Introduction
Helminth infections of livestock and humans cause serious economic loss and disease throughout the world. With the introduction of the anthelmintic benzimidazoles, and other drugs, there has been substantial progress in chemotherapy of helminth infections (Kohler, Reference Kohler2001). Control of Ascaris lumbricoides var. suum (A. suum) parasites, using the benzimidazole anthelmintic albendazole, is mediated through the selective binding to nematode β-tubulins (Lacey, Reference Lacey1990). This results in inhibition of polymerization and prevents the formation of microtubules and cell division (Shrestha et al., Reference Shrestha2016). Disruption of microtubules leads to loss of chromosome segregation and disruption of kinetochore function during meiosis resulting in aneuploidy, infertility and loss of fecundity (Dawson et al., Reference Dawson1984; Morrissette et al., Reference Morrissette2004; Lyons-Abbott et al., Reference Lyons-Abbott2010). The kinetochore is a specialized structure on the centromeric region of chromosomes comprising specific proteins which regulate the attachment of spindle microtubules during mitosis and meiosis. If the chromosomes are not segregated equally to the gametes during meiosis, there will be an unequal number of chromosomes (aneuploidy) in each gamete. Thus, the loss of the cytoplasmic microtubules results in the death of the nematodes (Shrestha et al., Reference Shrestha2016) and interferes with other cellular functions including metabolism and cellular transport of proteins (Amini et al., Reference Amini2014). Albendazole has also been used as anticancer and antiparasitic drugs (Jordan & Kamath, Reference Jordan and Kamath2007; Sant'anna et al., Reference Sant'anna2013).
In this study, the animal parasitic nematode Ascaris lumbricoides var. suum was exposed to albendazole through treatment of its host animal, Sus domesticus. It reproduces primarily via sexual reproduction (amphimixis). The two sexes are separate and there is distinct sexual dimorphism between the male and female. The adult female has a pair of ovaries and 12 pairs of autosomes with zero X chromosomes (2n = 24A). Nuclei during the pachytene stage are arranged peripherally around a central rachis which provides for synchronous development in that region of the gonad. Pachytene is a specific stage of the first meiotic prophase in which the homologous pairs of chromosomes undergo the process of crossing-over and recombination. The adult male has a single testis and 12 pairs of autosomes in each spermatocyte nucleus (thus, n = 12 for both the male and female). However, the male does have five univalent sex chromosomes, designated ‘Y’ to infer that they are not present in females (Goldstein & Moens, Reference Goldstein and Moens1976). These five Y chromosomes are linked together and distributed to the spermatocyte, which is destined to produce a male. Spermatocytes without the univalent Y chromosomes would produce a female. Thus, dimorphism of spermatozoa is associated with dimorphism of sex. The two sexes experience an unequal number of sex chromosomes, similar to humans. They must compensate for this state of aneuploidy and develop mechanisms for gene expression and dosage compensation.
In A. suum the telogonic gonad contains gametogonia which originate from the proximal end of the gonad and move towards the distal end. As they migrate, they undergo successive stages of gametogenesis and are arranged in a honeycomb pattern. It is only at pachytene that there is complete synchrony of the oocytes, since they are arranged peripherally around a central rachis and are in communication of each via cytoplasmic bridges (Amini et al., Reference Amini2014). Each of these oocytes are in prophase I of meiosis and contains synaptonemal complexes (SCs), which are tripartite, proteinaceous structures that are found between homologous paired chromosomes at pachytene (Goldstein, Reference Goldstein1981a, Reference Goldstein, Zuckerman and Rhodeb). The SC comprises two lateral elements, which are the axial cores of the homologues and a proteinaceous central element. The SC has been highly conserved throughout evolution and occurs in virtually all organisms that reproduce via meiosis. Its role is twofold: (1) maintenance of proximity of homologous chromosomal segments, such that the axial cores of the chromosome become the lateral elements of the SC; and (2) regulation of ordered meiotic disjunction, in which case the SC is maintained in the chiasma. Irregular chromosome segregation and non-disjunction results in the formation of non-viable gametes, aneuploidy and loss of fecundity.
This study represents the first examination of the changes in meiotic nuclear architecture and meiotic chromosomes after exposure to albendazole and provides the basis for the anthelmintic control of nematodes. In this paper, changes in meiotic nuclei, loss of the central rachis, formation of accessory nuclei and loss of SCs in albendazole-exposed nematodes are described.
Materials and methods
Ascaris suum worms were collected from 20 humanely slaughtered pigs as per Food Safety and Inspection Service guidelines (9 C.F.R. 313.1–313.90) (Sus domesticus), near El Paso, TX. Ten of these pigs had been treated per os with the recommended single dosage of 5 mg/kg albendazole. Ten pigs were selected that had not been treated with any anthelmintic. One female worm was obtained from the intestine of each of the treated and non-treated pigs and processed for electron microscopy.
For electron microscopy, the worms were placed into a phosphate-buffered 2% glutaraldehyde solution, pH 7.2, and the ovaries were immediately removed, transferred to fresh fixative and kept in the refrigerator overnight. Post fixation was in Dalton's osmium–chromic acid (Zickler & Olson, Reference Zickler and Olson1975) for 2 h at room temperature, followed by dehydration through an alcohol and propylene oxide series, embedded in Epon and stained with uranyl acetate and Fiske lead citrate (Fiske, Reference Fiske1966). Ultrathin sections were cut on a Porter–Blum ultramicrotome and examined with a Zeiss electron microscope. Light microscopy was performed using the technique of Goldstein & Braselton (Reference Goldstein and Braselton1975), which is a rapid whole-mount procedure using a single-solution Hoyer's mounting medium haematoxylin stain. This facilitates light microscopic examination of nuclear events.
Ten worms were randomly selected from each of the groups: (1) control-unexposed and (2) exposed. In each group, the following parameters were assessed: (1) presence of the SC; (2) presence of a normal bipartite nuclear envelope that was completely contiguous with the nucleoplasm; and (3) presence of the central rachis in the germinal zone at the stage of meiosis prophase I. All statistical analysis in this study were performed using the open-source RStudio software (Version 1.2.5033, 2019 RStudio, Inc.).
Results
The ultrastructure of oocytes in meiotic prophase I revealed numerous aberrations after exposure to albendazole. In untreated worms, the oocytes at meiotic prophase I were arranged peripherally around a central rachis (fig. 1). The epidermal cells of the ovary appeared normal with a contiguous cell membrane and all organelles. The double membrane of the nuclear envelope of each oocyte was completely contiguous with the nucleoplasm. In worms exposed to albendazole, the central rachis deteriorated and SCs were not present in the oocyte nuclei. Remnants of dissociated SCs were discarded into the central rachis (fig. 2). There were no secretory vesicles or vacuoles containing dissociated SCs. Normally, the SC in A. suum consists of two lateral elements and a central element (fig 3) and is essential for the production of viable gametes (Goldstein & Moens, Reference Goldstein and Moens1976).

Fig. 1. Meiotic prophase cross-section through the anterior portion of the ovary of an untreated female of Ascaris lumbricoides var. suum. Oocytes (O) surround the central rachis (R). Epithelial cell (E) of the ovarian wall (×2000).

Fig. 2. The branching rachis (R) contains remnants of dissociated synaptonemal complexes (arrowheads) following treatment with albendazole (×4900).
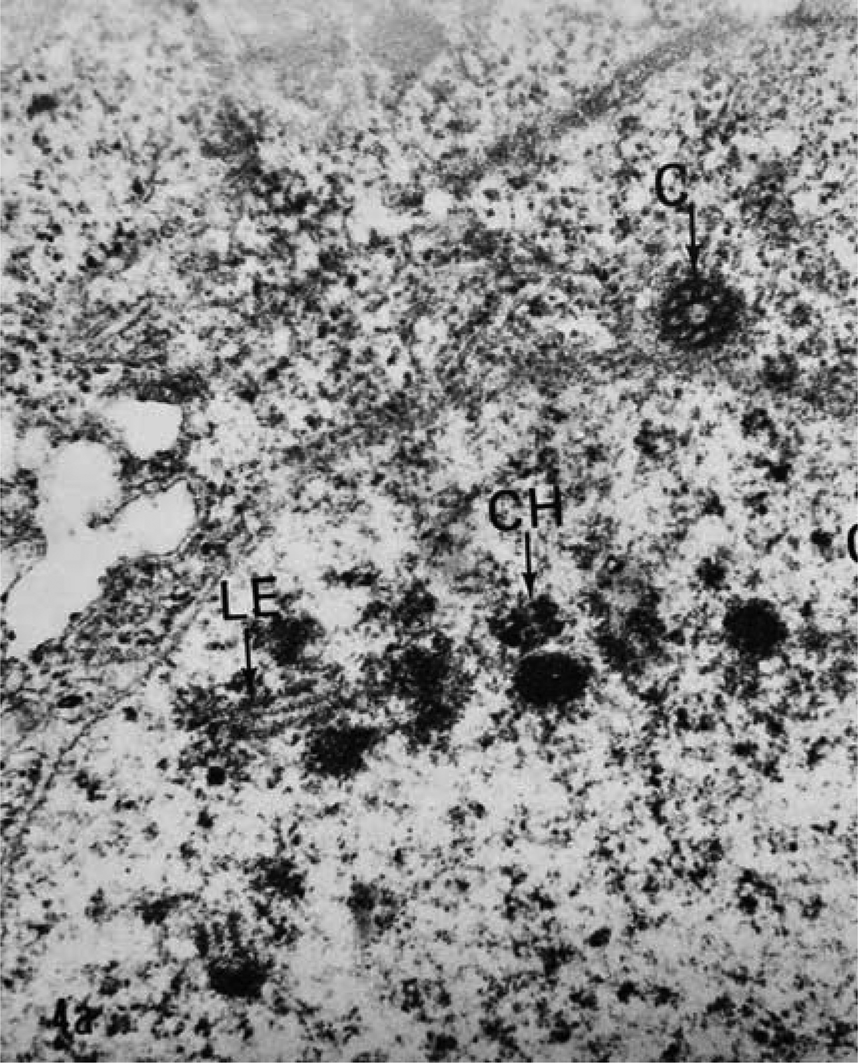
Fig. 3. The synaptonemal complex from an untreated female Ascaris lumbricoides var. suum oocyte is a tripartite structure. The lateral element (LE) central element is the line between the two lateral elements. CH, chromatin; C, centriole (×80,000).
Other changes in oocyte structure and function were noted after exposure to albendazole, including the observation of formation of accessory nuclei (fig. 4). These were observed in all ten of the exposed A. suum female worms. None was present in unexposed control female worms.

Fig. 4. Accessory nuclei (AN, arrow) in albendazole-treated oocytes of Ascaris lumbricoides var. suum. ON, oocyte nucleus; CH, chromatin; G, granular structures (×40,000).
Exposure of A. suum to albendazole increases the propensity of the loss of SCs at meiotic prophase, loss of the contiguous nuclear envelop, and loss of the central rachis in the ovary (table 1). Statistical analysis was performed on the data using the R program for statistical computing. Individual one-sided tests for two proportions were performed to compare the probability of each of the three outcomes between the exposure/control populations. For each of the three proportion tests, the resulting (identical) chi-squared (with Yates’ correction for continuity) values (16.2) and P-values (0.0000285) suggested that the null hypothesis of no difference in propensities could be rejected in favour of the alternative hypothesis of increased propensity in the treated population at both per comparison and family-wise significance level α = 0.05. Since the data were collected in a randomized controlled setting, a causal effect behind the treatment can be further inferred.
Table 1. Ascaris suum female worms exposed to albendazole resulted in complete loss of the synaptonemal complexes (SCs), rachis and a properly formed nuclear envelope (NE) around the developing oocyte.

Discussion
Exposure to albendazole results in abnormal chromosome segregation during meiosis. Meiosis is a specialized cell division that halves the chromosome number and results in the production of gametes (Goldstein, Reference Goldstein1981a, Reference Goldstein, Zuckerman and Rhodeb). Meiosis normally produces gametes containing exactly one copy of each chromosome. Meiotic errors lead to gametes with incorrect chromosome numbers, a major cause infertility and decreased fecundity (Mikwar et al., Reference Mikwar2020). A key step in meiosis I is the separation of homologous chromosomes, which is mediated by the SC. Homologous chromosomes first become physically linked by recombination, which keeps them together until they attach properly at their centromeres via microtubules to the apparatus that will pull them to opposite sides of the cell (Gladston et al., Reference Gladston2009). Disruption of microtubules leads to the production of aneuploidy nuclei and non-viable zygotes (Mikwar et al., Reference Mikwar2020).
In A. suum the central rachis provides the infrastructure for the peripherally arranged syncytial oocytes (Prestage, Reference Prestage1960). Each oocyte is connected via a cytoplasmic bridge to the central rachis. Within a restricted zone, all the oocytes are in meiotic prophase I and normally contain SCs between the homologous paired chromosomes. Recombination and disjunction of the chromosomes follow with equal distribution into developing oocytes. Loss of the central rachis, as observed in albendazole-exposed A. suum oocytes resulted in complete loss of the syncytium and synchrony of development. Loss of the SC results in the production of aneuploidy gametes (Goldstein, Reference Goldstein1981a, Reference Goldstein, Zuckerman and Rhodeb).
Accessory nuclei found in the A. suum oocyte nuclei contained chromosomal material as evidenced by the positive stain reaction for nucleic acids by either acridine orange or by the procedure of Goldstein & Braselton (Reference Goldstein and Braselton1975). The formation of accessory nuclei in A. suum was present only after exposure to albendazole. Their function is not clear, but they most likely represent premature senescence, as benzimidazoles are known to induce senescence. The accessory nuclei are eliminated starting with the fourth embryonic division through the process of chromatin diminution (Goldstein, Reference Goldstein1981a, Reference Goldstein, Zuckerman and Rhodeb).
Albendazole
Albendazole binds specifically to β-tubulin, which is a protein subunit of the microtubules that has a fundamental role in segregation of chromosomes during meiosis. Albendazole-specific binding sites on β-tubulin lead to local disruption of the protein resulting in inhibition of the formation of microtubules (Horton, Reference Horton2000; Kohler, Reference Kohler2001). The anthelmintic efficacy is due to the ability of albendazole to compromise the cytoskeleton through this selective unfolding of a small region within the β-tubulin monomer (Kohler, Reference Kohler2001). The effects of albendazoles on A. suum, and other nematodes, such as Litomosoides (Cardenas et al., Reference Cardenas2010) and Caenorhabditis elegans, (Sant'anna et al., Reference Sant'anna2013) include impaired locomotion, impaired microtubule formation leading to cellular disintegration affecting the germinal zone of the ovary and the oocytes. Since microtubules are involved in so many critical aspects of the cell and reproduction, the albendazole-induced destruction eventually leads to the death of the organism (Kohler, Reference Kohler2001). The sensitivity of the nematode C. elegans to albendazole is mediated by a single gene, ben-1, which encodes β-tubulin. This has provided a platform to investigate the molecular basis of albendazole resistance in parasitic nematodes (Holden-Dye & Walker, Reference Holden-Dye and Walker2007). Studies are in process to determine if A. suum has the same genetic sequence.
In this paper, the effectiveness of albendazole to reduce fecundity in A. suum is demonstrated by the changes in the oocytes and the rachis. The loss of SCs at meiotic prophase I is significant because viable oocytes cannot be formed. The result is the limitation of parasitic infections in individual livestock and human populations thereby controlling transmission (CDC, 2019).
Acknowledgement
I thank Michael Pokojovy, PhD, Assistant Professor of Statistics at the University of Texas at El Paso for his assistance in the statistical analysis of the data.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
The author declares none.







