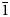Introduction
Rewitzerite was found in specimens of altered zwieselite in the Mineralogical State Collection, Munich, Germany (SNSB). Preliminary energy-dispersive X-ray analyses of rewitzerite crystals indicated that they were close in composition to the recently described mineral pleysteinite, [(H2O)K]Mn2Al3(PO4)4F2(H2O)10⋅4H2O (Grey et al., Reference Grey, Hochleitner, Rewitzer, Kampf, MacRae, Gable, Mumme, Keck and Davidson2023c). Single-crystal diffraction studies showed, however, that the crystals had monoclinic symmetry with space group P21/c, a maximal non-isomorphic subgroup of the space group Pbca for pleysteinite. The mineral and its name (symbol Rwz) have been approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA2023-005, Grey et al., Reference Grey, Hochleitner, Kampf, Boer, MacRae, Mumme and Keck2023b). The name honours Christian H.A. Rewitzer (born 1955) of Furth im Wald, Bavaria. Christian has been a passionate and dedicated mineral collector since he was 14 years old and he now has a collection of +20,000 specimens. His collection includes more than 1000 mineral specimens from the Hagendorf Süd phosphate pegmatite mine that he collected during weekly visits from 1972. After the mine closure in 1984, he turned his research interests to other phosphate pegmatites in Bavaria, Spain and Portugal and has published several papers on them. He has recently established his own mineralogical laboratory, equipped with a scanning electron microscope and powder X-ray diffractometer, so he can conduct preliminary characterisation studies on specimens to determine if they are potential new species. He has been involved in the discovery, characterisation and naming of hydroniumpharmacoalumite, fehrite, alcantarillaite, cuprozheshengite, whiteite-(CaMnFe) and pleysteinite. Christian Rewitzer has agreed to the naming of the mineral in his honour.
The holotype specimen is housed in the mineralogical collections of the Natural History Museum of Los Angeles County, catalogue number 76280. Portions of the original holotype specimen, used for the initial characterisation using energy-dispersive X-ray analysis, and for the colour images are registered as cotype specimens at the Mineralogical State Collection Munich (SNSB), registration number MSM38037.
Rewitzerite belongs to the Dana mineral classification 42.11.21 of hydrated phosphates containing hydroxyl or halogen. This classification group is called the paulkerrite group in Dana's New Mineralogy, edition 8 (Gaines et al., Reference Gaines, Skinner, Foord, Mason and Rosenzweig1997) and comprises paulkerrite (Peacor et al., Reference Peacor, Dunn and Simmons1984), mantienneite (Fransolet et al., Reference Fransolet, Oustriere, Fontan and Pillard1984) and benyacarite (Demartin et al., Reference Demartin, Gay, Gramaccioli and Pilati1997) that have all been reported to have orthorhombic symmetry, space group Pbca, with a ≈ 10.5, b ≈ 20.5 and c ≈ 12.4 Å. The group has been added to recently with the characterisation of two new orthorhombic members, pleysteinite (Grey et al., Reference Grey, Hochleitner, Rewitzer, Kampf, MacRae, Gable, Mumme, Keck and Davidson2023c) and hochleitnerite (Grey et al., Reference Grey, Keck, Kampf, MacRae, Gable, Numme, Glenn and Davidson2023d). The paulkerrite group has been approved as a new mineral group by the IMA-CNMNC, proposal 22-K-bis (Grey et al., Reference Grey, Bosi, Mumme and Boer2023a).
Occurrence and associated minerals
Rewitzerite occurs in specimens of altered zwieselite that were collected in 1971 by one of the authors (EK) from the 47 m level of the Hagendorf Süd pegmatite mine quarry in the Oberpfalz, northeast Bavaria (49°39′1″N, 12°27′35″E). The zwieselite is bleached to a very light beige colour and contains many millimetre-sized vugs. Colourless tabular crystals of rewitzerite and associated rockbridgeite are located on the walls of the vugs. No other minerals were found in the vugs. The leached zwieselite contains residual grains of older columbite, quartz, sphalerite and zircon which sometimes are protruding into the vugs.
Physical and optical properties
Rewitzerite forms clusters of colourless elongated hexagonal-shaped prisms, up to 0.1 mm long (Fig. 1). The crystals are flattened on {010} and elongated along [100], with forms {010}, {001}, {111} and {$\bar{1}$![]() 11} as shown in Fig. 2. The calculated density, for the empirical formula and single-crystal unit-cell volume, is 2.33 g⋅cm–3.
11} as shown in Fig. 2. The calculated density, for the empirical formula and single-crystal unit-cell volume, is 2.33 g⋅cm–3.

Figure 1. Rewitzerite crystals, dark green rockbridgeite and fine-grained zwieselite in vug. Field of view is 0.17 mm. Photo by C. Rewitzer, cotype specimen MSM38037.

Figure 2. Crystal drawing of rewitzerite; clinographic projection in nonstandard orientation, a vertical.
Optically, rewitzerite crystals are biaxial (+), with α = 1.585(2), β = 1.586(2) and γ = 1.615(2) (measured in white light). The measured 2V from extinction data analysed with EXCALIBR (Gunter et al., Reference Gunter, Bandli, Bloss, Evans, Su and Weaver2004) is 25(2)°, and the calculated 2V is 21.3°. Dispersion and pleochroism were not observed. The optical orientation is X = c, Y = b, Z = a. The Gladstone-Dale compatibility index (Mandarino, Reference Mandarino1981) is –0.025 (excellent) based on the empirical formula and the calculated density.
Chemical composition
Crystals of rewitzerite were analysed using wavelength-dispersive electron microprobe (EMP) spectrometry on a JEOL JXA 8500F Hyperprobe operated at an accelerating voltage of 15 kV and a beam current of 2.2 nA. The beam was defocused to 5 μm. Analytical results (average of 12 analyses on 4 crystals) are given in Table 1, where they are compared with the analyses for pleysteinite. There was insufficient material for direct determination of H2O, so it was based upon the crystal structure. The EMP results show a strong negative correlation between atoms of Fe and atoms of Al (Fig. 3).
Table 1. Analytical data (wt.%).

* All Fe as Fe3+ in rewitzerite based on BVS.
** Based on stoichiometry: 4P and 34(K+O+F).

Figure 3. Plot of EMP results, atoms of Fe vs. atoms of Al in rewitzerite.
The atomic proportions, normalised to 4P and 34(K+O+F) atoms per formula unit are:
The general structural formula for monoclinic paulkerrite-group minerals is A1A2M12M22M3(PO4)4X 2(H2O)10⋅4H2O, where A1 and A2 = K and H2O and X = F, OH and O (see Discussion section). Expressing the atomic proportions in this form and taking into account the refined site occupancies from the crystal structure, the empirical formula for rewitzerite is: A 1[K0.77(H2O)0.23]A 2[H2O] M 1(Mn2+0.82Mg0.64Fe3+0.43□0.11)Σ2.00 M 2(Al1.00Ti4+0.67Fe3+0.33)Σ2.00 M 3(Al3+0.51Ti4+0.39Fe3+0.10)Σ1.00 (PO4)4 X[(OH)0.54F0.42O1.04]Σ2.00 (H2O)10⋅4H2O, where □ = vacancy, corresponding to the end-member formula K(H2O)Mn2Al3(PO4)4(OH)2(H2O)10⋅4H2O.
Although this formula was approved by the CNMNC, it is in conflict with the empirical formula that has divalent O2– dominant at the X site. The problem arises because of the high degree of mixing of Ti and Al at the M2 and M3 sites, so that small changes in the distribution of the minor constituent Fe3+ between the two sites can result in different dominant cations at the sites. This is a problem common to all paulkerrite-group minerals, and to overcome it the compositions at the M2 and M3 sites are merged, (M2)2M3, and the composition is plotted on a ternary plot, Al3–Ti3–Fe3, that is divided into different composition fields based on different end-member compositions, Ti3, Al2Ti, Ti2Al etc. The details of this approach will be reported separately, but the outcome for rewitzerite is shown in the ternary plot in Fig. 4. This shows that the (M2)2M3 empirical composition for rewitzerite is located in the Al2Ti end-member compositional field. When this is combined with the dominant A, M1 and X site constituents the end-member formula for rewitzerite is K(H2O)Mn2(Al2Ti)(PO4)4[O(OH)](H2O)10⋅4H2O, which requires K2O 5.04, MnO 15.20, Al2O3 10.92, TiO2 8.56, P2O5 30.40, H2O 29.88, total 100.00 wt.%. The revised end-member formula for rewitzerite has been approved by the IMA-CNMNC (proposal revised 22-K-bis, Grey et al., Reference Grey, Bosi, Mumme and Boer2023a).

Figure 4. Ternary diagram for (M2)2M3 site Al–Ti–Fe3+ compositions, showing end-member compositions (Al2Ti, Ti2Al etc.) and location of the empirical compositions for rewitzerite and pleysteinite.
Raman spectroscopy
Raman spectroscopy was conducted on a Horiba XploRA PLUS spectrometer using a 532 nm diode laser, 100 μm slit and 1800 gr/mm diffraction grating and a 100× (0.9 NA) objective. The spectrometer was calibrated using the 520.7 cm–1 band of silicon. The sample was susceptible to thermal damage at high laser power, so the spectrum was recorded at a laser power of 4mW. The sample was examined after the spectrum was recorded to verify that no thermal damage had occurred.
The spectrum is shown in Fig. 5. The O–H stretch region has a broad band that can be assigned to H-bonded water, with a maximum at 3345 cm–1 and a shoulder at 3110 cm–1. Hydroxyl ion stretching is evident by a sharp shoulder at 3560 cm–1. The H–O–H bending region for water has a band at 1665 cm–1 and a shoulder at 1595 cm–1. Two strong bands at 955 and 1012 cm–1 in the P–O stretching region can be assigned to symmetric stretching modes whereas weaker bands at 1090 and 1135 cm–1 correspond to antisymmetric P–O stretching modes. Bending mode vibrations of the (PO4)3– groups are located at 605 and 455 cm–1. Bands at lower wavenumbers are related to lattice vibrations. A strong band at 845 cm–1 with a shoulder at 780 cm–1 can be assigned to metal–oxygen stretch vibrations for short Ti–O bonds that occur in linear trimers of corner-connected octahedra M2–M3–M2 in the structure, Fig. 6, by analogy with published Raman spectra for titanates containing short Ti–O distances (Tu et al., Reference Tu, Guo, Tao, Katiyar, Guo and Bhalla1996; Bamberger et al., Reference Bamberger, Begun and MacDougall1990, Stassen et al., Reference Stassen, Tarte and Rulmont1998; Su et al., Reference Su, Balmer and Bunker2000). We have observed similar strong bands in other paulkerrite-group minerals, pleysteinite and hochleitnerite that have in common a short M2–X distance of ~1.8 Å. Silva et al. (Reference Silva, Filho, Silva, Balzuweit, Bantiignies, Caetano, Moreira, Freire and Righi2018) have recently reported the modelling of the Raman spectrum of Na2Ti3O7 using first-principles calculations based on density functional theory. The spectrum has a strong band at 849 cm–1 that was assigned as a Ti–O stretching mode for Ti3–O2 and Ti1–O4 bonds with distances of 1.76 and 1.85 Å, respectively (Yakubovich and Kireev, (Reference Yakubovich and Kireev2003). For comparison, rewitzerite has similar M2–O bond distances of 1.77 and 1.82 Å. The shoulder at 780 cm–1 in the Raman spectrum for rewitzerite may be the corresponding Ti–F stretching vibrations for Ti at the M2 sites.

Figure 5. Raman spectrum of rewitzerite.

Figure 6. (010) slice of the rewitzerite structure. Prepared using ATOMS (Dowty, Reference Dowty2004).
Crystallography
Powder X-ray diffraction data were recorded using a Rigaku R-Axis Rapid II curved imaging plate microdiffractometer with monochromatised MoKα radiation. A Gandolfi-like motion on the φ and ω axes was used to randomise the sample. Observed d values and intensities were derived by profile fitting using JADE Pro software (Materials Data, Inc.). Data are given in Table 2. Refined monoclinic unit-cell parameters (space group P21/c (#14)) are a = 10.44(2) Å, b = 20.44(2) Å, c = 12.27(2) Å, β = 90.17(16)°, V = 2620(7) Å3 and Z = 4.
Table 2. Powder X-ray diffraction data (d in Å) for rewitzerite (I calc > 1.5).

Single-crystal diffraction data were collected at the Australian Synchrotron microfocus beamline MX2 (Aragao et al., Reference Aragao, Aishima, Cherukuvada, Clarken, Clift, Cowieson, Ericsson, Gee, Macedo, Mudie, Panjikar, Price, Riboldi-Tunnicliffe, Rostan, Williamson and Caradoc-Davies2018). Intensity data were collected using a Dectris Eiger 16M detector and monochromatic radiation with a wavelength of 0.7107 Å. The crystal was maintained at 100 K in an open-flow nitrogen cryostream during data collections. The diffraction data were collected using a single 36 second sweep of 360° rotation around phi. The resulting dataset consists of 3600 individual images with an approximate phi angle of each image being 0.1 degrees. The raw intensity dataset was processed using XDS software to produce data files that were analysed using SHELXT (Sheldrick, Reference Sheldrick2015) and JANA2006 (Petříček et al., Reference Petříček, Dušek and Palatinus2014). Refined unit-cell parameters and other data collection details are given in Table 3.
Table 3. Crystal data and structure refinement for rewitzerite.

* weight = 1/(σ(F0)2
Structure refinement
A structural model for rewitzerite was obtained in space group P21/c using SHELXT (Sheldrick, Reference Sheldrick2015). Inspection of the model showed that it had the same topology as the Pbca model for pleysteinite (Grey et al., Reference Grey, Hochleitner, Rewitzer, Kampf, MacRae, Gable, Mumme, Keck and Davidson2023c) but with the Pbca sites all split into pairs of sites at x,y,z and ½+x, ½–y, –z. To ensure that the same atom labelling was retained as for pleysteinite for ease of comparison, the pleysteinite atom coordinates were imported to JANA2006 and then transformed to the non-isomorphous subgroup P21/c. Twinning was implemented with 2-fold rotation about c*. Initial assignment of cations to different sites was based on the published site occupancies in the related minerals benyacarite (Demartin et al., Reference Demartin, Pilati, Gay and Gramaccioli1993) and pleysteinite (Grey et al., Reference Grey, Hochleitner, Rewitzer, Kampf, MacRae, Gable, Mumme, Keck and Davidson2023c). Manganese and Mg were incorporated at the M1 sites, Al and Ti at the M2 and M3 sites and K and O (for H2O) at the A sites and their occupancies were refined to obtain the site scattering values. At a later stage of the refinements, a fixed amount of Fe was included at the M2 and M3 sites, to take account of the Al-for-Fe substitution shown in Fig. 3. The amount of Fe in the two sites was adjusted in incremental steps and the Al and Ti occupancies were refined in a series of runs to optimise the agreement between the refined site occupancies and the EMP results. The resulting refined site occupancies and site-scattering values are given in Table 4. Refinement with anisotropic displacement parameters for all atoms in JANA2006 converged at wR obs = 0.068 for 5894 reflections with I > 3σ(I). Details of the data collection and refinement are given in Table 3. The refined coordinates, equivalent isotropic displacement parameters and bond-valence sums (BVS) (using Gagné and Hawthorne, Reference Gagné and Hawthorne2015) from the refinement are reported in Table 5. Selected interatomic distances are reported in Table 6. Although the H atoms were not located, the low BVS in Table 5 for O9 through to O15 are consistent with them being H2O molecules. Possible H-bonded O⋅⋅⋅O pairs involving the water molecules at O9 to O15 and at the A1 and A2 sites are listed in Table 7. Most of the distances are greater than 2.7 Å and correspond to weak H-bonds according to Libowitzky (Reference Libowitzky1999), consistent with the broad Raman band extending from 3200 to 3600 cm–1. Only a few of the distances in Table 7 are below 2.7 Å (2.67–2.68 Å) corresponding to strong H-bonds, consistent with the shoulder at 3110 cm–1 in the Raman spectrum. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 4. Refined site-occupation factors and site scattering for rewitzerite.

Table 5. Refined atom coordinates, equivalent isotropic displacement parameters (Å2) and bond valence sums (BVS, in valence units) for rewitzerite.

Table 6. Polyhedral bond lengths [Å] for rewitzerite.

Table 7. Possible H-bonded O⋅⋅⋅O distances (< 3 Å) in rewitzerite.

Discussion
The monoclinic crystal structure of rewitzerite is closely related to the orthorhombic, Pbca, structures of benyacarite (Demartin et al., Reference Demartin, Pilati, Gay and Gramaccioli1993) and pleysteinite (Grey et al., Reference Grey, Hochleitner, Rewitzer, Kampf, MacRae, Gable, Mumme, Keck and Davidson2023c). The structures are based on heteropolyhedral layers of corner-connected M2(Op)4X(H2O) and M3(Op)4X2 octahedra and PO4 tetrahedra parallel to (010) (Fig. 6), that are connected into 3D frameworks by corner-sharing between the PO4 tetrahedra and M1(Op)2(H2O)4 octahedra along [010] (Fig. 7). A feature of the (010) layers is linear trimers of corner-connected octahedra M2–M3–M2 oriented along ~ [102] and [$\bar{1}$![]() 02]. The M2-site cations are strongly displaced from the centres of the octahedra along <001> towards the bridging X anions to give short M2–X distances of ~1.8 Å (Table 6), manifested by strong Raman M–O stretching modes (Fig. 5). The corner-connected octahedra and tetrahedra form 10-member rings, elongated along [100] (Fig. 6) in which are located the A-site species (K+ and H2O) and zeolitic H2O molecules (O14 and O15). The A1 sites, containing dominant K, and A2 sites, containing dominant H2O, are located in different 10-member rings, centred at 0,y,0 and ½,y,½, respectively in Fig. 6.
02]. The M2-site cations are strongly displaced from the centres of the octahedra along <001> towards the bridging X anions to give short M2–X distances of ~1.8 Å (Table 6), manifested by strong Raman M–O stretching modes (Fig. 5). The corner-connected octahedra and tetrahedra form 10-member rings, elongated along [100] (Fig. 6) in which are located the A-site species (K+ and H2O) and zeolitic H2O molecules (O14 and O15). The A1 sites, containing dominant K, and A2 sites, containing dominant H2O, are located in different 10-member rings, centred at 0,y,0 and ½,y,½, respectively in Fig. 6.

Figure 7. [100] projection of the structure of rewitzerite. Prepared using ATOMS (Dowty, Reference Dowty2004).
Figure 8 shows an (001) slice through the crystal structure centred at z = ¼. It illustrates a different aspect of the structure, viz. the corner-shared linkage of M2-centred octahedra and PO4 tetrahedra into 4-member rings that are fused into kröhnkite-type ribbons (Hawthorne, Reference Hawthorne1985) along [100]. The bridging between adjacent kröhnkite ribbons via the M1(Op)2(H2O)4 octahedra gives 8-member rings that bound [001] channels. The heteropolyhedral (001) layer in rewitzerite (Fig. 8) has the same topology as (001) layers of kröhnkite ribbons interconnected via M1(Op)2(H2O)4 octahedra in laueite-group minerals (Mills and Grey, Reference Mills and Grey2015). Adjacent (001) layers in rewitzerite at z = ¼ and z = ¾ (Fig. 8) are interconnected via corner sharing of the M2-centred octahedra with isolated M3-centred octahedra located in (001) planes at z = 0 and ½.

Figure 8. (001) slice through the rewitzerite structure, at z = ¼. Prepared using ATOMS (Dowty, Reference Dowty2004).
The refined site-occupation factors for the A sites in Table 5 show that there is a high degree of ordering of the K and H2O in different A sites, with 96% of the K at site A1 while the site A2 is occupied 97% by H2O. In related minerals benyacarite and pleysteinite, with orthorhombic symmetry, the A1 and A2 sites are merged into a single A site containing approximately equal amounts of K and H2O. In contrast to the ordering at the A1 and A2 sites, the pairs of M sites in the monoclinic structure show only minor variations in the site scattering (Table 4), bond distances (Table 6) and BVS values (Table 5). This situation is similar to that for jahnsite-group minerals, with general formula XM1M22M32(H2O)8(OH)2(PO4)4 (Kampf et al., Reference Kampf, Alves, Kasatkin and Škoda2019). The M2 and M3 sites in jahnsites each comprise pairs of crystallographically independent sites, but in all jahnsite-group minerals, the pairs of sites have the same or almost the same occupancy, and so in the general formula they are not distinguished. Applying the same reasoning to monoclinic paulkerrite-group minerals the general formula can be written as A1A2M12M22M3(PO4)4X 2(H2O)10⋅4H2O, where the K/H2O-containing sites are separated as A1 and A2, but the pairs of M1, M2 and M3 sites are merged.
Based on their crystal structure refinement for benyacarite, Demartin et al. (Reference Demartin, Pilati, Gay and Gramaccioli1993) considered that the M1 site was occupied by divalent cations, Mn2+, Mg2+ and Fe2+ and in the refinement of pleysteinite (Grey et al., Reference Grey, Hochleitner, Rewitzer, Kampf, MacRae, Gable, Mumme, Keck and Davidson2023c) the BVS value for site M1 was consistent with divalent cations at this site. In contrast, rewitzerite has elevated BVS values for sites M1a (2.22 vu) and M1b (2.20 vu) that require the Fe at the site (after allocation of all Mn2+ and Mg to the site) to be in the trivalent state. This situation is common in mineral groups such as schoonerites and rockbridgeites at Hagendorf Süd, where both reduced species (green Fe2+-bearing) and oxidised (red, Fe3+-bearing) species occur (Grey et al., Reference Grey, Kampf, Keck, MacRae, Cashion and Gozukara2018, Reference Grey, Kampf, Keck Cashion, MacRae, Gozukara and Shanks2019) in close spatial association. The oxidised species generally have M to P ratios that are lower than required by the structural formulae, due to cation vacancies. The EMP analyses for rewitzerite also had lower M:P atomic ratios than the 5:4 required by the structure, corresponding to just over 2% cation vacancies. In the empirical formula, these have been assigned to the M1 sites, but this assignment is somewhat arbitrary – it being difficult to confirm with multiple cations at each site.
Rewitzerite and pleysteinite occupy adjacent (M2)2M3 composition fields corresponding to Al2Ti and Al3, respectively, as shown in Fig. 4, and they have somewhat similar analyses (Table 1), although rewitzerite has only about one third the F content of pleysteinite. There are also significant differences in the contents of M-site cations, with rewitzerite containing 50% less Al and Mn and up to 80% more Fe and Ti than pleysteinite. The EMP data for rewitzerite shows a weak positive correlation between F and Al (R 2 = 0.32) but no correlation of F with Fe or Ti. The crystal-chemical properties of the two minerals are compared in Table 8. Despite the minerals having different symmetries and space groups, their crystallographic parameters (unit-cell parameters and powder diffraction peaks) are closely matched, with the only significant difference being a larger b parameter for pleysteinite due to a higher percentage of large cations (Mn2+ and Fe2+) at the M2 sites. The main crystal-chemical difference associated with the lowering of symmetry in rewitzerite is an ordering of K+ and H2O at the A sites (Fig. 6).
Table 8. Comparison of rewitzerite and pleysteinite.

Acknowledgements
We thank Cameron Davidson for preparing polished mounts for EMP analyses. This research was undertaken in part using the MX2 beamline at the Australian Synchrotron, part of ANSTO, and made use of the Australian Cancer Research detector. Thanks to an anonymous reviewer for bringing the Silva et al. (Reference Silva, Filho, Silva, Balzuweit, Bantiignies, Caetano, Moreira, Freire and Righi2018) reference to our attention.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1180/mgm.2023.55.
Competing interest
The authors declare none.