INTRODUCTION
Pertussis is a rapidly progressive infectious disease of the respiratory tract [Reference Yeh1]. There are 20–40 million cases reported each year, of which 200 000–400 000 are fatal. The mortality rate of the disease in the infancy period is 4% [2]. Vaccination plays an important role in protection against pertussis. Due to routine vaccination programmes, the incidence and mortality rates of the disease have decreased significantly [Reference Petersen3]. However, an increase in the number of cases has recently been reported, most of which were in the population aged >4 years [4, Reference Levy-Bruhl5]. In addition, owing to pertussis being disregarded as a presumptive diagnosis, the exact number of cases might be higher than reported [Reference Hutchins6]. Waning of vaccine-acquired immunity, which occurs 5–10 years following vaccination, can be the factor in the occurrence of pertussis [7]. In Turkey, some studies have shown evidence of pertussis in schoolchildren suffering from prolonged cough. Serosurveillance studies have shown the presence of high antibody levels against pertussis in adolescents and adults, indicating circulation of the bacteria [Reference Aksakal8–Reference Esen10]. Moreover, an estimated level of antibody that would be expected to protect infants, at least until the first application of a pertussis vaccine, was absent in 31·2–42·9% of women of childbearing age, which is indicative of a lack of immunity in infants [Reference Vatansever9, Reference Esen10]. Thus, schoolchildren, adolescents and adults are considered an important source of infection for infants who have not completed primary immunization [Reference Greenberg11].
In this study, we aimed to investigate the immune status of primary and secondary schoolchildren who have been immunized by whole-cell vaccine and to determine the risk for pertussis in a district of Ankara.
MATERIAL AND METHODS
The study included 997 healthy volunteer children attending primary and secondary school, aged 9–17 years. Serum samples were collected between September 2006 and November 2007. Students who had been immunized with four doses of whole-cell pertussis vaccine were included in the study. Students who had been vaccinated with less than four doses, those with an unknown number of doses or those immunized via acellular pertussis vaccine and who had a chronic disease or drug use history were excluded at the sample collection step. Informed consent was obtained from the families of all children. The study was approved by the Gulhane Military Medical Hospital Local Ethics Committee.
Age, gender, household size, monthly income and previous pertussis history as diagnosed by a doctor were recorded. To determine immunity against the disease, serum levels of IgG anti-pertussis toxin (aPT) antibodies were measured.
Venous blood samples (3–5 ml) were taken and centrifuged at 3000 rpm for 3–5 min. Serum samples were stored at −80°C and the serum levels of aPT were measured by in-house ELISA in the Refik Saydam National Hygiene Centre as previously described [Reference Coplu12]. Briefly, this was performed using purified pertussis toxin (PT) (JNIH-5, Japan), Fc-specific alkaline phosphatase-conjugated goat anti-human IgG (Seikagaku Kogyo, Japan), and p-nitrophenyl phosphate (Wako, Japan). Serum aPT concentrations were calculated using a parallel line assay and expressed in ELISA units (EU)/ml according to the reference anti-sera for PT (JNIH-10; 250 EU/ampoule for anti-PT antibody and 400 EU/ml). The limit of detection for aPT antibody was 1·0 EU/ml.
Some studies suggest that serum levels of aPT antibody ⩾10 EU/ml indicate immunity against the disease, and a value ⩾100 EU/ml indicates acute/recent infection with a mean persistence of 4·5 months [Reference de Melker13–Reference Giammanco16]. Therefore, we arbitrarily classified the subjects according to serum levels of aPT: <10 EU/ml (non-immune group), 10–100 EU/ml (immune group), and ⩾100 EU/ml (recent infection group).
The relationship between immunity and gender, age [primary school age (9–14 years), secondary school age (15–17 years)], household size (1–4 and >4), and the monthly total income of the household [<1000 and ⩾1000 Turkish lira (TL)] was evaluated. The last two parameters were considered reasonable for the Turkish population for comparison of socioeconomic conditions. Statistical analysis was performed using SPSS for Windows 15.0 (SPSS Inc., USA). Descriptive statistics were given as mean±standard deviation, median, frequency and percentage. For the evaluation of the relationships the χ2 test was applied.
RESULTS
The study group consisted of 997 students (559 female, 438 male) at primary and secondary school, aged 9–17 years (mean 13·02±2·25, median 13 years). Mean serum levels of aPT antibody were 64·62±141·74 EU/ml and 72·7% of the students had a level of ⩾10 EU/ml. Serum aPT antibody levels of <10 EU/ml, 10–100 EU/ml and ⩾100 EU/ml were 27·3%, 59·3%, and 13·4%, respectively.
aPT antibody levels ⩾10 EU/ml were found in 72·1% and 73·5% of female and male serum samples, respectively, and there was no statistical difference between these two groups (P=0·68). However, the percentage of females with 10–100 EU/ml aPT antibody was significantly higher than males, while the number of males with recent infection was significantly higher than females (P<0·001, P<0·001, respectively) (Table l). Of those in the study, 20 students had a clinical disease history diagnosed by a clinician and there was no statistical difference between males and females (P=0·370). Serum aPT antibody levels of these children were compared in three groups as <10 EU/ml, 10–100 EU/ml and ⩾100 EU/ml and the levels were 40·0%, 45·0%, and 15·0%, respectively. In addition, 25·0% had a cough, 50·0% had sinusitis and 45·0% had an allergy history. Only seven of the children could give a date for the diagnosis of pertussis, although it was during the years 1993–2007.
Table 1. Anti-pertussis toxin (aPT) levels according to gender
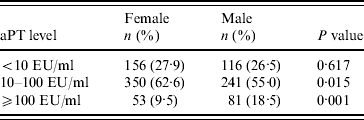
EU, ELISA units.
<10 EU/ml, Non-immunized; ⩾10–100 EU/ml, immune; ⩾100 EU/ml, past or current infection.
Students who had serum levels ⩾10 EU/ml in primary school [9–14 years age group, n=598 (60·0%)] and in secondary school [15–17 years age group, n=399 (40·0%)] were 73·1% and 72·2%, respectively, and showed no statistical difference (P=0·81). Students had higher immunity rates against pertussis in the 15–17 years age group and recent infection history was high in the 9–14 years age group (P<0·001) (Table 2). The ratio of non-immune to immune students had a peak at age 9 years, which diminished gradually and reached its lowest value at 12–13 years, then increased again. In contrast, the lowest percentage of recent infection was at 9 years, then increased, with a peak at age 12 years and then decreased again (Table 3) (Fig. 1).
Table 2. Anti-pertussis toxin (aPT) levels according to age group
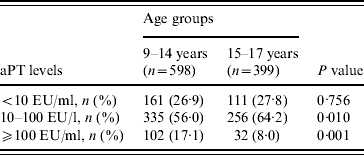
EU, ELISA units.
<10 EU/ml, Non-immunized; ⩾10–100 EU/ml, immune; ⩾100 EU/ml, past or current infection.
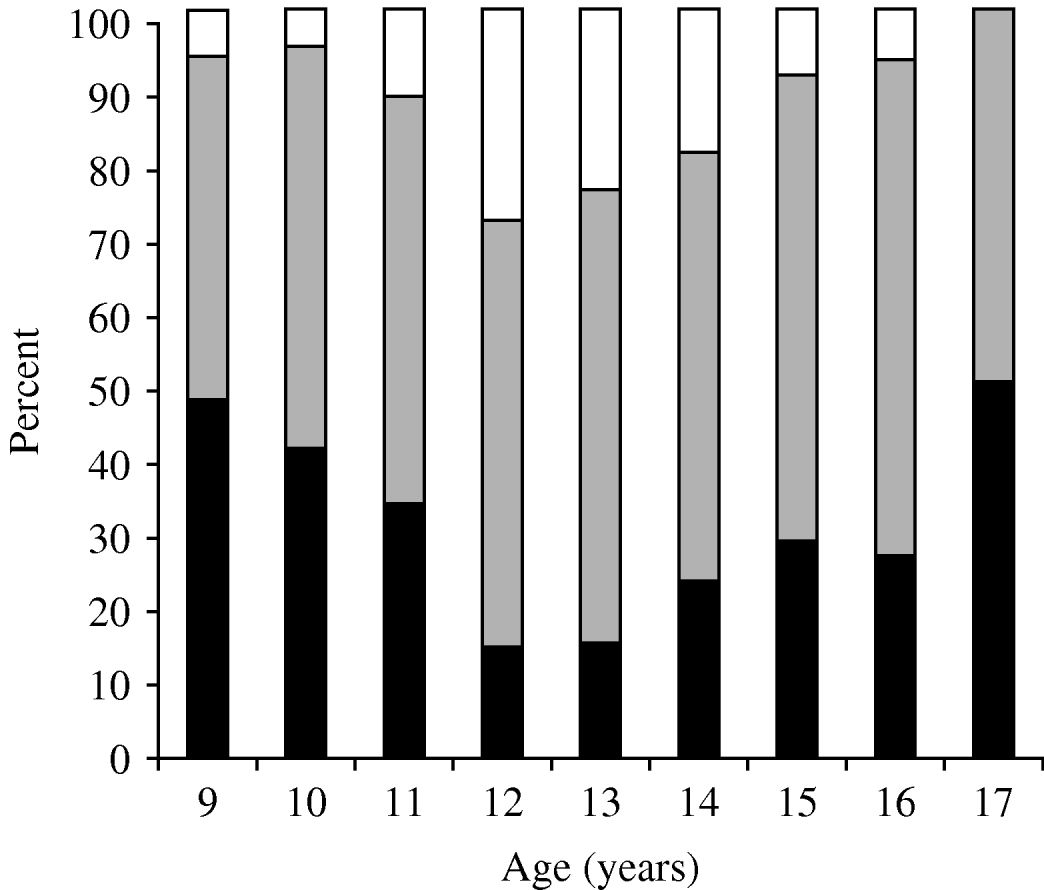
Fig. 1. Immunity status in relation to serum anti-pertussis toxin levels for different age groups. ▪, <10 ELISA units (EU)/ml; ![]() , 10–100 EU/ml; □, ⩾100 EU/ml.
, 10–100 EU/ml; □, ⩾100 EU/ml.
Table 3. Anti-pertussis toxin levels according to age
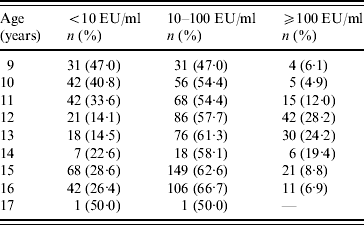
EU, ELISA units.
<10 EU/ml, Non-immunized; ⩾10–100 EU/ml, immune; ⩾100 EU/ml, past or current infection.
The range of household size in the study groups varied from 2 to 13 (median 4). Household size was grouped according to number of family members, as 1–4 and >4. Regarding household size, those with aPT antibody levels ⩾10 EU/ml were 73·5% in the 1–4 member group and 72·0% in the >4 member group (P=0·65).
Of the students where the monthly income was <1000 TL, 72·0% had serum aPT antibody levels ⩾10 EU/ml, and for monthly income ⩾1000 TL, 75·1% had the same level. There was no correlation of monthly income with serum aPT antibody levels (P=0·37).
DISCUSSION
PT is important in the pathogenesis of pertussis. The level of antibody against PT is measured for serological purposes, e.g. evaluation of the immune status of populations [7, Reference Giammanco16]. In addition, a high aPT titre has been proposed as an indicator of acute/recent infection by some authors [Reference de Melker13, Reference Giammanco16]. There are several studies that report pertussis cases in schoolchildren, adolescents and adults, even in highly vaccinated areas [4, Reference Coplu12, Reference Celentano17, Reference Skowronski18]. Vaccine-induced immunity lasts 5–10 years and a waning of immunity makes schoolchildren in particular, as well as the older population, sensitive to the disease. In Turkey, a serosurveillance study comprising all age groups from 6 months to >60 years showed an increasing number of subjects with high levels of aPT antibody, indicating circulation of bacteria. In addition, there was a considerable population with a low titre of aPT antibody, indicating a group at risk of infection, especially the younger age groups, including infants [Reference Esen10].
In our study, 27·3% of students had serum levels <10 EU/ml, which shows there is a considerable portion of the population lacking sufficient immunity. In addition, 13·4% had levels ⩾100 EU/ml, which is indicative of a recent reaction to the antigen, which can be either a symptomatic or an asymptomatic infection. The former has risk for transmission of bacteria to the sensitive population, not only in their age group, but also to siblings who may be infants, while the latter has no risk for transmission. It is generally accepted that passively acquired immunity protects infants up to 6 months, by which time primary immunization should be completed. However, results of recent studies in Turkey have shown that aPT antibody levels can be so low in adolescents and women of childbearing age that they cannot provide protective antibody against pertussis, therefore their infants are at risk [Reference Vatansever9, Reference Esen10]. In order to protect these infants, improvement of primary immunization is not sufficient, and additional vaccination of schoolchildren by acellular vaccine should be discussed, implementing this would be useful in protecting schoolchildren against pertussis. On the other hand, when additional vaccination is discussed, its influence on the whole population should be considered. Vaccination of schoolchildren may cause even lower titres of antibody in mothers due to waning of antibodies after a certain period, and the ratio of sensitive infants will be even more than at present. In addition, those young mothers could become infected and cause transmission of bacteria to their infants. Therefore, additional vaccination discussions should also include adolescents and preventive measures should be adopted.
Interestingly, despite the fact that 13·4% of the subjects had serum aPT antibody levels ⩾100 EU/ml, only 2·0% of them had a past disease history. That may be a result of misdiagnosis in the evaluation of cough, which may be a symptom of pertussis, especially in older children or adolescents. On the other hand, there were 20 students with pertussis history, as diagnosed by a clinician, and aPT levels of these children were variable as well as the date of the history. Lack of laboratory confirmation of pertussis history is a limitation of this study, which makes it difficult to determine if the disease actually was pertussis.
In this study, some demographic determinants were evaluated together with aPT antibody levels, including gender, household size and monthly income. Regarding gender, the percentage of immune females was higher than males, while males who had a past or current infection was significantly higher (P<0·001). In Turkey, the age of first marriage in the female population aged 18–24 years is reported to be 58·7% [19]. Some authors have also found that females have infections more frequently than males [Reference Elliott20–Reference Ozkan and Pekcan22]. Household size showed no statistical difference in serum aPT antibody levels when <4 and ⩾4 members were compared in our study (P=0·65). Although household size has been found to have no influence on the rate of infection [Reference Giammanco23, Reference Plotkin24], Strofolini et al. concluded that for children from a household of ⩾9 members, aPT antibody values were 2·2 times higher than those with <9 members [Reference Stroffolini25].
In conclusion, additional pertussis vaccination should be discussed and this discussion should not only focus on schoolchildren but also on adolescents and adults. However, further multicentre studies in all age groups are needed.
DECLARATION OF INTEREST
None.






