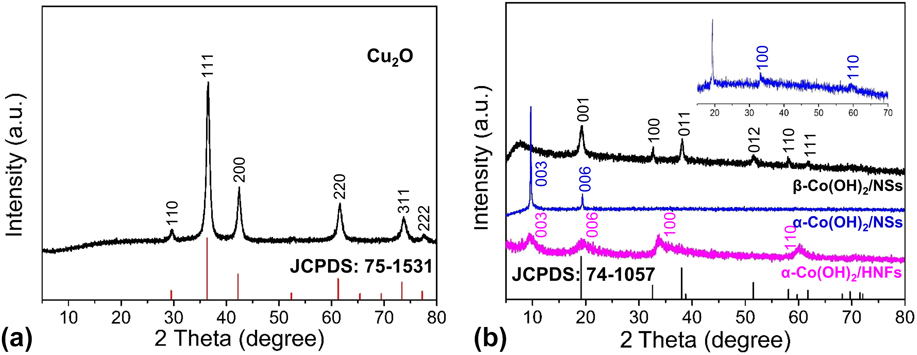
FIG. 2. XRD patterns of Cu2O templates (a) and α-Co(OH)2/HNFs, α-Co(OH)2/NSs, and β-Co(OH)2/NSs (b).
Generally, XPS is considered as a credible method to study the chemical states and coordination environment information of atoms in the material surfaces. In this study, to determine the surface oxidation states of the as-obtained α-Co(OH)2 sample, an XPS survey was carried out and displayed in Fig. 3. In the Co 2p spectrum [Fig. 3(a)], the peaks of 2p 3/2 and 2p 1/2, due to the spin–orbit coupling, are located at a binding energy of 780.7 and 796.4 eV with the shake-up satellites of the CoII, respectively. This is ascribed to the CoII oxidation state in Co(OH)2. Reference Yang, Liu, Martens and Frost94 The corresponding shake-up satellite bands owing to plasmon losses and mulitelectron excitation appear in the higher binding energy region, further indicating the CoII oxidation state in the as-obtained α-Co(OH)2 material. Generally, not only the general photoelectron lines but also satellite lines appear in the XPS spectrum of the latter 3d transition metal complex. As for the cobalt compound, CoII is distinguished from CoIII due to the shake-up satellites for CoII but due to very weak or missing satellites for CoIII. In addition, the spin–orbit splitting value of 2p 1/2 and 2p 3/2 is 15.7 eV, which is consistent with that in Co(OH)2. Reference Xue, Wang and Lee95 In the O 1s spectrum [Fig. 3(b)], a single component locates at 531.2 eV corresponding to the oxygen in hydroxides as reported.

FIG. 3. XPS spectra of the α-Co(OH)2/HNFs: (a) Co 2p spectrum and (b) O 1s spectrum.
The porous structure and the specific surface area are always regarded as key factors beneficial for excellent electrocatalytic activity. The N2 adsorption–desorption isotherms of the α-Co(OH)2/HNFs is shown in Fig. 4(a). The surface area of the α-Co(OH)2/HNFs is calculated as high as 78.13 m2/g by the Brunauer–Emmett–Teller (BET) method. The high surface area is originated from the self assembly of ultrathin nanosheets and well-formed hollow structures. The high surface area offers superior surface sites for OER. Figure 4(b) displays the pore size distribution of α-Co(OH)2/HNFs with the main pore diameter between 20 and 130 nm. Generally, polyporous materials possess excellent electrochemical activity due to the exposure of active sites. Moreover, if the pore diameters are abundant, the corresponding polyporous materials will have superior OER performance owning to the quick mass diffusion. The experimental result from N2 adsorption–desorption isotherms confirms the structural feature of α-Co(OH)2 hollow nanoflowers.

FIG. 4. (a) N2 adsorption/desorption isotherms and (b) pore size distribution of α-Co(OH)2/HNFs.
B. Electrocatalytic performance
The electrocatalytic OER performance of α-Co(OH)2 hollow nanoflowers and contrast samples were evaluated by CV measurements in 1 M KOH solutions using a traditional three-electrode system. Figure 5(a) presents the CV curves of α-Co(OH)2/HNFs, α-Co(OH)2/NSs, and β-Co(OH)2/NSs. The overpotential is a crucial parameter for estimating the electrocatalytic water oxidation activity of the materials. As for α-Co(OH)2/HNFs, a current density of 10 mA/cm2 was achieved at an overpotential of 310 mV, which is lower than the values of 324 and 360 mV required for Co(OH)2/NSs and β-Co(OH)2/NSs, respectively. The excellent OER activity of α-Co(OH)2 hollow nanoflowers results from the following reasons. Generally speaking, the distinctive structure and amorphous characteristic of α-Co(OH)2/HNFs influence the electrochemical activity. Firstly, the unique hollow structure affords large spaces that are beneficial for fast diffusion of the electrolyte and releasing of gases. Beyond that, the outer ultrathin nanoflakes offer larger surface areas and more active sites giving rise to a high electrochemical OER activity. In other words, the superior surface areas from the unique hollow architecture and outer ultrathin nanoflakes play a significant role on the excellent activity. Secondly, the surfaces of α-Co(OH)2/HNFs are three-dimensional due to the vertical ultrathin nanoflakes, which are more favorable to adsorb electrolytic ions in a reaction system than that of smooth nanospheres. Furthermore, a disordered structure and amorphous characteristic of the α-Co(OH)2 make contributions to the high performance. As mentioned above, the amorphous CoO x (OH) y surface layer rather than inner crystalline Co3O4 made the main contribution to the outstanding activity in other work. Thus, the amorphous characteristic of α-Co(OH)2/HNFs bulk will account for the superior performance. Last, the porous structure resulted from hollow characteristic also be significant for the large surface areas, which further increase active sites. Additionally, the Tafel analytical method has been commonly used in the research of the OER process because reliable mechanistic and dynamics information can be obtained from the Tafel plot. The Tafel study gives the vital parameters of overpotential and exchange current density. Normally, high exchange current density is of equal importance with a low Tafel slope during the whole OER process. Reference Lai, Li, Jiang, Xu, Lv, Li and Liu96 As shown in Fig. 5(b), the corresponding Tafel plots demonstrate that the Tafel slope of α-Co(OH)2/HNFs is 68.9 mV/dec, which indicates a fast mass and electron transfer process. This value is much smaller than that of α-Co(OH)2/NSs (90.7 mV/dec) and β-Co(OH)2/NSs (94.9 mV/dec).
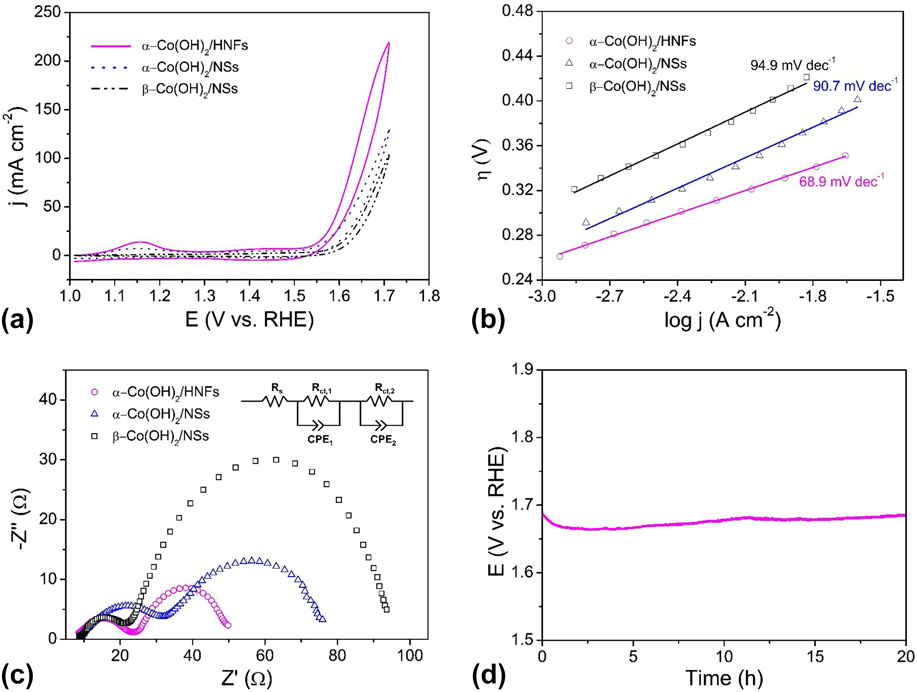
FIG. 5. (a) CV curves of α-Co(OH)2/HNFs, α-Co(OH)2/NSs and β-Co(OH)2/NSs in 1 M KOH; (b) Tafel plots for α-Co(OH)2/HNFs, α-Co(OH)2/NSs and β-Co(OH)2/NSs at scan rate of 1 mV/s; (c) EIS spectra of α-Co(OH)2/HNFs, α-Co(OH)2/NSs, and β-Co(OH)2/NSs. The inset is the equivalent circuit; (d) Chronopotentiometry curves at constant current density of 10 mA/cm2 for the α-Co(OH)2/HNFs without iR compensation with an ITO working electrode.
To investigate electrode surface properties and reaction kinetics, EIS was carried out. Figure 5(c) displays the Nyquist plots of three catalysts, the first semicircle represents solution resistance including the resistance of the electrode material, electrolyte from the working electrode to the counter electrode and other possible resistance, Reference Jin, Mao, Zhan, Xu, Bao and Wang97 which is independent of the potential. Reference Qi, Zhang, Xiang, Liu, Wang, Chen, Han and Cao32 The OER efficiency can be evaluated by the second semicircle, which corresponds to the resistance of the charge transfer during the redox reactions. Generally, a smaller semicircle equals to a lower resistance value, which means a faster charge transfer rate. As shown in Fig. 5(c), α-Co(OH)2/HNFs possesses the lowest resistance during OER due to the distinctive structural features, which suggest a quicker oxygen evolution rate of α-Co(OH)2/HNFs than that of α-Co(OH)2/NSs and β-Co(OH)2/NSs. The equivalent circuit was displayed, in which R s represents the solution resistance, R ct means the charge-transfer resistance, and CPE is a constant-phase element. Stability is another critical index for evaluating electrocatalysts especially for large-scale commercial applications. The long-term stability of Co(OH)2/HNFs was carried out by chronopotentiometry at a current density of 10 mA/cm2 to maintain a constant potential of ∼1.69 V for 20 h [Fig. 5(d)], which indicates that the as-prepared material is highly durable in an alkaline environment. Noteworthy, the unsmooth trace resulted from the formation of oxygen bubbles, which diffuse from the surface of the electrode to the electrolyte. Moreover, the required overpotential to realize a current density of 10 mA/cm2 during the electrolysis was higher than that in the CV experiments. The higher overpotential in the electrolysis is reasonable with the following interpretations. On the one hand, CV studies were carried out with iR compensation, which was absent during electrolysis. On the other hand, an ITO electrode was introduced during electrolysis for convenient O2 release. However, slower mass transfer restricts the whole reaction, leading to higher overpotential for electrolysis. For the same reason, the current density in Fig. 5(d) is lower than that in Fig. 5(a) at the same potential, which is due to the absence of iR compensation during electrolysis. Of course, the lower current density was also caused by the slower mass transfer due to ITO working electrode with higher surface area used in electrolysis.
The ECSAs of the Co(OH)2/HNFs and the counterparts were obtained through investigating the double-layer capacitance (C dl) in the nonFaradaic potential region because the ECSA is in direct proportion to the C dl. The calculation about ECSA is according to the following equations:
j c is the charging current density, ν is the scan rate, C dl is the double layer capacitance, A is the geometric area (0.07 cm2) of working electrode, and C s is the specific capacitance value. Figure 6(a) shows the CV curves of Co(OH)2/HNFs with different scan rates at a potential window from 1.18 to 1.28 V versus RHE. Those of Co(OH)2/NSs and β-Co(OH)2/NSs can be seen in Fig. S2. As displayed in Fig. 6(b), the slope of the charging capacitive current versus the scan rate is equivalent to C dl/A. The C dl of Co(OH)2/HNFs is 2780 μF through equations. Those of Co(OH)2/NSs and β-Co(OH)2/NSs are 1533 μF and 861 μF, respectively. Generally, C s of Co-based materials is 27 μF/cm2 in 1 M KOH. Thus, the corresponding ECSAs are 103, 57, and 32 cm2 for Co(OH)2/HNFs, Co(OH)2/NSs, and β-Co(OH)2/NSs in proper order. This result reveals that Co(OH)2/HNFs possesses the highest active surface area, which is mainly attributed to the unique hollow architecture and outer ultrathin nanoflakes that are electrochemically accessible. Reference Song and Hu98
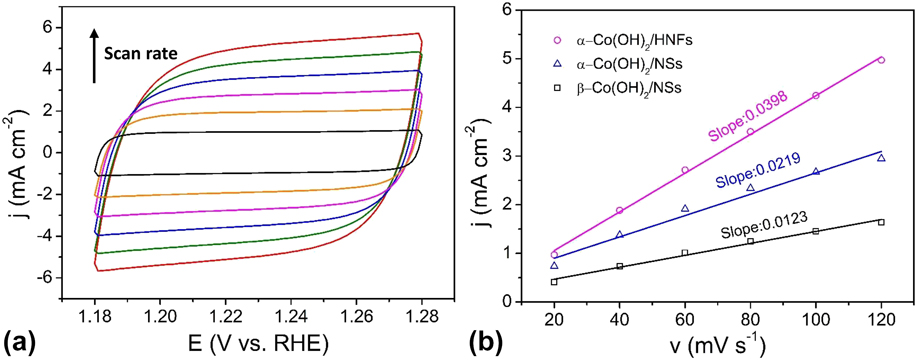
FIG. 6. (a) CV curves of the α-Co(OH)2/HNFs in 1 M KOH at different scan rates from 20 to 120 mV/s; (b) Charging current densities at 1.23 V versus RHE plotted against the scan rate. The linear slope (equivalent to the C dl) was used to represent the ECSA.
IV. CONCLUSIONS
In summary, we used a simple and highly efficient template-based method to synthesize amorphous α-Co(OH)2/HNFs as highly active electrocatalysts for water oxidation in an alkaline solution. Moreover, the template-based method is superior to other traditional template synthesis because no other etching agents are used to remove the templates. Na2S2O3 played roles as the alkali source and etching agent at the same time. Hard and soft acids and bases principle offers the theoretical basis for the choice of reaction reagent and solvent. The whole synthetic was accomplished under mild conditions. The 3D hollow nanoflower architecture, amorphous phase characteristic, surface information, and porous structure were expressed through a series of physical characterizations. The α-Co(OH)2/HNFs exhibited a low overpotential of 310 mV at a current density of 10 mA/cm2, a low Tafel slope of 68.9 mV/dec, and at high electrochemical surface areas. Furthermore, the α-Co(OH)2/HNFs displayed excellent durability for OER in 1 M KOH solution. The long-term chronopotentiometry at a current density of 10 mA/cm2 can provide evidence with a stable potential of ∼1.69 V for 20 h. The oxygen evolution kinetics was revealed from Tafel and EIS techniques. Significantly, the remarkable performance of α-Co(OH)2/HNFs can be attributed to the unique hollow structure that is beneficial for fast mass and electron transfer process and the ultrathin nanoflakes that provide larger surface areas and more catalytic active sites. Additionally, the amorphous characteristic of α-Co(OH)2/HNFs also made a contribution to the outstanding catalytic activity due to the anisotropy, defect, and unordered structure. Therefore, this work provides researchers with a facile method to fabricate amorphous metal hydroxides or oxides with potential electrochemical applications.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1557/jmr.2017.390.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (21101170, 21503126, and 21573139), the Fundamental Research Funds for the Central Universities (GK201603037), the Starting Research Funds of Shaanxi Normal University, and the “Thousand Talents Program” of China.